
Video Upload Options
Swimming motors navigating in complex fluidic environments have received tremendous attention over the last decade. In particular, liquid metal (LM) as a new emerging material has shown considerable potential in furthering the development of swimming motors, due to their unique features such as fluidity, softness, reconfigurability, stimuli responsiveness, and good biocompatibility. LM motors can not only achieve directional motion but also deformation due to their liquid nature, thus providing new and unique capabilities to the field of swimming motors.
1. Introduction
Motors and machines play a major role in the development and evolution of modern industry. Since 1959, when Richard Feynman predicted the flourishing research field of nanoscience and nanotechnology, abundant research about the miniaturization of motors and machines to micro/nanoscale have been executed. Micro/nanomotors (MNMTs) have been developed to perform delicate tasks in micro/nanoscale from drug/cargo delivery, tumor therapy, biodetoxification, precision surgery [1] to environmental remediation [2]. These applications are based on the autonomous motion of MNMTs that can convert chemical or physical energy into kinetic energy. In fact, the performance of MNMTs mostly relies on the intrinsic features of adopted materials. At first, most MNMTs are made of metals and metal oxides such as Au, Pt, ZnO, and Cu2O to achieve propulsion by a chemical gradient in hydrogen peroxide (H2O2) [3]. When applied in biomedicine, the chemical fuel is toxic to human. Furthermore, most of them are rigid and inflexible. It is easy to harm the soft tissue in the human body where intricate and soft channels are present everywhere. In addition, polymer-based and bio-hybrid MNMTs have good biocompatibility and low toxicity. However, low stability, fast degradation, and quick clearance are other hurdles for their long-time use. To address these challenges, there is still a strong desire for the incorporation of attractive materials to further expand the application fields of MNMTs.
The room temperature liquid metals (LMs) are a unique group of metals that behave like liquids near room temperature. The pure LMs include cesium (Cs, melting point = 28.5 °C), francium (Fr, 27 °C), rubidium (Rb, 39.3 °C), mercury (Hg, −38.8 °C), and gallium (Ga, 29.8 °C). Among these types of LMs, Hg is highly toxic, Cs and Rb are very reactive, and Fr is radioactive. In comparison, Ga and Ga-based alloys such as EGaIn (75.5 wt% gallium and 24.5 wt% indium) and Galinstan (68.5 wt% gallium, 21.5 wt% indium, and 10 wt% tin), have high chemical stability and negligible toxicity. The Ga-based LMs have recently shown great values in various scopes of applications owing to their unique properties (Table 1), such as basic metallic characteristics (high thermal conductivity, good electrical conductivity, radiopacity, and electromagnetic properties), amorphous properties (superb fluidity, excellent flexibility, shape transformability, self-healing capability, reconfigurability, and low viscosity), and several featured properties (facile functionalization accessibility, good biocompatibility, biodegradability, low toxicity, catalytic properties, photothermal/photodynamic capability, and stimuli responsiveness). Because of these unique properties, LMs hold a great possibility to supplement the vacant applications of conventional materials-based MNMTs, such as microfluidics [4][5], repairing nanonetwork [6], imaging [7], cancer-targeting [8][9], vascular embolism, and so on [10][11].
| Properties | Application | Refs. | |
|---|---|---|---|
| Basic metallic characteristics |
High thermal conductivity | Thermal interface materials | [12][13] |
| Good electrical conductivity | Electronics | [14] | |
| Electromagnetic properties | Electromagnetic shielding material |
[15][16][17] | |
| Radiopacity | Radiocontrast agent | [7][11][18] | |
| Amorphous properties | Superb fluidity | Microfluidics | [4][5] |
| Excellent flexibility | Stretchable and soft electronics | [19] | |
| Shape transformability | Cancer therapy | [8][9][20][21][22] | |
| Self-healing capability | Self-healing e-skin systems | [23] | |
| Reconfigurability | Soft robotics | [24][25] | |
| Facile functionalization accessibility |
Metal composite | [26][27] | |
| Featured properties | Biocompatibility and biodegradability |
Biomedical applications | [28] |
| Catalytic properties | Catalyst | [29][30] | |
| Photo-thermal/photodynamic capability |
Cancer therapy | [31][32][33][34][35][36] | |
| Stimuli responsiveness | Robotics | [37] | |
Liquid metal motors can be powered by various energy sources, including chemical fuels (water, NaOH solution, and H2O2 solution), external stimuli (electrical, acoustic, magnetic, and light fields), and hybrid sources (Figure 1A). A brief introduction of different propulsion methods of liquid metal motors is summarized in Table 2. Under the motivation of these driving sources, liquid metal motors can not only perform a directional movement, circular motion, and self-rotation, but also a unique large-scale deformation. It is worth mentioning that the mechanisms behind the movement and deformation of LM motors highly depend on the size of LM. Figure 1B shows the relationship between the size of LM and the corresponding driving force. For macroscopic LM motors, the large-scale deformations induced by surface tension are very intriguing [38][39], but the corresponding propulsion is still a challenge due to the requirement of the large driving force. Additionally, the definition of motors emphasizes the significance of directional translational motion [40]. As the size of the LM decreases to less than 1 cm, the propulsion has been successfully and extensively reported. Thus, LM motors below 1 cm, named mini/micro/nano scale liquid metal motors (MLMTs) (MCLMTs for micro/nano LM motors; MILMTs for mini LM motors) will be discussed in this paper. When the size of MLMT is between 1 cm and 1 mm, it is still in a fully liquid state, so it can realize a self-deformation and adapt to its surroundings [41]. At this length scale, the movement of MLMTs requires less driving force, and the main driving force is bubbles recoil force and surface tension. Once the size of the LM declines to the micro-nano field, asymmetric structure designs of LM are essential to achieve effective propulsion. Although the surface oxide makes the MLMTs in a core-shell structure, it is helpful to maintain its shape, separate state, and prevent fusion [6][8][25], as it can also cause deformation. So far, although great progress has been achieved in MLMTs, it is still a significant challenge to quickly mass-produce MLMTs, precisely control the motion, perform more delicate tasks, and achieve more potential applications. Based on the extensive exploration of the tremendous ubiquitous characteristics of LM over past decades, there are vast opportunities to endow motors with new vitality and impart fresh life in more possible filed by combining the prominent properties of LM with MNMTs.
