
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mosab Kaseem | + 1197 word(s) | 1197 | 2021-03-29 11:04:43 | | | |
| 2 | Rita Xu | Meta information modification | 1197 | 2021-03-30 05:37:53 | | |
Video Upload Options
Plasma electrolytic oxidation (PEO) is an effective surface modification method for producing ceramic oxide layers on metals and their alloys. Although inorganic electrolytes are widely used in PEO, the organic additives have received considerable interest in the last decade due to their roles in improving the final voltage and controlling spark discharging, which lead to significant improvements in the performance of the obtained coatings.
1. Introduction
Plasma electrolytic oxidation (PEO) is an effective coating method of valve metals to fabricate well-adherent oxide layers, which impart improved wear and corrosion performances in many aggressive environments [1][2][3][4][5][6][7]. The formation of PEO coatings is usually accompanied by a series of simultaneous events, such as acoustic emission, luminescence, and heat release, which are associated with a localized electric breakdown of the growing oxide layer [1]. Typically, the resultant PEO coating exhibited a large thickness up to ~200 μm depending on processing conditions, which certainly improves corrosion resistance [8]. Earlier works on PEO have reported that the surface properties of PEO coatings are mainly affected by the composition of the electrolyte, current mode, coating time, as well as the electrical parameters, namely, current density, current frequency, duty cycle, and cell potential [9][10][11][12][13][14][15][16][17][18][19][20]. Therefore, optimizing the processing parameters during PEO would be important as these parameters affect the characteristics of plasma discharges thereby, affecting the quality of the PEO coatings [1]. It should be pointed out that the modification of plasma discharges via the utilization of electrolyte composition has been in the spotlight since the combination of available elements generates many different compounds and potentially leads to different plasma behavior. Two major types of electrolytes are those containing organic compounds and those containing inorganic compounds [21][22][23][24][25][26]. While numerous investigations showed that organic compounds tend to be precluded in the coating layer during coating growth, the use of inorganic compounds generally showed successful incorporation [21].
PEO treatment as a kind of surface treatment of valve metals has previously been reviewed by several works [27][28][29][30][31]. For example, a critical review discussing the principle, structure, and performance of PEO coatings was reported recently by Kaseem et al. [1]. A review discussing the mechanism and performance of PEO-coated Mg alloys was documented by Darband et al. [27]. An interesting review very recently reported on micro-discharge characteristics during PEO treatment [28]. The impacts of different particles on the characteristics of PEO coating have been reviewed by Lu et al. [30]. To date, however, the types of organic additives added to the electrolyte during PEO and their roles in fabricating compact coatings with improved protective properties have not been given sufficient attention.
2. Influence of Organic Additive on the Electrical Response of PEO
Generally, organic additives tend to adsorb on the surface of metallic substrates during PEO treatment through their functional groups (−OH, –COOH, –NH–CO–, etc.), which would prevent the inorganic additives—the main components of the electrolyte—and dissolved oxygen from transferring to the anodic substrate, leading to easy increments in the cell voltage [32][33][34][35][36][37]. For example, Pak et al. [38] examined the influence of organic polar liquids, such as glycerol (GLY), triethanolamine (TEA), and 3-aminopropyltrimethoxysilane (APTMS) on the voltage-time response during PEO treatment of AZ31B Mg alloy. They reported that organic compounds interacted with the Mg surface through their –OH groups, which separated the substrate surface from the electrolyte and increased the resistance of the coating. Similar findings were reported during PEO treatment of Mg alloys using other organic additives, such as starch [39][40], benzotriazole (BTA) [41], sodium oxalate (SOx) [42], and phytic acid [43]. However, the effect of organic additives on the voltage response during PEO was still controversial [44][45][46]. For instance, Zhu et al. [44] reported that the breakdown and final voltages during PEO treatment of AZ31B Mg alloy in electrolytes containing 10 g/L ethylene glycol (EG) and polyethylene glycol (PEG) as organic additives were almost identical to the counterparts obtained in electrolytes without organic additives. The adsorption of these additives by their segments –CH2–CH2–O– on the surface of the Mg alloy, with carbon atoms aligned toward the substrate surface and the oxygen atoms oriented toward the electrolyte, was found to have an insignificant effect on the voltage response as compared to the sample without additives. The authors ascribed this result to the high molecular weight of PEG which reduces the adsorption ability of this additive. On the other hand, some organic additives, like ethanol (EtOH) would have no obvious increase in the voltage during PEO. Sun and co-workers [45] also reported that only minor changes in the voltage-time curves of PEO processes conducted in silicate electrolytes without or with an addition of 10 g/L of either halloysite nanotubes (HNT) or BT- loaded HNT. According to Asoh et al. [46], the PEO treatment of AZ31B Mg alloy using a phosphate electrolyte (0.25 M Na3PO4) containing 5 vol.% EtOH as an organic additive had no obvious increase in voltage after reaching the value of breakdown voltage (Figure 1a), suggesting that ethanol was not desirable for coating formation presumably due to its somewhat low pH (12.65) and weak base compared with that comprising only Na3PO4 (12.73). This result indicated that ethanol could suppress the dissociation of the electrolytes during PEO. In contrast, when other additives, such as GLY and EG, were included in the phosphate electrolyte, the rates of the increase of voltage were higher than when the PEO process was performed in electrolytes without GLY and EG. The electrolyte conductivity would be reduced by the inclusion of alcohol into the Na3PO4-based electrolyte. In other words, the electrolyte resistance could be increased as the addition of EG and GLY led to an increase in the electrolyte viscosity. Microstructural observations revealed that EG or GLY utilized as an additive could act as an enhancer for film qualities, leading to the development of coatings with fewer micropores.
