Plasma electrolytic oxidation (PEO) is an effective surface modification method for producing ceramic oxide layers on metals and their alloys. Although inorganic electrolytes are widely used in PEO, the organic additives have received considerable interest in the last decade due to their roles in improving the final voltage and controlling spark discharging, which lead to significant improvements in the performance of the obtained coatings.
- plasma electrolytic oxidation
- soft plasma
- organic additive
1. Introduction
Plasma electrolytic oxidation (PEO) is an effective coating method of valve metals to fabricate well-adherent oxide layers, which impart improved wear and corrosion performances in many aggressive environments [1][2][3][4][5][6][7][1,2,3,4,5,6,7]. The formation of PEO coatings is usually accompanied by a series of simultaneous events, such as acoustic emission, luminescence, and heat release, which are associated with a localized electric breakdown of the growing oxide layer [1]. Typically, the resultant PEO coating exhibited a large thickness up to ~200 μm depending on processing conditions, which certainly improves corrosion resistance [8]. Earlier works on PEO have reported that the surface properties of PEO coatings are mainly affected by the composition of the electrolyte, current mode, coating time, as well as the electrical parameters, namely, current density, current frequency, duty cycle, and cell potential [9][10][11][12][13][14][15][16][17][18][19][20][9,10,11,12,13,14,15,16,17,18,19,20]. Therefore, optimizing the processing parameters during PEO would be important as these parameters affect the characteristics of plasma discharges thereby, affecting the quality of the PEO coatings [1]. It should be pointed out that the modification of plasma discharges via the utilization of electrolyte composition has been in the spotlight since the combination of available elements generates many different compounds and potentially leads to different plasma behavior. Two major types of electrolytes are those containing organic compounds and those containing inorganic compounds [21][22][23][24][25][26][21,22,23,24,25,26]. While numerous investigations showed that organic compounds tend to be precluded in the coating layer during coating growth, the use of inorganic compounds generally showed successful incorporation [21].
PEO treatment as a kind of surface treatment of valve metals has previously been reviewed by several works [27][28][29][30][31][27,28,29,30,31]. For example, a critical review discussing the principle, structure, and performance of PEO coatings was reported recently by Kaseem et al. [1]. A review discussing the mechanism and performance of PEO-coated Mg alloys was documented by Darband et al. [27]. An interesting review very recently reported on micro-discharge characteristics during PEO treatment [28]. The impacts of different particles on the characteristics of PEO coating have been reviewed by Lu et al. [30]. To date, however, the types of organic additives added to the electrolyte during PEO and their roles in fabricating compact coatings with improved protective properties have not been given sufficient attention.
2. Influence of Organic Additive on the Electrical Response of PEO
Generally, organic additives tend to adsorb on the surface of metallic substrates during PEO treatment through their functional groups (−OH, –COOH, –NH–CO–, etc.), which would prevent the inorganic additives—the main components of the electrolyte—and dissolved oxygen from transferring to the anodic substrate, leading to easy increments in the cell voltage [32][33][34][35][36][37][32,33,34,35,36,37]. For example, Pak et al. [38] examined the influence of organic polar liquids, such as glycerol (GLY), triethanolamine (TEA), and 3-aminopropyltrimethoxysilane (APTMS) on the voltage-time response during PEO treatment of AZ31B Mg alloy. They reported that organic compounds interacted with the Mg surface through their –OH groups, which separated the substrate surface from the electrolyte and increased the resistance of the coating. Similar findings were reported during PEO treatment of Mg alloys using other organic additives, such