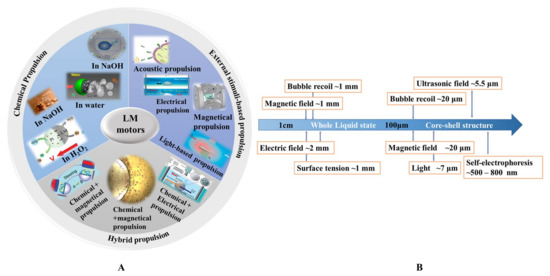
| - | - | Key Features | Pros | Cons |
|---|---|---|---|---|
| Chemical propulsion |
In water | MLMTs are powered by bubbles generated from the chemical reactions of LM with water. | High speed good biocompatibility low cost |
Lack of directional motion short lifetime |
| In NaOH | MLMTs are powered by bubbles generated from the chemical reactions of LM with NaOH. | High speed low cost |
Poor biocompatibility Lack of directional motion short lifetime |
|
| In H2O2 | MLMTs are powered by electrophoresis due to the electron transfer between LM and other metals which are capable of catalyzing the decomposition of H2O2. | Low cost | Poor biocompatibility Lack of directional motion short lifetime |
|
| External stimuli-based propulsion | Electrical propulsion | MLMTs are powered by an external electrical field. | Precise motion control long lifetime |
Poor biocompatibility require special experimental setup |
| Acoustic propulsion | MLMTs are powered by an external high-frequency acoustic (or ultrasonic) field. | Good biocompatibility non-invasive precise motion control. |
The requirement for special and complex experimental devices to control the motion |
|
| Magnetic propulsion | MLMTs are powered by an external magnetic field. | |||
| Light-based propulsion | MLMTs are powered by an external light source. | |||
| Hybrid propulsion | Combination of various propulsion mechanisms to propel motors. | Multi-stimuli responsive capability | (depends on the methods) |
In this paper, we will first introduce the properties of micro/nanoscale LMs to guide researchers interested in the utilization of LMs to meet their specific needs. Then, the preparation, driving mechanism, and application of MLMTs are summarized. In particular, the different principles and methods behind the fabrication and propulsion of MLMTs of different sizes will be highlighted in this part. Lastly, the challenge and future outlook will be given.
2. Physicochemical Characteristics of Micro/Nanoscale LMs
LM micro/nanomaterials (LMMMs), especially LM micro/nanoparticles (LMNPs), pose unique physicochemical characteristics and wide applications, which will endow novel properties and functions to motors. Here, the properties of LMMMs related to motors will be discussed for better utilization of LM in MNMTs. More specific investigation and discussion about LMNPs such as surface microenvironment modification [42], nanocomposite [27], and biomedical [43] or chemical [44] applications can be found in recent reviews.
2.1. Shape
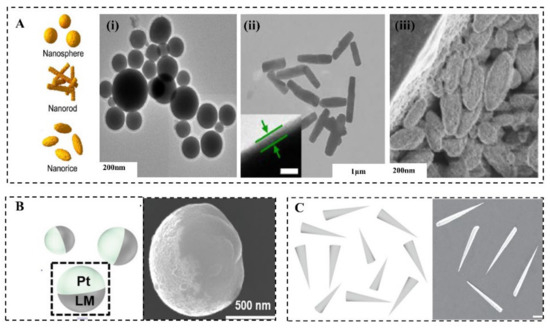
LMMMs are generally in the shape of spheres [45], rods [46], and rice (Figure 2A) [47], which is determined by fabrication methods and parameters [46][48]. There are three common methods to prepare MCLMTs: Sonication [25], transfer printing [49], and pressure-filter-template [9] methods. The former one can produce LMMMs in the sphere, rod, and rice shape by varying parameters. The latter two can prepare the sphere, rod, or needle, and even dumbbell-shaped LMMMs by changing templates. For the sonication method, different parameters lead to various shapes of the LM nanostructure. The ultrasonication time [50], temperature [51], medium [46], and additive [45][47][51][52] affect the shape and size of LMMMs significantly. The rod shape formation can be attributed to the formation of flake oxide GaO(OH) [50], in the presence of dissolved oxygen with a consistently high temperature induced by ultrasonication. Further, the equation for GaO(OH) formation can be given by [50]:
In addition to the ultrasonic method, LM nanorods can also be prepared by the pressure-filter-template method [8][9]. It should be noted that this method produces nanorods by generating surface oxides of Ga2O3 instead of GaO(OH) mentioned above because the reaction of Ga with oxygen at mild temperatures forms Ga2O3. Additionally, Ga2O3 on the surface is necessary to stabilize the rod shape because of the inner amorphous liquid Ga.
The shape of LMMMs highly affects the effective propulsion and function of MCLMTs [40], thus should be carefully considered to meet specific requirements in various potential applications. It is worth mentioning that the relation between the shape or structure and propulsion mechanism is universal to MNMTs of other materials, and more information can be found in these reviews [3][53][54]. Fundamentally, the shape of LMMMs significantly influences the propulsion mechanism [40]. For instance, the Janus-sphere constructed by applying other materials such as Pt on half of it (Figure 2B) can be propelled in H2O2 by bubbles ejected from one side of it [6]; rods with two ends that differ in size are the prerequisite for MCLMTs to achieve movement by producing enough pressure difference along the long axis under the ultrasonic field or light field (Figure 2C). When increasing the size difference of two ends, this rod motor can achieve a higher speed than the normal rod one [9]. By contrast, spheres can be hardly propelled by these two fields, because the symmetry lengths of diameters cannot produce enough pressure difference. For MCLMTs motivated by magnetic fields, special designs such as helical tails and dumbbell-like shapes are required [49]. Besides, the function of LMNPs can also be influenced by their shape. The LM nanomotors in rod [9] and needle [8] shape are more likely to be developed to open the cell membrane mechanically. Compared with the spherical counterpart, they can move in a rotating way and have a smaller contact area with the cell. Furthermore, these rod and needle LM nanomotors would transform into spheres in the acid environment such as endosomes [9], which is beneficial for further fuses and degrades in cells. Moreover, LMNPs can disrupt the endosomal membrane to achieve effective drug delivery by the transformation to the hollow rod by light irradiation [20]. Another similar transformation is utilized to break the bacterial biofilm, which can achieve a 99% killing rate of bacterial [22].
2.2. Electrical/Thermal Properties
LMNPs are normally non-conductive because of their semi-conductive Ga2O3 oxide skin. However, external stimuli could remove or break this oxide and high conductivity can be regained. Many methods are adapted to form a highly conductive path, including laser sintering [55], mechanical sintering [46], and peeling off [56]. During these methods, mechanical sintering [57] is a facile way to induce a conductive path by introducing particle interface rupture, allowing the inner LM to flow out to coalesce into a continuous liquid conductive circuit. It is operated at room temperature without requiring any complex equipment. In addition, the acid vapor [58] and mechanical fracturing [59] can be used to remove the LM oxide and achieve the conductive pathway as well. For example, Kim et al. proved that the hydrochloric acid (HCl) vapor can be applied to react with the oxide skin of Galinstan (Ga2O3 and Ga2O) and change it into InCl3 and GaCl3, which poses a better conductivity than the initial LM particles [58]; Wang et al. used this method to remove the oxide skin on LM Janus nanomotors to achieve a conducive state, thus having the function of repairing silver nanowire networks [6].