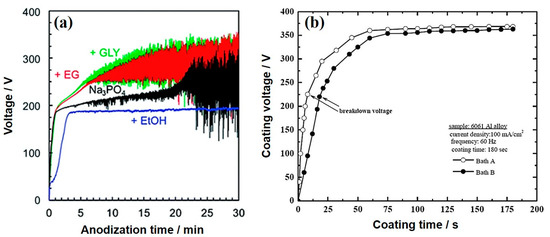
Figure 1. (a) Voltage-time curves of AZ31 Mg alloy coated via plasma electrolytic oxidation (PEO) in electrolytes without and with organic additives, such as ethanol (EtOH), ethylene glycol (EG), and glycerol (GLY). [46] (b) Voltage-time curves of 6061 Al alloy coated in the electrolytes without and with NaBz. Reprinted from permission from [47]. Copyright 2015 Elsevier
Kaseem et al. [47] claimed that the addition of 1 g/L sodium benzoate (NaBz) into the alkaline-aluminate (5.61 KOH + 4.098 g/L NaAlO2) electrolyte during PEO treatment of 6061 Al alloy would not greatly affect the growth rate and values of breakdown and final voltages as no changes in the electrolyte conductivity have been reported with the inclusion of NaBz into the solution. However, the addition of NaBZ into the alkaline-aluminate electrolyte caused a delay in the appearance of plasma discharges, as shown in Figure 1b. It was evident from Figure 1b that a delay in the appearance of plasma discharges can occur during PEO in the electrolyte containing NaBz. Moreover, the voltage in the case of NaBz was slightly lower than that without NaBz. This result was linked to the competition between the dissolution and oxidation of Al alloy substrate in the electrolyte with NaBz [48]. To sum up, the organic additives could affect the characteristics of the PEO process by increasing the voltage. However, controversial results can be found in the literature that are ascribed to differences in the pH, conductivity, concentration, composition, and viscosity of the electrolytes, as well as electrical parameters, used during PEO [1][44][45][46].
References
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2020, 117, 100735.
- Kaseem, M.; Ko, Y.G. Morphological modification and corrosion response of MgO and Mg3(PO4)2 composite formed on magnesium alloy. Compos. Part B Eng. 2019, 176, 107225.
- Kaseem, M.; Ko, Y.G. A Novel hybrid composite composed of albumin, WO3, and LDHs film for smart corrosion protection of Mg alloy. Compos. Part B Eng. 2021, 204, 108490.
- Narayanan, T.S.N.; Song, I.P.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71.
- Li, C.-Y.; Fan, X.-L.; Zeng, R.-C.; Cui, L.-Y.; Li, S.-Q.; Zhang, F.; He, Q.-K.; Kannan, M.B.; Jiang, H.-W.; Chen, D.-C.; et al. Corrosion resistance of in-situ growth of nano-sized Mg(OH)2 on micro-arc oxidized magnesium alloy AZ31—Influence of EDTA. J. Mater. Sci. Technol. 2019, 35, 1088–1098.
- Kaseem, M.; Hussain, T.; Baek, S.H.; Ko, Y.G. Formation of stable coral reef-like structures via self-assembly of functionalized polyvinyl alcohol for superior corrosion performance of AZ31 Mg alloy. Mater. Des. 2020, 193, 108823.
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119.
- Curran, J.A.; Kalkanci, H.; Magurova, Y.; Clyne, T.W. Mullite-rich plasma electrolytic oxide coatings for thermal barrier applications. Surf. Coat. Technol. 2007, 201, 8683–8687.
- Kaseem, M.; Ko, Y.G. Electrochemical response of Al2O3–MoO2–TiO2 oxide films formed on 6061 Al alloy by plasma electrolytic oxidation. J. Electrochem. Soc. 2016, 163, C587–C592.
