
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yuhei Nishimura | + 1311 word(s) | 1311 | 2021-04-01 10:56:10 | | | |
| 2 | Vivi Li | -17 word(s) | 1294 | 2021-04-01 12:02:10 | | | | |
| 3 | Yuhei Nishimura | + 53 word(s) | 1364 | 2021-04-01 12:07:09 | | | | |
| 4 | Yuhei Nishimura | + 53 word(s) | 1364 | 2021-04-01 14:59:25 | | | | |
| 5 | Yuhei Nishimura | + 53 word(s) | 1364 | 2021-04-01 15:00:48 | | | | |
| 6 | Vivi Li | + 10 word(s) | 1374 | 2021-04-02 04:53:48 | | |
Video Upload Options
Cisplatin is widely used to treat various types of cancers, but it is often limited by nephrotoxicity. In this study, we employed an integrated in silico and in vivo approach to identify potential treatments for cisplatin-induced nephrotoxicity (CIN). We were able to find that palonosetron, a serotonin type 3 receptor (5-HT3R) antagonist, can suppress CIN. This study supports the use of in silico and in vivo approaches in drug repositioning studies.
1. Introduction
Cisplatin, an inorganic platinum derivative, is one of the most effective anticancer drugs and is widely used for the treatment of many malignancies, including brain, head and neck, lung, breast, and ovarian cancers [1][2]. Cisplatin acts by crosslinking purine bases in DNA. If the resulting damage cannot be reversed by dedicated DNA repair pathways, the cell undergoes apoptosis [1][2]. However, cisplatin can also damage normal tissues, including the kidney, which may lead to severe adverse effects, such as nephrotoxicity [3]. Cisplatin is imported into renal proximal tubule epithelial cells mainly through organic cation transporter 2, localized on the basolateral membrane, and it is excreted into the urine mainly through multidrug and toxin extrusion protein transporter 1, localized on the apical membrane [4][5][6]. In addition to DNA repair pathways, cisplatin activates other signaling cascades that lead to oxidative stress and inflammation in renal proximal tubule epithelial cells, tubular cell death, decline of glomerular filtration rate, and acute kidney failure [3][7][8]. Cisplatin-induced nephrotoxicity (CIN) is often observed within 14 days after initiation of cisplatin treatment [3]. CIN is generally treated and/or prevented by increasing hydration through the administration of isotonic saline, magnesium supplementation, or by mannitol-induced forced diuresis [9]. The latter approach is considered for patients treated with high doses of cisplatin, but mannitol itself may cause dehydration by over-diuresis [9]. Therefore, there is a pressing need for safer and more effective renoprotective therapies for CIN [3][7][8][10].
In recent years, repurposing of approved drugs for new indications, or drug repositioning, has gained considerable interest as a source of new therapies [11][12][13]. Because the candidate drugs have usually passed phase I safety trials, they can directly enter phase II/III safety/efficacy trials for the new indication, greatly reducing the cost and time associated with novel drug development. Drug repositioning has successfully identified therapies for various diseases, including Parkinson’s disease and dry eye [14], and has been aided by the concept of The Connectivity Map [15][16], which is based on the hypothesis that the mechanism of action of two drugs may be similar if they have similar gene expression signature (GES) and that a drug may be therapeutic if it elicits the opposite GES to that induced by the disease [17][18]. The data-driven approach has previously shown success when applied to drug repositioning [18][19].
2. Identification of a Gene Expression Signature Associated with Cisplatin-Induced Nephrotoxicity
To identify a GES associated with CIN, which we termed GES-CIN, we analyzed transcriptome datasets of cisplatin-exposed mouse kidneys [20][21] and human kidney organoid [22] from the Gene Expression Omnibus [23]. These studies analyzed transcriptome alterations at 2 days [21] or 3 days [20][22] after cisplatin treatment. We identified a GES-CIN of 152 and 56 genes that were significantly upregulated and downregulated, respectively, by cisplatin treatment in all three datasets (Figure 1, Tables S1 and S2, supplementary link is https://www.mdpi.com/1424-8247/13/12/480#supplementary). To examine the functions related to this expression signature, we performed enrichment analysis using the Kyoto Encyclopedia of Genes and Genomes (KEGG) [24]. The analysis identified significant enrichment of the GES-CIN in a number of signaling pathways and biological functions, including “p53 signaling”, “TNF signaling”, and “tight junction” (Figure 2, Table S3). Notably, these functions are known to be involved in the mechanisms underlying CIN [7][25], thereby supporting the validity of the 152 and 56 genes identified in this study as a GES-CIN.