The melting point of various LMNPs is generally influenced by their alloying elements and size. Ga and most LM have low melting points and high boiling points, keeping them in the liquid state across a wide temperature range (8–2200 °C). Generally, the melting point of Ga-based alloy is even lower than pure Ga and is influenced by the composition ratio of different elements [44]. Moreover, the size of LMNPs is proven to change the melting point of LM significantly [60], as the melting temperature was found to be decreased relative to values of bulk LM. According to Ghigna et al. [55], the analysis of extended X-ray absorption fine structure spectra of Ga particles shows the melting point of LMNPs (diameter ~150 nm) was 300 K, similar to that of the bulk value (303 K), while an approximate 110 K and 20 K decrease was observed for LMNPs with diameters around 10 nm and 30 nm, respectively. Similarly, recent research [48][56] on the size dependence of the melting point of LMNPs supports the aforementioned trends. Based on the supercooling nature and high conductivity of LMNPs, Simge et al. reported a facile heat-free joining technique [59]. This technology breaks the outer oxide layers of LMNPs through mechanical stressing or shearing, thus triggering the inside fluid flow accompanied by deformation, alloying, and solidification.
2.3. Optical Properties
Localized surface plasmon resonance (LSPR) is a surface charge density oscillation at certain optical frequencies, which can occur on most kinds of metals. For Ga-based LMNPs, the oscillation frequency of an electron cloud lies in the range of the ultraviolet to the visible light [61][62][63]. Strategies involving particle size and shape control [64][65], surface oxide thickness adjustment [66], alloy composition changing [67], and substoichiometric doping levels of metal oxide on the LM surface [68] can be utilized to control UV-plasmonic of Ga-based LMPs.
In the near-infrared (NIR) light range, although a strong absorption peak has not been observed in LM, it turns out LMNPs have a high photothermal conversion efficacy with the irradiation of NIR light. The photothermal properties of LM treated with NIR light have received increasing attention since the Miyako group’s investigation on it, where they prepared EGaIn nanocapsule encapsulated by DSPE-PEG2000-Amine and DC (8,9) PC with core-shell structure [69]. Under 1 W (80 mW/mm2) irradiation with a 785 nm NIR laser, it shows a quick temperature rise, superior photothermal conversion efficiency, and photothermal stability. The surface temperature of the 1 mg LM droplet quickly rose from 20 °C to 43 °C after 5 min irradiation (Figure 3A). In addition, the photothermal conversion efficiency of the LM nanocapsules is almost 3 times that of commercial Au-NR1 (52% and 17%, respectively). Moreover, the optical absorbance of the LM capsule can last for 1 h without observed degradation.
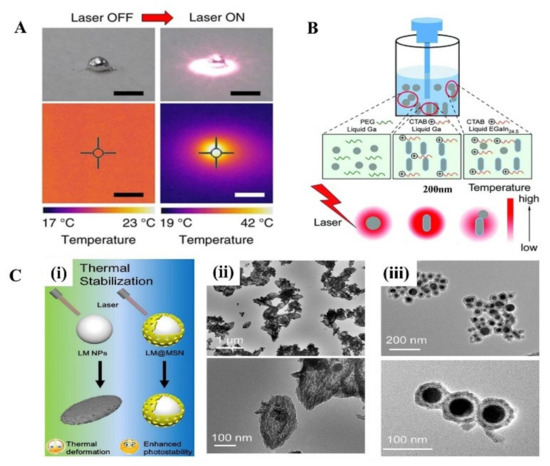
Then, a great number of researchers explore the photothermal application in cancer therapy and prove it has an excellent therapeutic effect. Sun et al. systematically evaluated the photothermal properties of various LMMMs, such as Ga nanorods (GaNR), Ga nanospheres (GaNS), and gallium-indium alloy nanorods (LMNR) [31]. Additionally, results show that GaNR exhibited an outstanding photothermal conversion efficiency and distinct temperature elevation compared to GaNS and LMNR. However, the poor thermal stability of pure LMMMs after irradiation impedes their broader applications. Hu et al. proposed a surface mesoporous silica coating strategy on LMNPs (LMNPs@MSN) (Figure 3B) [34], which significantly improved the stability and sustainability of the photothermal performance of LMNPs (Figure 3C). With the modification of HA and loading of doxorubicin (Dox), LMNPs showed significant inhibition of solid tumor growth by the combination of photothermal and chemotherapy both in vitro and in vivo experiments. Similarly, another group proved [35] that LMNPs coated with silica and modified with RGD peptides are an efficient photothermal conversion nano agent. Besides, when LMNPs embedded in poly (NIPAm-co-MBA) hydrogels (PNM) the photothermal conversion efficiency was improved, causing the temperature rise to exceed the shrinkage threshold of the hydrogels, thus promoting the controlled release of drugs (Dox) [32]. In addition, LMNPs and drugs can be loaded on other nanoparticles and show excellent photothermal and chemical synergistic therapy as well [33].
2.4. Oxidability
LMNPs easily form a thin oxide skin on the surface even in an extremely low-oxygen environment. The thickness of this oxide skin varies from 0.7 nm to 3 nm [45][52] depending on but not limited to oxygen concentration [70], vapor concentration, surfactant [45][47][70], and oxide-forming time [52]. In most cases, the composition of this oxide skin is mostly Ga2O3, with a little fraction of In or Sn. The co-existence of LM and this oxide exhibits unique physiochemical properties such as facilitating electron extraction [71], improving photocatalytic activity [72], and providing a strategy for adjusting surface plasmon resonances [66][73]. Besides, the size, shape, morphology, thermal, electrical, and optical properties of LMNPs are influenced by the LM oxide significantly, which is described in the respective sections. Furthermore, LM oxide provides a surface conjugation site for functionalization. Surfactants including thiols [70][74], PEGylating [75], and positive-charged molecules [51] and other strategies such as membrane coating [8] and metal particles coating [76] are successfully assembled on the surface of LM, which is vitally important for the application of LMNPs in biomedicine, on-demand design of MLMTs and other fields.