- Su, P.; Wu, X.; Jiang, Z.; Guo, Y. Effects of working frequency on the structure and corrosion resistance of plasma electrolytic oxidation coatings formed on a ZK60 Mg alloy. Int. J. Appl. Ceram. Technol. 2009, 8, 112–119.
- Kaseem, M.; Kamil, M.; Ko, Y. Electrochemical response of MoO2–Al2O3 oxide films via plasma electrolytic oxidation. Surf. Coat. Technol. 2017, 322, 163–173.
- Songur, F.; Dikici, B.; Niinomi, M.; Arslan, E. The plasma electrolytic oxidation (PEO) coatings to enhance in-vitro corrosion resistance of Ti–29Nb–13Ta–4.6Zr alloys: The combined effect of duty cycle and the deposition frequency. Surf. Coat. Technol. 2019, 374, 345–354.
- Hussein, R.; Zhang, P.; Nie, X.; Xia, Y.; Northwood, D. The effect of current mode and discharge type on the corrosion resistance of plasma electrolytic oxidation (PEO) coated magnesium alloy AJ62. Surf. Coat. Technol. 2011, 206, 1990–1997.
- Kaseem, M.; Kim, M.J.; Ko, Y.G. Hydration-dehydration behavior induced densification of porous plasma electrolysis coating. J. Alloys Compd. 2019, 798, 220–226.
- Kaseem, M.; Ko, Y.G. Benzoate intercalated Mg–Al-layered double hydroxides (LDHs) as efficient chloride traps for plasma electrolysis coatings. J. Alloys Compd. 2019, 787, 772–778.
- Kaseem, M.; Min, J.H.; Ko, Y.G. Corrosion behavior of Al-1 wt.% Mg-0.85 wt.%Si alloy coated by micro-arc-oxidation using TiO2 and Na2MoO4 additives: Role of current density. J. Alloys Compd. 2017, 723, 448–455.
- Kaseem, M.; Yang, H.W.; Ko, Y.G. Toward a nearly defect-free coating via high-energy plasma sparks. Sci. Rep. 2017, 7, 1–10.
- Srinivasan, P.B.; Liang, J.; Blawert, C.; Störmer, M.; Dietzel, W. Effect of current density on the microstructure and corrosion behaviour of plasma electrolytic oxidation treated AM50 magnesium alloy. Appl. Surf. Sci. 2009, 255, 4212–4218.
- Vakili-Azghandi, M.; Fattah-Alhosseini, A. Effects of duty cycle, current frequency, and current density on corrosion behavior of the plasma electrolytic oxidation coatings on 6061 Al alloy in artificial seawater. Met. Mater. Trans. A 2017, 48, 4681–4692.
- Fattah-Alhosseini, A.; Keshavarz, M.K.; Molaei, M.; Gashti, S.O. Plasma electrolytic oxidation (PEO) process on commercially pure Ti surface: Effects of electrolyte on the microstructure and corrosion behavior of coatings. Met. Mater. Trans. A 2018, 49, 4966–4979.
- Babaei, K.; Fattah-Alhosseini, A.; Molaei, M. The effects of carbon-based additives on corrosion and wear properties of Plasma electrolytic oxidation (PEO) coatings applied on Aluminum and its alloys: A review. Surf. Interfaces 2020, 21, 100677.
- Kaseem, M.; Ko, Y.G. On the compactness of the oxide layer induced by utilizing a porosification agent. Appl. Surf. Sci. 2019, 473, 715–725.
- Kaseem, M.; Ko, Y.G. A novel composite system composed of zirconia and LDHs film grown on plasma electrolysis coating: Toward a stable smart coating. Ultrason. Sonochem. 2018, 49, 316–324.
- Barati, N.; Yerokhin, A.; Golestanifard, F.; Rastegari, S.; Meletis, E.I. Alumina- zirconia coatings produced by plasma electrolytic oxidation on Al alloy for corrosion resistance improvement. J. Alloys Compd. 2017, 724, 435–442.
- Fatimah, S.; Kamil, M.; Kwon, J.; Kaseem, M.; Ko, Y. Dual incorporation of SiO2 and ZrO2 nanoparticles into the oxide layer on 6061 Al alloy via plasma electrolytic oxidation: Coating structure and corrosion properties. J. Alloys Compd. 2017, 707, 358–364.
- Kaseem, M.; Lee, Y.H.; Ko, Y.G. Incorporation of MoO2 and ZrO2 particles into the oxide film formed on 7075 Al alloy via micro-arc oxidation. Mater. Lett. 2016, 182, 260–263.
- Darband, G.B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 74–132.