as starch [39][40][39,40], benzotriazole (BTA) [41], sodium oxalate (SOx) [42], and phytic acid [43]. However, the effect of organic additives on the voltage response during PEO was still controversial [44][45][46][44,45,46]. For instance, Zhu et al. [44] reported that the breakdown and final voltages during PEO treatment of AZ31B Mg alloy in electrolytes containing 10 g/L ethylene glycol (EG) and polyethylene glycol (PEG) as organic additives were almost identical to the counterparts obtained in electrolytes without organic additives. The adsorption of these additives by their segments –CH2–CH2–O– on the surface of the Mg alloy, with carbon atoms aligned toward the substrate surface and the oxygen atoms oriented toward the electrolyte, was found to have an insignificant effect on the voltage response as compared to the sample without additives. The authors ascribed this result to the high molecular weight of PEG which reduces the adsorption ability of this additive. On the other hand, some organic additives, like ethanol (EtOH) would have no obvious increase in the voltage during PEO. Sun and co-workers [45] also reported that only minor changes in the voltage-time curves of PEO processes conducted in silicate electrolytes without or with an addition of 10 g/L of either halloysite nanotubes (HNT) or BT- loaded HNT. According to Asoh et al. [46], the PEO treatment of AZ31B Mg alloy using a phosphate electrolyte (0.25 M Na3PO4) containing 5 vol.% EtOH as an organic additive had no obvious increase in voltage after reaching the value of breakdown voltage (Figure 1a), suggesting that ethanol was not desirable for coating formation presumably due to its somewhat low pH (12.65) and weak base compared with that comprising only Na3PO4 (12.73). This result indicated that ethanol could suppress the dissociation of the electrolytes during PEO. In contrast, when other additives, such as GLY and EG, were included in the phosphate electrolyte, the rates of the increase of voltage were higher than when the PEO process was performed in electrolytes without GLY and EG. The electrolyte conductivity would be reduced by the inclusion of alcohol into the Na3PO4-based electrolyte. In other words, the electrolyte resistance could be increased as the addition of EG and GLY led to an increase in the electrolyte viscosity. Microstructural observations revealed that EG or GLY utilized as an additive could act as an enhancer for film qualities, leading to the development of coatings with fewer micropores.
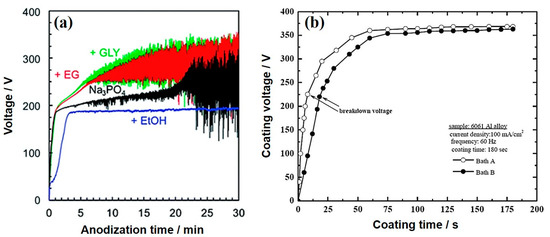
Figure 1. (a) Voltage-time curves of AZ31 Mg alloy coated via plasma electrolytic oxidation (PEO) in electrolytes without and with organic additives, such as ethanol (EtOH), ethylene glycol (EG), and glycerol (GLY). [46] (b) Voltage-time curves of 6061 Al alloy coated in the electrolytes without and with NaBz. Reprinted from permission from [47]. Copyright 2015 Elsevier
Kaseem et al. [47] claimed that the addition of 1 g/L sodium benzoate (NaBz) into the alkaline-aluminate (5.61 KOH + 4.098 g/L NaAlO2) electrolyte during PEO treatment of 6061 Al alloy would not greatly affect the growth rate and values of breakdown and final voltages as no changes in the electrolyte conductivity have been reported with the inclusion of NaBz into the solution. However, the addition of NaBZ into the alkaline-aluminate electrolyte caused a delay in the appearance of plasma discharges, as shown in Figure 1b. It was evident from Figure 1b that a delay in the appearance of plasma discharges can occur during PEO in the electrolyte containing NaBz. Moreover, the voltage in the case of NaBz was slightly lower than that without NaBz. This result was linked to the competition between the dissolution and oxidation of Al alloy substrate in the electrolyte with NaBz [48]. To sum up, the organic additives could affect the characteristics of the PEO process by increasing the voltage. However, controversial results can be found in the literature that are ascribed to differences in the pH, conductivity, concentration, composition, and viscosity of the electrolytes, as well as electrical parameters, used during PEO [1][44][45][46][1,44,45,46].