Among many surfactants, thiol can not only help to stabilize LMNPs, but also can serve for drug delivery and tumor targeting. Hohman et al. investigated the function of thiol-containing molecules in the process of EGaIn nanodroplets formation [45]. The results show that the thiols further decrease the size of LMNPs and prevent rapid agglomeration, which is attributed to the formation of a metal thiolate complex. Besides, EGaIn functionalized with thiolated (2-hydroxypropyl)-b-cyclodextrin and thiolated hyaluronic acid have the function of targeting drug delivery and enhanced chemotherapeutic inhibition towards the tumor [77]. These two thiols not only cap EGaIn, but more importantly serves as a drug (Dox)-loading and active targeting moiety, respectively. However, there are still some defects for these thiol-terminated surfactants. For example, some thiol groups tend to couple with reactive oxygen species (ROS) and inactivate metalloenzymes in the human body. Many thiols are water-insoluble, which is hard to stabilize LMNPs in water. Apart from thiols, PEGylating provides effective strategies to improve the water-stability of LMNPs. For example, Chechetka prepared LM nanodroplets with PEGylating modification, showing high water dispersibility and no precipitations for at least three days at 20 °C [69]. Sun et al. added thiol-polyethylene glycol-thiol (HS-PEG-HS) into LMNPs preparation solution to obtain well water-dispersed LMNPs, stable for months [31]. Moreover, some surfactants can contribute to the formation of LMNPs in various shapes. For example, melanin helps the formation of rice-shaped LMNPs, which turns out to have the best photothermal conversion efficiency among the LMNPs in the sphere, rod, and rice shape [47]. In addition, lysozyme (positively charged surfactants) play a significant role in the sphere-rod transformation of LMNPs while no rod structure was observed with negatively charged surfactants.
Other surface modification strategies have also been implemented on LMNPs. The cell membrane can be assembled onto the surface of LMNPs through an acoustically assisted nanovesicle fusion method [8], which enables MLMTs capable of avoiding bio-fouling and actively seeking cancer cells. In addition, the other metal particles can also be added to the surface of the metal to form “liquid marbles”. Galinstan coated with insulators (including Tefl on and silica) and semiconductors (including WO3, TiO2, MoO3, In2O3, and carbon nanotubes) have mechanical stability and new functionality [76]. Further, Zhang et al. reported a LM marble with WO3 coating in microscale, which has high sensitivity towards low concentrations of heavy metal ions, and enhanced solar light-driven photocatalytic activities [73].
2.5. Stimulus–Responsive Transformable Properties
2.5.1. Acid-Induced Responsive Properties
LMMMs with a thin oxide can react with acid slowly, which triggers its transformation (Figure 4A). Lu et al. first investigated the acid-triggered conformational transformation of LMNPs and proposed the potential function of promoting drug release and X-ray imaging [77]. When exposed to an acid environment (pH = 5.0), LMNPs gradually fused within the first 5 min and then aggregated into larger droplets over time. This transformation is induced by the acid removal of oxide Ga2O3. The inside pristine LMs exits as droplets and quickly merge into large droplets due to the high surface tension when contacting with other nearby droplets. Then the LM microdroplets (LMMDs) were observed to be degraded into the hollow polymeric shells (Figure 4B). The transformability of LMNPs in the acid environment such as tumor sites can significantly promote drug release, which is experimentally proved in Wang’s research [8]. The drug release rate from MCLMTs in the acidic buffer (47.4%) is 8 times higher than that in the neutral buffer (5.3%) within 4 h. Furthermore, the drug release from the membrane-coated MCLMTs can achieve 72.6% in the acidic buffer within 4 h. In comparison, the drug release of rigid metal-based MNMTs mostly rely on external stimuli such as magnetic interaction and ultrasound triggering, and the in-situ acid-triggered drug release of LMNPs does not require complex external equipment and delicate operations. Besides, the fusion of LMNPs accumulated at the tumor site could enhance the contrast of tumor tissue during X-ray imaging. Such acid-induced responsive transformability offers great promise for the future in vivo drug release and cargo delivery fields.
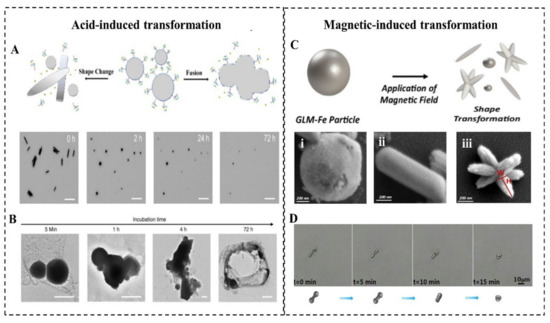
2.5.2. Magnetic-Induced Responsive Properties
The transformation of LMNPs induced by magnetic usually involved the addition of magnetic materials into LM, such as Fe, Ni, and so on. Elbourne et al. reported a magnetic-responsive Galinstan-Fe micro/nanoparticle (GLM-Fe) that can transform from particles to nanorod and nanostars with shaping edges (Figure 4C) [22]. When placed in contact with a bacterial biofilm, GLM-Fe with sharping edges activated by the magnetic field can simultaneously rupture the biofilm matrix and lead to 99% nonviable bacteria. This transformability can be contributed to the liquid nature of the inner core of particles. The drag force experienced by a GLM-Fe when subjected to the magnetic field actuates the physical distortion of particles, leading to an increase of the surface area of LM particles. With local heating induced by the magnetic field, it facilitates the oxidation of the surface to be a nanosheets-shaped GaO(OH) and then rolls up to form rods. Subsequently, the rods aggregate to form the star-like shape with sharping edges. Significantly, the strength of the magnetic field is crucial for this transformation. When GLM-Fe was exposed to a rotating ferrite rare-earth magnet, with a magnetic field strength of 775 milligauss, it shows an obvious morphological change. In contrast, a smaller magnetic field created by a neodymium magnet fails to induce this shape transformation. When the heat is insufficient to promote the oxidation and the drag force is too weak to change the surface area, another type of shape transforms of LM nanomaterial will be triggered [49]. With an alternating magnetic field (AMF) irradiation, the EGaIn MNMTs underwent a morphological transformation from a bowling-pin-like shape to a sphere in an aqueous environment (Figure 4D). It is believed that this transformation is attributed to the local heat generated by eddy currents, resulting in rapturing and removed oxide skin on the LM surface. Then the inner pristine LM flows out to form a droplet due to the high surface tension.
2.5.3. Temperature-Induced Responsive Properties
LM with a low melting temperature allows temperature-induced responsive manipulation by temperature variation. The temperature-triggered transformation is more easily achieved in LM than other rigid metals with a much higher melting point. Until now, high and low temperatures have been utilized to induce the transformation of LMNPs and endow their potential in the application of nanomedicine.