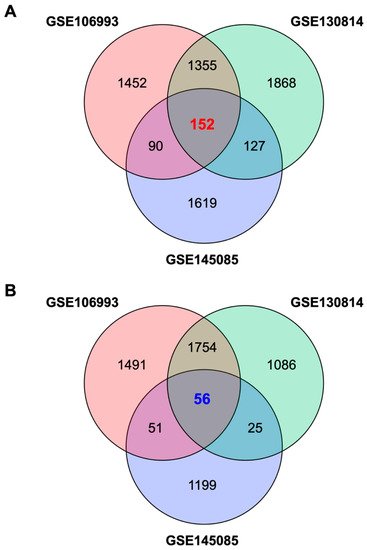
Figure 1. Venn diagrams of genes contained in the gene expression signature associated with cisplatin-induced nephrotoxicity (GES-CIN).
Venn diagrams of unique and shared differentially expressed genes in untreated vs. cisplatin-treated mouse kidneys (GSE106993 and GSE130814) or human kidney organoids (GSE145085). Differentially expressed genes were identified using a false discovery rate threshold of 1% (Table S1). (A,B) show genes upregulated and downregulated by cisplatin treatment, respectively.
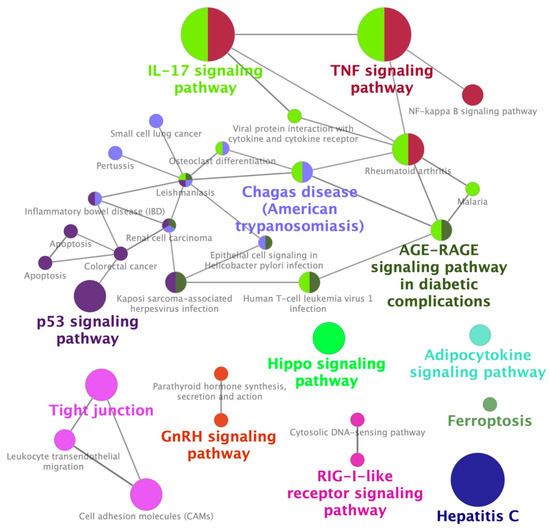
Figure 2. Enrichment of CIN-associated differentially expressed genes in Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
The 208 genes in the GES-CIN (Table S2) were analyzed with ClueGO using KEGG as the reference database to identify significantly enriched functional networks.
3. Identification of Palonosetron as a Potential Therapeutic Drug for CIN
GESs may be causally related to a particular disease or drug side effect; therefore, it is plausible that drugs that induce the opposite pattern of gene expression to the disease/drug might have therapeutic effects [15]. To identify chemicals that may have a GES opposite to that observed for CIN, we used Cmap and Lincs Unified Environment (CLUE), a database that contains 476,251 expression signatures generated by 27,927 perturbagens, including 19,811 small molecule chemicals [16]. This analysis identified 303 chemicals that were predicted to cause a GES opposite to the GES-CIN (Table S4). In parallel, we searched Food and Drug Administration Adverse Event Reporting System (FAERS) database [26] to identify drugs that could potentially suppress adverse events caused by other drugs [27][28][29]; in this case, CIN. Using this database, we screened 23,028 patients who experienced acute kidney injury following cisplatin treatment (Table S5) and identified 16 co-administered drugs that significantly reduced the reporting odds ratio (ROR) of developing acute kidney injury (Table S5). Palonosetron, a 5-HT3R antagonist in clinical use as an antiemetic [30], was included in the 303 chemicals, identified using CLUE, and the 16 drugs, identified using FAERS. These findings suggested that palonosetron may suppress CIN.
4. Suppression of CIN in Patients with Head and Neck Cancer by Palonosetron
To test the validity of our hypothesis, we retrospectively analyzed clinical data from 103 patients with head and neck cancers who received cisplatin and 5-fluorouracil for the first time at Mie University Hospital. Among the 103 patients, 26 and 77 patients received the 5-HT3R antagonists ramosetron and palonosetron, respectively, for the treatment of chemotherapy-induced nausea and vomiting. None of the baseline characteristics differed significantly between the patients receiving ramosetron and palonosetron (Table 1).