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2019, 64, 127–162.
- Toorani, M.; Aliofkhazraei, M. Review of electrochemical properties of hybrid coating systems on Mg with plasma electrolytic oxidation process as pretreatment. Surf. Interfaces 2019, 14, 262–295.
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182.
- Molaei, M.; Nouri, M.; Babaei, K.; Fattah-Alhosseini, A. Improving surface features of PEO coatings on titanium and titanium alloys with zirconia particles: A review. Surf. Interfaces 2021, 22, 100888.
- Liu, Y.; Zhang, D.; Chen, C.; Zhang, J.; Cui, L. Adsorption orientation of sodium of polyaspartic acid effect on anodic films formed on magnesium alloy. Appl. Surf. Sci. 2011, 257, 7579–7585.
- Chen, M.; Li, Y.; Lian, J.S.; Jiang, Q. Study of the formation and growth of tannic acid based conversion coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2009, 204, 736–747.
- Chen, X.; Li, G.; Lian, J.; Jiang, Q. An organic chromium-free conversion coating on AZ91D magnesium alloy. Appl. Surf. Sci. 2008, 255, 2322–2328.
- Ashassi-Sorkhabi, H.; Ghalebsaz-Jeddi, N.; Hashemzadeh, F.; Jahani, H. Corrosion inhibition of carbon steel in hydrochloric acid by some polyethylene glycols. Electrochim. Acta 2006, 51, 3848–3854.
- Dou, J.; Chen, Y.; Yu, H.; Chen, C. Research status of magnesium alloys by micro-arc oxidation: A review. Surf. Eng. 2017, 33, 731–738.
- Zhang, W. Research progress on organic additives in the anodic oxidation process of magnesium and its alloys. Appl. Mech. Mater. 2013, 341–342, 187–190.
- Pak, S.-N.; Yao, Z.; Ju, K.-S.; Ri, C.-N.; Xia, Q. Effect of organic additives on structure and corrosion resistance of MAO coating. Vacuum 2018, 151, 8–14.
- Kaseem, M.; Ko, Y.G. Effect of starch on the corrosion behavior of Al–Mg–Si alloy processed by micro arc oxidation from an ecofriendly electrolyte system. Bioelectrochemistry 2019, 128, 133–139.
- Fang, Y.; Tu, X.; Miao, C.; Xu, Y.; Xie, W.; Chen, F.; Zhang, Y.; Li, J. Effect of starch addition in alkaline electrolyte on the characteristics of plasma electrolytic oxidation coating on AZ31B Mg alloy. Int. J. Electrochem. Sci. 2017, 12, 11473–11478.
- Guo, X.; An, M.; Yang, P.; Li, H.; Su, C. Effects of benzotriazole on anodized film formed on AZ31B magnesium alloy in environmental-friendly electrolyte. J. Alloys Compd. 2009, 482, 487–497.
- Kaseem, M.; Kwon, J.H.; Ko, Y.G. Modification of a porous oxide layer formed on an Al–Zn–Mg alloy via plasma electrolytic oxidation and post treatment using oxalate ions. RSC Adv. 2016, 6, 107109–107113.
- Zhang, R.; Zhang, S.; Duo, S. Influence of phytic acid concentration on coating properties obtained by MAO treatment on magnesium alloys. Appl. Surf. Sci. 2009, 255, 7893–7897.
- Zhu, F.; Wang, J.; Li, S.; Zhang, J. Preparation and characterization of anodic films on AZ31B Mg alloy formed in the silicate electrolytes with ethylene glycol oligomers as additives. Appl. Surf. Sci. 2012, 258, 8985–8990.
- Su, M.; Yerokhin, A.; Bychkova, M.Y.; Shtansky, D.V.; Levashov, E.A.; Matthews, A. Self-healing plasma electrolytic oxidation coatings doped with benzotriazole loaded halloysite nanotubes on AM50 magnesium alloy. Corros. Sci. 2016, 111, 753–769.
- Asoh, H.; Asakura, K.; Hashimoto, H. Effect of alcohol addition on the structure and corrosion resistance of plasma electrolytic oxidation films formed on AZ31B magnesium alloy. RSC Adv. 2020, 10, 9026–9036.
- Kaseem, M.; Kamil, M.; Kwon, J.; Ko, Y. Effect of sodium benzoate on corrosion behavior of 6061 Al alloy processed by plasma electrolytic oxidation. Surf. Coat. Technol. 2015, 283, 268–273.
- Hsu, C.-H.; Teng, H.-P.; Lu, F.-H. Effects of addition of Al(NO3)3 to electrolytes on alumina coatings by plasma electrolytic oxidation. Surf. Coat. Technol. 2011, 205, 3677–3682.