The rising temperature on LMNPs can induce the morphology transition from a sphere to a rod. This is attributed to the generation of rod-shaped GaO(OH). Several researchers suggest that heating is a necessary condition for (GaO)OH formation [20][21][51]. When LMMDs in an aqueous solution are heated up to about 70 °C for 30 min, the spherical nanoparticles will be oxidized, leading to the formation of rod-like GaOOH. Moreover, the rising temperature induced by light irradiation can also effectively promote this sphere-to-rod shape transformation. Lu et al. proposed an EGaIn-based LMNPs can undergo a dramatic morphological transformation in an aqueous environment with light irradiation (Figure 5A) [20]. With 635 nm light at a power density of 100 mW/cm2, these LMNPs coated with graphene quantum dots (GQDs) gradually experienced the shape transformation from nanoparticles (350 nm) to rod-like nanostructures (900 nm) (Figure 5B). It worth noting that the heat and ROS generated by GQDs with light irradiation created the necessary conditions for oxidizing spherical EGaIn nanoparticles to rod-like GaO(OH). In contrast, LMNPs with light at the same intensity, but without GQD coating, subsequently showed no sign of morphological transition. This sphere-rod transformation turned out to be an effective method to physically disrupt the endosomal membrane thus promoting endosomal escape of payloads. Similarly, Gan et al. reported that LMMDs coated with polydopamine (PDA) (Figure 5C) can transform from sphere to ellipsoid by NIR laser irradiation (Figure 5D) [21]. This transformation depends on the significant heating caused by PDA, which has a high photothermal conversion capability. With 808 nm laser at a power density of 2 W/cm2 for 20 min, the temperature of suspension of LMMDs coated with polydopamine (PDA) gradually increased 10 °C, at least 5 °C higher than that of pure LMMDs. Besides, the thickness of the PDA shell should be less than 15 nm to guarantee the shape transformation, because the thicker and stronger PDA shell may prevent oxidation of Ga by impeding oxygen and water diffusion.
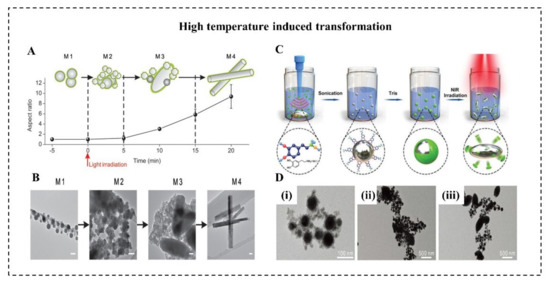
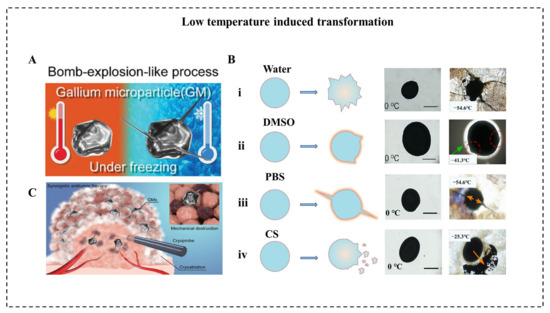
The low temperature can also induce LM transformation to achieve various applications. When the ambient temperature is reduced to a low level, the volume of conventional metals tends to decrease due to their positive thermal expansion coefficient. Unlike conventional metals, LM expands in volume after solidification, and the volume expansion ratio of bulk LM was reported to reach 3% [15]. In micro/nanoscale, Sun et al. reported a large-scale transformation of LMMDs with a deformable area increase of 13.8% due to the liquid-solid phase transition in an aqueous solution [79]. As temperature decreased at a speed of 10 °C/s to a final temperature of −60 °C, LMMDs transform from an oval shape into an amorphous shape with sharp edges within 1 ms. In addition, the same group reported a drastic “bomb-explosion-like” deformation of LMMDs in chitosan (CS) or PBS solution (Figure 6A) [18]. Many factors influence the success of shape transformation of LMNPs induced by decreasing temperature, including the types of solution, the size of particles, and the cooling rate. Significantly, the deformation degree of LMNPs varies in various mediums, such as water, dimethyl sulfoxide (DMSO), PBS, and CS solution (Figure 6B). Different solutions influence the formation of ice crystals and the properties of LMMDs, and it is essential for the shape transformation that the LMMDs phase change happened after the ice crystal formation of a solution, leaving little space for deformation. For example, DMSO can inhibit the formation of ice crystals and smooth the edge of the crystals [80] while chitosan solution can enhance the oxidization property and the plasticity of LMMDs. Thus, the deformation behaviors are different in these two solutions. In addition, to guarantee the shape deformation of LMMDs, the cooling rate should be higher than 2 °C/min. This large-scale deformation of LMMDs can be applied to temperature-controlled sensors, which pave the way for human basic equipment to explore extremely cold environments on earth or outer space. Furthermore, this shape transformation can exert mechanical destruction to tumor cells, which shows an enhanced tumor cryoablation effect (Figure 6C).
2.5.4. Toxicity
Although the toxicology profiles of LMNPs have not been thoroughly tested, an increasing number of studies have shown that LMNPs could be biocompatible both in vitro and in vivo.
In an in vitro experiment, Wang et al. demonstrated that the Ga-LMNPs without any modification have negligible cytotoxicity to both normal cells and cancer cells (over 80% of human L-02 hepatocytes and HeLa cells remained alive at 300 mg/L) [9]. In addition, the cytotoxicity of LMNPs with modification is also investigated by many researchers. Ga-LMNPs encapsulated in glucan particles were demonstrated to have low toxicity to cells, by adding GP-Ga to the murine B6 macrophage cells at a ratio of 10: 1 GP: cell [81]. What is more, compared with other mature but rigid nanomaterials such as single-walled carbon nanotubes (SWCNTs), multi-walled CNTs (MWCNTs), and gold nanorods (Au-NRs), LMNPs displayed lower toxicity at high concentrations (>90% cells were alive at 1600 μg/mL) (Figure 7A) [69]. Recently, other research involving LMNPs also proved its low cytotoxicity at normal treatment dose, such as LM-loaded polymeric hydrogels [32], injectable LM, methotrexate-loaded microsphere [33], and LM coated with SiO2 (Figure 7B) [34][35].
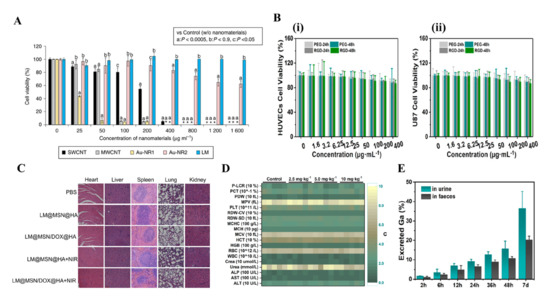
In an in vivo experiment, Chechetka et al. demonstrated that high concentrated LMNPs (up to 320 mg/mL) in injections had no negative effect on the viability and body weight of mice up to 19 days [69]. Furthermore, Lu et al. systematically evaluated the toxicity of LMNPs in mice and concluded that LMNPs were highly encouraging for the application of nanomedicine, based on the results that it displayed no obvious toxicity at the treatment dose [77]. Recently, more work on the in vivo toxicity showed the low toxicity of LMNPs. Hu et al. proved that LMNPs coated with mesoporous silica loaded with Dox showed low systemic toxicity on BALB/c mice. The H&E images of major organs showed no obvious pathological abnormalities in the heart, liver, spleen, lung, and kidney, and the parameters of hematological assessment and blood biochemistry assay were all within normal ranges (Figure 7C) [34]. Similarly, Zhu et al. proved the LMNPs coated with SiO2 presented negligible systemic side effects since it induces no obvious renal and hepatic toxicity and no inflammatory lesions or damage in the treatment group (Figure 7D) [35].
