Cisplatin is widely used to treat various types of cancers, but it is often limited by nephrotoxicity. In this study, we employed an integrated in silico and in vivo approach to identify potential treatments for cisplatin-induced nephrotoxicity (CIN). We were able to find that palonosetron, a serotonin type 3 receptor (5-HT3R) antagonist, can suppress CIN. This study supports the use of in silico and in vivo approaches in drug repositioning studies.
- cisplatin
- nephrotoxicity
- drug repositioning
- data-driven approach
- gene expression signature
- adverse events
- zebrafish
1. Introduction
Cisplatin, an inorganic platinum derivative, is one of the most effective anticancer drugs and is widely used for the treatment of many malignancies, including brain, head and neck, lung, breast, and ovarian cancers [1,2]. Cisplatin acts by crosslinking purine bases in DNA. If the resulting damage cannot be reversed by dedicated DNA repair pathways, the cell undergoes apoptosis [1,2]. However, cisplatin can also damage normal tissues, including the kidney, which may lead to severe adverse effects, such as nephrotoxicity [3]. Cisplatin is imported into renal proximal tubule epithelial cells mainly through organic cation transporter 2, localized on the basolateral membrane, and it is excreted into the urine mainly through multidrug and toxin extrusion protein transporter 1, localized on the apical membrane [4,5,6]. In addition to DNA repair pathways, cisplatin activates other signaling cascades that lead to oxidative stress and inflammation in renal proximal tubule epithelial cells, tubular cell death, decline of glomerular filtration rate, and acute kidney failure [3,7,8]. Cisplatin-induced nephrotoxicity (CIN) is often observed within 14 days after initiation of cisplatin treatment [3]. CIN is generally treated and/or prevented by increasing hydration through the administration of isotonic saline, magnesium supplementation, or by mannitol-induced forced diuresis [9]. The latter approach is considered for patients treated with high doses of cisplatin, but mannitol itself may cause dehydration by over-diuresis [9]. Therefore, there is a pressing need for safer and more effective renoprotective therapies for CIN [3,7,8,10].
In recent years, repurposing of approved drugs for new indications, or drug repositioning, has gained considerable interest as a source of new therapies [11,12,13]. Because the candidate drugs have usually passed phase I safety trials, they can directly enter phase II/III safety/efficacy trials for the new indication, greatly reducing the cost and time associated with novel drug development. Drug repositioning has successfully identified therapies for various diseases, including Parkinson’s disease and dry eye [14], and has been aided by the concept of The Connectivity Map [15,16], which is based on the hypothesis that the mechanism of action of two drugs may be similar if they have similar gene expression signature (GES) and that a drug may be therapeutic if it elicits the opposite GES to that induced by the disease [17,18]. The data-driven approach has previously shown success when applied to drug repositioning [18,19].
2. Identification of a Gene Expression Signature Associated with Cisplatin-Induced Nephrotoxicity
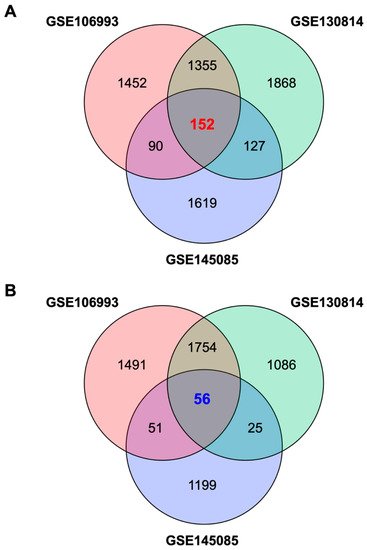
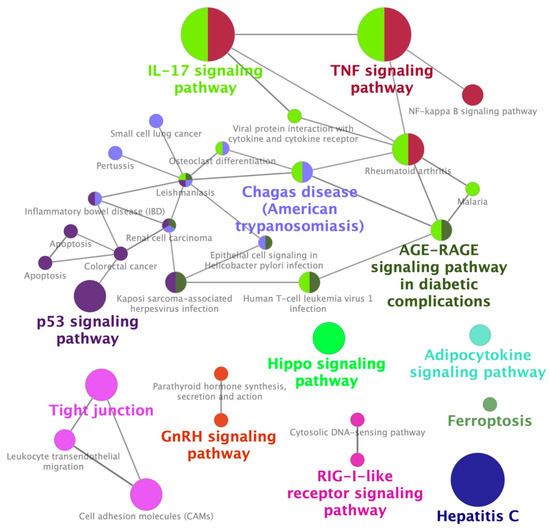
3. Identification of Palonosetron as a Potential Therapeutic Drug for CIN
4. Suppression of CIN in Patients with Head and Neck Cancer by Palonosetron
To test the validity of our hypothesis, we retrospectively analyzed clinical data from 103 patients with head and neck cancers who received cisplatin and 5-fluorouracil for the first time at Mie University Hospital. Among the 103 patients, 26 and 77 patients received the 5-HT3R antagonists ramosetron and palonosetron, respectively, for the treatment of chemotherapy-induced nausea and vomiting. None of the baseline characteristics differed significantly between the patients receiving ramosetron and palonosetron (Table 1).