Table 1. Characteristics of patients treated with ramosetron or palonosetron.
| Patients’ Characteristics | All Patients (n = 103) |
Ramosetron (n = 26) |
Palonosetron (n = 77) |
p Value |
|---|---|---|---|---|
| Male | 88 (85) | 22 (85) | 66 (86) | 0.891 |
| Age (years) | 64 (33–78) | 66 (33–78) | 64 (34–76) | 0.111 |
| Body weight (kg) | 52 (34–85) | 52 (34–72) | 51 (34–85) | 0.499 |
| Body surface area (m2) | 1.56 (1.29–2.02) | 1.57 (1.29–1.85) | 1.56 (1.22–2.02) | 0.463 |
| Smoking history | 86 (83) | 21 (81) | 65 (84) | 0.606 |
| Drinking history | 67 (65) | 17 (65) | 50 (65) | 0.995 |
| 5-FU dose (mg/day) | 1260 (900–1640) | 1225 (1000–1500) | 1270 (900–1640) | 0.204 |
| Cisplatin dose (mg/day) | 125 (80–164) | 120 (95–150) | 125 (80–164) | 0.105 |
| Baseline biological parameters | ||||
| BUN (mg/dL) | 11.0 (6.5–19.0) | 12.0 (7.0–19.0) | 11 (6.5–19.0) | 0.445 |
| Scr (mg/dL) | 0.73 (0.44–1.02) | 0.77 (0.45–1.01) | 0.72 (0.44–1.02) | 0.538 |
| Hemoglobin (g/dL) | 12.4 (8.2–16.9) | 12.1 (8.9–15.1) | 12.5 (8.2–16.9) | 0.463 |
| Platelet (×109 L) | 220 (58–417) | 217 (82–407) | 220 (58–417) | 0.566 |
| White blood cells (×109 L) | 5.27 (2.10–14.9) | 6.20 (2.10–14.9) | 5.14 (2.21–14.4) | 0.477 |
| Co-administrated | ||||
| NSAIDs | 12 (12) | 4 (15) | 8 (10) | 0.396 |
| Magnesium oxide | 26 (25) | 7 (27) | 19 (25) | 0.776 |
| Proton pump inhibitors | 14 (14) | 4 (15) | 10 (13) | 0.632 |
5-fluorouracil (5-FU); blood urea nitrogen (BUN); serum concentrations of creatinine (Scr); non-steroidal anti-inflammatory drugs (NSAIDs).
Analysis of the maximum serum concentrations of creatinine (Scr) and blood urea nitrogen (BUN) in the first 14 days of cisplatin treatment showed that both variables were significantly lower in the palonosetron group compared with the ramosetron group (Figure 3), whereas the overall survival rates of the patients were not significantly different (Figure 4).
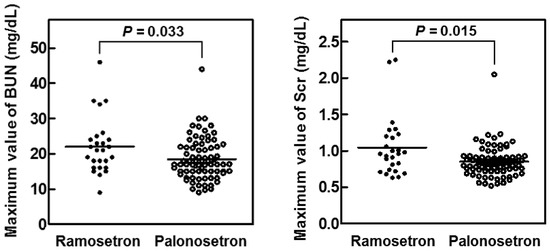
Figure 3. Maximum values of serum creatinine (Scr) and blood urea nitrogen (BUN) in patients treated for 14 days with cisplatin and either ramosetron or palonosetron.
Patients with head and neck cancer (n = 103) received cisplatin and 5-fluorouracil together with ramosetron (n = 26) or palonosetron (n = 77). p values determined by the Mann–Whitney U test.