The mechanism of the low toxicity of LMNPs has not been well investigated. However, the degradability and excretion ability of LMNPs seems to be strongly related to it, as shown in many types of research [8][9][34][35][69][77]. Many kinds of research [8][9][77] showed that LM nanomaterials can be gradually fused and degraded when exposed to an acidic environment such as in acidic lysosomes/endosomes or acid tumor microenvironment [20]. In addition, the products of degradation are Ga3+, which proved to be an anti-drug resistance ion in drug-resistant cancer cells. After degradation, LMNPs could be excreted efficiently through urine and feces over time. Zhu et al. demonstrated that the cumulative excretion of Ga-LMNPs increased to be 55.7% after intravenous injection for seven days (Figure 7E) [35]. Similar results were shown in the LMNPs coated with mesoporous silica [34] and LMNPs encapsulated in polymeric [69].
References
- Abdelmohsen, L.; Peng, F.; Tu, Y.; Wilson, D.A. Micro- and nano-motors for biomedical applications. J. Mater. Chem. B 2014, 2, 2395–2408.
- Gao, W.; Wang, J. The environmental impact of micro/nanomachines: A review. ACS Nano 2014, 8, 3170–3180.
- Sanchez, S.; Soler, L.; Katuri, J. Chemically powered micro- and nanomotors. Angew. Chem. Int. Ed. Engl. 2015, 54, 1414–1444.
- Khoshmanesh, K.; Tang, S.Y.; Zhu, J.Y.; Schaefer, S.; Mitchell, A.; Kalantar-Zadeh, K.; Dickey, M.D. Liquid metal enabled microfluidics. Lab Chip 2017, 17, 974–993.
- Tang, S.Y.; Lin, Y.; Joshipura, I.D.; Khoshmanesh, K.; Dickey, M.D. Steering liquid metal flow in microchannels using low voltages. Lab Chip 2015, 15, 3905–3911.
- Wang, Y.; Duan, W.; Zhou, C.; Liu, Q.; Gu, J.; Ye, H.; Li, M.; Wang, W.; Ma, X. Phoretic Liquid Metal Micro/Nanomotors as Intelligent Filler for Targeted Microwelding. Adv. Mater. 2019, 31, e1905067.
- Wang, Q.; Yu, Y.; Pan, K.; Liu, J. Liquid metal angiography for mega contrast X-ray visualization of vascular network in reconstructing in-vitro organ anatomy. IEEE Trans. Biomed. Eng. 2014, 61, 2161–2166.
- Wang, D.; Gao, C.; Zhou, C.; Lin, Z.; He, Q. Leukocyte Membrane-Coated Liquid Metal Nanoswimmers for Actively Targeted Delivery and Synergistic Chemophotothermal Therapy. Research 2020, 2020, 3676954.
- Wang, D.; Gao, C.; Wang, W.; Sun, M.; Guo, B.; Xie, H.; He, Q. Shape-Transformable, Fusible Rodlike Swimming Liquid Metal Nanomachine. ACS Nano 2018, 12, 10212–10220.
- Kim, D.; Hwang, J.; Choi, Y.; Kwon, Y.; Jang, J.; Yoon, S.; Choi, J. Effective Delivery of Anti-Cancer Drug Molecules with Shape Transforming Liquid Metal Particles. Cancers 2019, 11, 1666.
- Fan, L.; Duan, M.; Xie, Z.; Pan, K.; Wang, X.; Sun, X.; Wang, Q.; Rao, W.; Liu, J. Injectable and Radiopaque Liquid Metal/Calcium Alginate Hydrogels for Endovascular Embolization and Tumor Embolotherapy. Small 2020, 16, e1903421.
- Chen, S.; Deng, Z.; Liu, J. High performance liquid metal thermal interface materials. Nanotechnology 2020, 32, 092001.
- Mei, S.F.; Gao, Y.X.; Deng, Z.S.; Liu, J. Thermally Conductive and Highly Electrically Resistive Grease Through Homogeneously Dispersing Liquid Metal Droplets Inside Methyl Silicone Oil. J. Electr. Packag. 2014, 136, 011009.
- Wang, C.; Wang, C.; Huang, Z.; Xu, S. Materials and Structures toward Soft Electronics. Adv. Mater. 2018, 30, e1801368.
- Zhang, M.K.; Zhang, P.J.; Zhang, C.L.; Wang, Y.S.; Chang, H.; Rao, W. Porous and anisotropic liquid metal composites with tunable reflection ratio for low -temperature electromagnetic interference shielding. Appl. Mater. Today 2020, 19.
- Zhang, M.K.; Zhang, P.J.; Wang, Q.; Li, L.; Dong, S.J.; Liu, J.; Rao, W. Stretchable liquid metal electromagnetic interference shielding coating materials with superior effectiveness. J. Mater. Chem. C 2019, 7, 10331–10337.
- Yu, D.; Liao, Y.; Song, Y.; Wang, S.; Wan, H.; Zeng, Y.; Yin, T.; Yang, W.; He, Z. A Super-Stretchable Liquid Metal Foamed Elastomer for Tunable Control of Electromagnetic Waves and Thermal Transport. Adv. Sci. 2020, 7.
- Sun, X.; Cui, B.; Yuan, B.; Wang, X.; Fan, L.; Yu, D.; He, Z.; Sheng, L.; Liu, J.; Lu, J. Liquid Metal Microparticles Phase Change Medicated Mechanical Destruction for Enhanced Tumor Cryoablation and Dual-Mode Imaging. Adv. Funct. Mater. 2020, 30.
- Dickey, M.D. Stretchable and Soft Electronics using Liquid Metals. Adv. Mater. 2017, 29, 1606425.
- Lu, Y.; Lin, Y.; Chen, Z.; Hu, Q.; Liu, Y.; Yu, S.; Gao, W.; Dickey, M.D.; Gu, Z. Enhanced Endosomal Escape by Light-Fueled Liquid-Metal Transformer. Nano Lett. 2017, 17, 2138–2145.
- Gan, T.; Shang, W.; Handschuh-Wang, S.; Zhou, X. Light-Induced Shape Morphing of Liquid Metal Nanodroplets Enabled by Polydopamine Coating. Small 2019, 15, e1804838.