| Patients’ Characteristics | All Patients (n = 103) |
Ramosetron (n = 26) |
Palonosetron (n = 77) |
p Value |
|---|---|---|---|---|
| Male | 88 (85) | 22 (85) | 66 (86) | 0.891 |
| Age (years) | 64 (33–78) | 66 (33–78) | 64 (34–76) | 0.111 |
| Body weight (kg) | 52 (34–85) | 52 (34–72) | 51 (34–85) | 0.499 |
| Body surface area (m2) | 1.56 (1.29–2.02) | 1.57 (1.29–1.85) | 1.56 (1.22–2.02) | 0.463 |
| Smoking history | 86 (83) | 21 (81) | 65 (84) | 0.606 |
| Drinking history | 67 (65) | 17 (65) | 50 (65) | 0.995 |
| 5-FU dose (mg/day) | 1260 (900–1640) | 1225 (1000–1500) | 1270 (900–1640) | 0.204 |
| Cisplatin dose (mg/day) | 125 (80–164) | 120 (95–150) | 125 (80–164) | 0.105 |
| Baseline biological parameters | ||||
| BUN (mg/dL) | 11.0 (6.5–19.0) | 12.0 (7.0–19.0) | 11 (6.5–19.0) | 0.445 |
| Scr (mg/dL) | 0.73 (0.44–1.02) | 0.77 (0.45–1.01) | 0.72 (0.44–1.02) | 0.538 |
| Hemoglobin (g/dL) | 12.4 (8.2–16.9) | 12.1 (8.9–15.1) | 12.5 (8.2–16.9) | 0.463 |
| Platelet (×109 L) | 220 (58–417) | 217 (82–407) | 220 (58–417) | 0.566 |
| White blood cells (×109 L) | 5.27 (2.10–14.9) | 6.20 (2.10–14.9) | 5.14 (2.21–14.4) | 0.477 |
| Co-administrated | ||||
| NSAIDs | 12 (12) | 4 (15) | 8 (10) | 0.396 |
| Magnesium oxide | 26 (25) | 7 (27) | 19 (25) | 0.776 |
| Proton pump inhibitors | 14 (14) | 4 (15) | 10 (13) | 0.632 |
Analysis of the maximum serum concentrations of creatinine (Scr) and blood urea nitrogen (BUN) in the first 14 days of cisplatin treatment showed that both variables were significantly lower in the palonosetron group compared with the ramosetron group (Figure 3), whereas the overall survival rates of the patients were not significantly different (Figure 4).
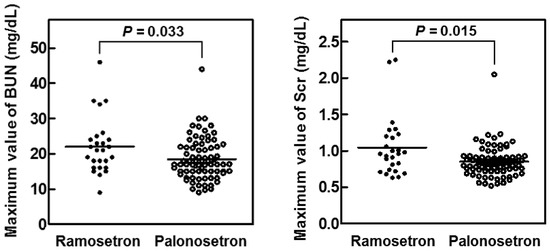
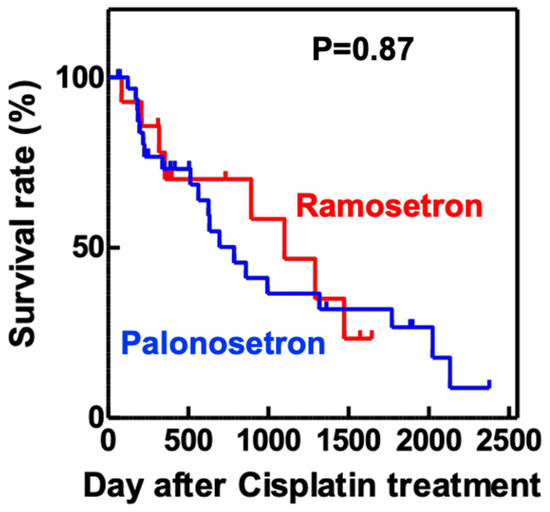
Figure 4. Kaplan–Meier overall survival analysis of patients treated with cisplatin and either ramosetron or palonosetron. Patients with head and neck cancer (n = 103) received cisplatin and 5-fluorouracil, together with either ramosetron (n = 26) or palonosetron (n = 77), to treat chemotherapy-induced nausea and vomiting. p value determined using the log-rank test.
5. Palonosetron Treatment Increases the Survival of Cisplatin-Exposed Zebrafish
Finally, we confirmed the protective effect of palonosetron on cisplatin-induced toxicity using a zebrafish model, in which cisplatin was co-administered with various 5-HT3R antagonists. This analysis revealed that palonosetron, granisetron, and ondansetron did not affect the survival of zebrafish when administered alone; however, palonosetron, but not granisetron or ondansetron, significantly increased the survival rate of zebrafish exposed to a lethal dose of cisplatin (Figure 5). These results suggest not only that palonosetron may suppress cisplatin-induced toxicity but also that the mechanism of action may not be through inhibition of 5-HT3R.
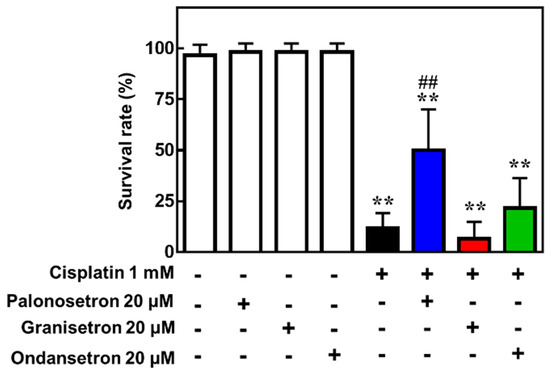
Figure 5. Effects of 5-hydroxytryptamine receptor 3 (5-HT3R) antagonists on the survival of zebrafish exposed to cisplatin. Zebrafish (5 days post-fertilization) were treated with the indicated combinations of drugs for 24 h, and survival was assessed. Mean ± SEM of n = 6. ** p < 0.01 vs. no drug group, ## p < 0.01 vs. cisplatin only group by Dunnett’s test.
This entry is adapted from the peer-reviewed paper 10.3390/ph13120480