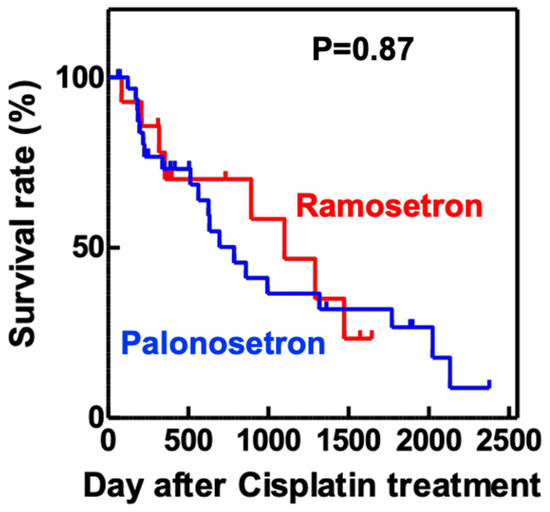
Figure 4. Kaplan–Meier overall survival analysis of patients treated with cisplatin and either ramosetron or palonosetron.
Patients with head and neck cancer (n = 103) received cisplatin and 5-fluorouracil, together with either ramosetron (n = 26) or palonosetron (n = 77), to treat chemotherapy-induced nausea and vomiting. p value determined using the log-rank test.
5. Palonosetron Treatment Increases the Survival of Cisplatin-Exposed Zebrafish
Finally, we confirmed the protective effect of palonosetron on cisplatin-induced toxicity using a zebrafish model, in which cisplatin was co-administered with various 5-HT3R antagonists. This analysis revealed that palonosetron, granisetron, and ondansetron did not affect the survival of zebrafish when administered alone; however, palonosetron, but not granisetron or ondansetron, significantly increased the survival rate of zebrafish exposed to a lethal dose of cisplatin (Figure 5). These results suggest not only that palonosetron may suppress cisplatin-induced toxicity but also that the mechanism of action may not be through inhibition of 5-HT3R.
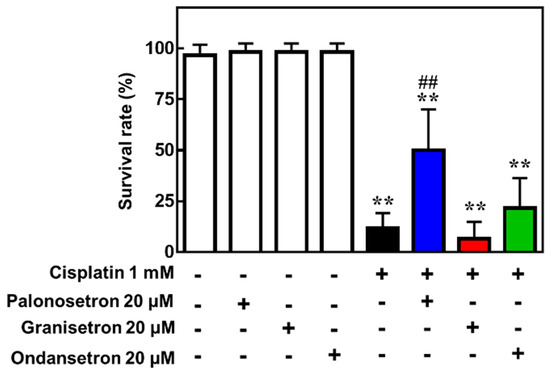
Figure 5. Effects of 5-hydroxytryptamine receptor 3 (5-HT3R) antagonists on the survival of zebrafish exposed to cisplatin.
Zebrafish (5 days post-fertilization) were treated with the indicated combinations of drugs for 24 h, and survival was assessed. Mean ± SEM of n = 6. ** p < 0.01 vs. no drug group, ## p < 0.01 vs. cisplatin only group by Dunnett’s test.
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378.
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925.
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007.
- Perazella, M.A. Pharmacology behind Common Drug Nephrotoxicities. Clin. J. Am. Soc. Nephrol. 2018, 13, 1897–1908.
- Oda, H.; Mizuno, T.; Ikejiri, M.; Nakamura, M.; Tsunoda, A.; Ishihara, M.; Saito, K.; Tamaru, S.; Yamashita, Y.; Nishimura, Y.; et al. Risk factors for cisplatin-induced acute kidney injury: A pilot study on the usefulness of genetic variants for predicting nephrotoxicity in clinical practice. Mol. Clin. Oncol. 2020, 13, 58.
- Hiramatsu, S.I.; Ikemura, K.; Fujisawa, Y.; Iwamoto, T.; Okuda, M. Concomitant lansoprazole ameliorates cisplatin-induced nephrotoxicity by inhibiting renal organic cation transporter 2 in rats. Biopharm. Drug Dispos. 2020, 41, 239–247.
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3011.
- Volarevic, V.; Djokovic, B.; Jankovic, M.G.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N. Molecular mechanisms of cisplatin-induced nephrotoxicity: A balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019, 26, 25.
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619.
- Casanova, A.G.; Hernández-Sánchez, M.T.; López-Hernández, F.J.; Martinez-Salgado, C.; Prieto, M.; Vicente-Vicente, L.; Morales, A.I. Systematic review and meta-analysis of the efficacy of clinically tested protectants of cisplatin nephrotoxicity. Eur. J. Clin. Pharmacol. 2020, 76, 23–33.
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58.
- Nishimura, Y.; Hara, H. Editorial: Drug Repositioning: Current Advances and Future Perspectives. Front. Pharmacol. 2018, 9, 1068.