- Elbourne, A.; Cheeseman, S.; Atkin, P.; Truong, N.P.; Syed, N.; Zavabeti, A.; Mohiuddin, M.; Esrafilzadeh, D.; Cozzolino, D.; McConville, C.F.; et al. Antibacterial Liquid Metals: Biofilm Treatment via Magnetic Activation. ACS Nano 2020, 14, 802–817.
- Yang, J.; Cheng, W.L.; Kalantar-Zadeh, K. Electronic Skins Based on Liquid Metals. Proc. IEEE 2019, 107, 2168–2184.
- Wang, X.; Guo, R.; Liu, J. Liquid Metal Based Soft Robotics: Materials, Designs, and Applications. Adv. Mater. Technol. 2018.
- Li, Z.S.; Zhang, H.Y.; Wang, D.L.; Gao, C.Y.; Sun, M.M.; Wu, Z.G.; He, Q. Reconfigurable Assembly of Active Liquid Metal Colloidal Cluster. Angew. Chem. Int. Ed. 2020.
- Chen, S.; Wang, H.-Z.; Zhao, R.-Q.; Rao, W.; Liu, J. Liquid Metal Composites. Matter 2020, 2, 1446–1480.
- Malakooti, M.H.; Bockstaller, M.R.; Matyjaszewski, K.; Majidi, C. Liquid metal nanocomposites. Nanoscale Adv. 2020, 2, 2668–2677.
- Yan, J.; Lu, Y.; Chen, G.; Yang, M.; Gu, Z. Advances in liquid metals for biomedical applications. Chem. Soc. Rev. 2018, 47, 2518–2533.
- Liang, S.T.; Wang, H.Z.; Liu, J. Progress, Mechanisms and Applications of Liquid-Metal Catalyst Systems. Chem. Eur. J. 2018.
- Allioux, F.-M.; Merhebi, S.; Ghasemian, M.B.; Tang, J.; Merenda, A.; Abbasi, R.; Mayyas, M.; Daeneke, T.; O’Mullane, A.P.; Daiyan, R.; et al. Bi–Sn Catalytic Foam Governed by Nanometallurgy of Liquid Metals. Nano Lett. 2020, 20, 4403–4409.
- Sun, X.; Sun, M.; Liu, M.; Yuan, B.; Gao, W.; Rao, W.; Liu, J. Shape tunable gallium nanorods mediated tumor enhanced ablation through near-infrared photothermal therapy. Nanoscale 2019, 11, 2655–2667.
- Fan, L.L.; Sun, X.Y.; Wang, X.L.; Wang, H.Z.; Liu, J. NIR laser-responsive liquid metal-loaded polymeric hydrogels for controlled release of doxorubicin. RSC Adv. 2019, 9, 13026–13032.
- Fan, L.; Duan, M.; Sun, X.; Wang, H.; Liu, J. Injectable Liquid Metal- and Methotrexate-Loaded Microsphere for Cancer Chemophotothermal Synergistic Therapy. ACS Appl. Bio Mater. 2020, 3, 3553–3559.
- Hu, J.J.; Liu, M.D.; Chen, Y.; Gao, F.; Peng, S.Y.; Xie, B.R.; Li, C.X.; Zeng, X.; Zhang, X.Z. Immobilized liquid metal nanoparticles with improved stability and photothermal performance for combinational therapy of tumor. Biomaterials 2019, 207, 76–88.
- Zhu, P.; Gao, S.; Lin, H.; Lu, X.; Yang, B.; Zhang, L.; Chen, Y.; Shi, J. Inorganic Nanoshell-Stabilized Liquid Metal for Targeted Photonanomedicine in NIR-II Biowindow. Nano Lett. 2019, 19, 2128–2137.
- Kulkarni, S.; Pandey, A.; Mutalik, S. Liquid metal based theranostic nanoplatforms: Application in cancer therapy, imaging and biosensing. Nanomedicine 2020, 26, 102175.
- Ren, L.; Xu, X.; Du, Y.; Kalantar-Zadeh, K.; Dou, S.X. Liquid metals and their hybrids as stimulus–responsive smart materials. Mater. Today 2020, 34, 92–114.
- Chen, S.; Yang, X.; Cui, Y.; Liu, J. Self-Growing and Serpentine Locomotion of Liquid Metal Induced by Copper Ions. ACS Appl. Mater. Interfaces 2018, 10, 22889–22895.
- Chen, S.; Wang, L.; Zhang, Q.L.; Liu, J. Liquid metal fractals induced by synergistic oxidation. Sci. Bull. 2018, 63, 1513–1520.
- Wang, W.; Duan, W.T.; Ahmed, S.; Mallouk, T.E.; Sen, A. Small power: Autonomous nano- and micromotors propelled by self-generated gradients. Nano Today 2013, 8, 531–554.
- Wang, D.; Lin, Z.; Zhou, C.; Gao, C.; He, Q. Liquid Metal Gallium Micromachines Speed Up in Confining Channels. Adv. Intell. Syst. 2019, 1.
- Liu, Y.; Zhang, W.; Wang, H. Synthesis and application of core–shell liquid metal particles: A perspective of surface engineering. Mater. Horiz. 2021, 8, 56–77.
- Li, H.; Qiao, R.; Davis, T.P.; Tang, S.-Y. Biomedical Applications of Liquid Metal Nanoparticles: A Critical Review. Biosensors 2020, 10, 196.
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-Zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111.
- Hohman, J.N.; Kim, M.; Wadsworth, G.A.; Bednar, H.R.; Jiang, J.; LeThai, M.A.; Weiss, P.S. Directing substrate morphology via self-assembly: Ligand-mediated scission of gallium-indium microspheres to the nanoscale. Nano Lett. 2011, 11, 5104–5110.
- Kumar, V.B.; Gedanken, A.; Kimmel, G.; Porat, Z. Ultrasonic cavitation of molten gallium: Formation of micro- and nano-spheres. Ultrason. Sonochem. 2014, 21, 1166–1173.
- Yan, J.J.; Zhang, X.D.; Liu, Y.; Ye, Y.Q.; Yu, J.C.; Chen, Q.; Wang, J.Q.; Zhang, Y.Q.; Hu, Q.Y.; Kang, Y.; et al. Shape-controlled synthesis of liquid metal nanodroplets for photothermal therapy. Nano Res. 2019, 12, 1313–1320.
- Yamaguchi, A.; Mashima, Y.; Iyoda, T. Reversible Size Control of Liquid-Metal Nanoparticles under Ultrasonication. Angew. Chem. Int. Ed. Engl. 2015, 54, 12809–12813.