- Ikemura, K.; Hiramatsu, S.; Okuda, M. Drug Repositioning of Proton Pump Inhibitors for Enhanced Efficacy and Safety of Cancer Chemotherapy. Front. Pharmacol. 2017, 8, 911.
- Nishimura, Y.; Tagawa, M.; Ito, H.; Tsuruma, K.; Hara, H. Overcoming Obstacles to Drug Repositioning in Japan. Front. Pharmacol. 2017, 8, 729.
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using Gene-Expression Signatures to Connect Small Molecules, Genes, and Disease. Science 2006, 313, 1929.
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.
- Montero-Melendez, T.; Perretti, M. Connections in pharmacology: Innovation serving translational medicine. Drug Discov. Today 2014, 19, 820–823.
- Musa, A.; Ghoraie, L.S.; Zhang, S.D.; Glazko, G.; Yli-Harja, O.; Dehmer, M.; Haibe-Kains, B.; Emmert-Streib, F. A review of connectivity map and computational approaches in pharmacogenomics. Brief. Bioinform. 2018, 19, 506–523.
- Gns, H.S.; Gr, S.; Murahari, M.; Krishnamurthy, M. An update on Drug Repurposing: Re-written saga of the drug’s fate. Biomed. Pharmacother. 2019, 110, 700–716.
- Späth, M.R.; Bartram, M.P.; Palacio-Escat, N.; Hoyer, K.J.R.; Debes, C.; Demir, F.; Schroeter, C.B.; Mandel, A.M.; Grundmann, F.; Ciarimboli, G.; et al. The proteome microenvironment determines the protective effect of preconditioning in cisplatin-induced acute kidney injury. Kidney Int. 2019, 95, 333–349.
- Cao, Y.; Mi, X.; Zhang, D.; Wang, Z.; Zuo, Y.; Tang, W. Transcriptome sequencing of circular RNA reveals a novel circular RNA-has_circ_0114427 in the regulation of inflammation in acute kidney injury. Clin. Sci. 2020, 134, 139–154.
- Digby, J.L.M.; Vanichapol, T.; Przepiorski, A.; Davidson, A.J.; Sander, V. Evaluation of cisplatin-induced injury in human kidney organoids. Am. J. Physiol. Renal. Physiol. 2020, 318, F971–F978.
- Wang, Z.; Lachmann, A.; Ma’ayan, A. Mining data and metadata from the gene expression omnibus. Biophys. Rev. 2019, 11, 103–110.
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.; Pages, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093.
- Trujillo, J.; Molina-Jijón, E.; Medina-Campos, O.N.; Rodriguez-Munoz, R.; Reyes, J.L.; Loredo, M.L.; Barrera-Oviedo, D.; Pinzon, E.; Rodriguez-Rangel, D.S.; Pedraza-Chaverri, J. Curcumin prevents cisplatin-induced decrease in the tight and adherens junctions: Relation to oxidative stress. Food Funct. 2016, 7, 279–293.
- FAERS. FDA Adverse Event Reporting System. Available online: (accessed on 19 December 2020).
- Nagashima, T.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Prevention of antipsychotic-induced hyperglycaemia by vitamin D: A data mining prediction followed by experimental exploration of the molecular mechanism. Sci. Rep. 2016, 6, 26375.
- Okada, N.; Niimura, T.; Zamami, Y.; Hamano, H.; Ishida, S.; Goda, M.; Takechi, K.; Chuma, M.; Imanishi, M.; Ishizawa, K. Pharmacovigilance evaluation of the relationship between impaired glucose metabolism and BCR-ABL inhibitor use by using an adverse drug event reporting database. Cancer Med. 2019, 8, 174–181.
- Ikemura, K.; Hiramatsu, S.I.; Shinogi, Y.; Nakatani, Y.; Tawara, I.; Iwamoto, T.; Katayama, N.; Okuda, M. Concomitant febuxostat enhances methotrexate-induced hepatotoxicity by inhibiting breast cancer resistance protein. Sci. Rep. 2019, 9, 20359.
- Sanger, G.J.; Andrews, P.L.R. A History of Drug Discovery for Treatment of Nausea and Vomiting and the Implications for Future Research. Front. Pharmacol. 2018, 9, 913.