- Liu, M.; Wang, Y.; Kuai, Y.; Cong, J.; Xu, Y.; Piao, H.G.; Pan, L.; Liu, Y. Magnetically Powered Shape-Transformable Liquid Metal Micromotors. Small 2019, 15, e1905446.
- Kumar, V.B.; Gedanken, A.; Porat, Z. Facile synthesis of gallium oxide hydroxide by ultrasonic irradiation of molten gallium in water. Ultrason. Sonochem. 2015, 26, 340–344.
- Lin, Y.; Liu, Y.; Genzer, J.; Dickey, M.D. Shape-transformable liquid metal nanoparticles in aqueous solution. Chem. Sci. 2017, 8, 3832–3837.
- Farrell, Z.J.; Tabor, C. Control of Gallium Oxide Growth on Liquid Metal Eutectic Gallium/Indium Nanoparticles via Thiolation. Langmuir 2018, 34, 234–240.
- Wang, H.; Pumera, M. Fabrication of Micro/Nanoscale Motors. Chem. Rev. 2015, 115, 8704–8735.
- Xu, T.; Gao, W.; Xu, L.P.; Zhang, X.; Wang, S. Fuel-Free Synthetic Micro-/Nanomachines. Adv. Mater. 2017, 29.
- Ghigna, P.; Spinolo, G.; Parravicini, G.B.; Stella, A.; Migliori, A.; Kofman, R. Metallic versus covalent bonding: Ga nanoparticles as a case study. J. Am. Chem. Soc. 2007, 129, 8026–8033.
- Tang, L.; Cheng, S.; Zhang, L.; Mi, H.; Mou, L.; Yang, S.; Huang, Z.; Shi, X.; Jiang, X. Printable Metal-Polymer Conductors for Highly Stretchable Bio-Devices. iScience 2018, 4, 302–311.
- Boley, J.W.; White, E.L.; Kramer, R.K. Mechanically sintered gallium-indium nanoparticles. Adv. Mater. 2015, 27, 2355–2360.
- Kim, D.; Thissen, P.; Viner, G.; Lee, D.W.; Choi, W.; Chabal, Y.J.; Lee, J.B. Recovery of nonwetting characteristics by surface modification of gallium-based liquid metal droplets using hydrochloric acid vapor. ACS Appl. Mater. Interfaces 2013, 5, 179–185.
- Çınar, S.; Tevis, I.D.; Chen, J.; Thuo, M. Mechanical Fracturing of Core-Shell Undercooled Metal Particles for Heat-Free Soldering. Sci. Rep. 2016, 6.
- Kumar, V.B.; Porat, Z.; Gedanken, A. DSC measurements of the thermal properties of gallium particles in the micron and sub-micron sizes, obtained by sonication of molten gallium. J. Therm. Anal. Calorim. 2015, 119, 1587–1592.
- Song, H.; Kim, T.; Kang, S.; Jin, H.; Lee, K.; Yoon, H.J. Ga-Based Liquid Metal Micro/Nanoparticles: Recent Advances and Applications. Small 2020, 16, e1903391.
- Yang, Y.; Callahan, J.M.; Kim, T.H.; Brown, A.S.; Everitt, H.O. Ultraviolet nanoplasmonics: A demonstration of surface-enhanced Raman spectroscopy, fluorescence, and photodegradation using gallium nanoparticles. Nano Lett. 2013, 13, 2837–2841.
- Yang, Y.; Akozbek, N.; Kim, T.-H.; Sanz, J.M.; Moreno, F.; Losurdo, M.; Brown, A.S.; Everitt, H.O. Ultraviolet–Visible Plasmonic Properties of Gallium Nanoparticles Investigated by Variable-Angle Spectroscopic and Mueller Matrix Ellipsometry. ACS Photonics 2014, 1, 582–589.
- Knight, M.W.; Coenen, T.; Yang, Y.; Brenny, B.J.; Losurdo, M.; Brown, A.S.; Everitt, H.O.; Polman, A. Gallium plasmonics: Deep subwavelength spectroscopic imaging of single and interacting gallium nanoparticles. ACS Nano 2015, 9, 2049–2060.
- Reineck, P.; Lin, Y.; Gibson, B.C.; Dickey, M.D.; Greentree, A.D.; Maksymov, I.S. UV plasmonic properties of colloidal liquid-metal eutectic gallium-indium alloy nanoparticles. Sci. Rep. 2019, 9, 5345.
- Catalan-Gomez, S.; Redondo-Cubero, A.; Palomares, F.J.; Nucciarelli, F.; Pau, J.L. Tunable plasmonic resonance of gallium nanoparticles by thermal oxidation at low temperaturas. Nanotechnology 2017, 28, 405705.
- Vivekchand, S.R.; Engel, C.J.; Lubin, S.M.; Blaber, M.G.; Zhou, W.; Suh, J.Y.; Schatz, G.C.; Odom, T.W. Liquid plasmonics: Manipulating surface plasmon polaritons via phase transitions. Nano Lett. 2012, 12, 4324–4328.
- Zhang, W.; Naidu, B.S.; Ou, J.Z.; O’Mullane, A.P.; Chrimes, A.F.; Carey, B.J.; Wang, Y.C.; Tang, S.Y.; Sivan, V.; Mitchell, A.; et al. Liquid Metal/Metal Oxide Frameworks with Incorporated Ga2O3 for Photocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 1943–1948.
- Chechetka, S.A.; Yu, Y.; Zhen, X.; Pramanik, M.; Pu, K.; Miyako, E. Light-driven liquid metal nanotransformers for biomedical theranostics. Nat. Commun. 2017, 8, 15432.
- Finkenauer, L.R.; Lu, Q.; Hakem, I.F.; Majidi, C.; Bockstaller, M.R. Analysis of the Efficiency of Surfactant-Mediated Stabilization Reactions of EGaIn Nanodroplets. Langmuir 2017, 33, 9703–9710.
- Yu, H.; Zhao, W.; Ren, L.; Wang, H.; Guo, P.; Yang, X.; Ye, Q.; Shchukin, D.; Du, Y.; Dou, S.; et al. Laser-Generated Supranano Liquid Metal as Efficient Electron Mediator in Hybrid Perovskite Solar Cells. Adv. Mater. 2020, 32, e2001571.
- Syed, N.; Zavabeti, A.; Mohiuddin, M.; Zhang, B.Y.; Wang, Y.C.; Datta, R.S.; Atkin, P.; Carey, B.J.; Tan, C.; van Embden, J.; et al. Sonication-Assisted Synthesis of Gallium Oxide Suspensions Featuring Trap State Absorption: Test of Photochemistry. Adv. Funct. Mater. 2017, 27, 1702295.
- Zhang, W.; Ou, J.Z.; Tang, S.Y.; Sivan, V.; Yao, D.D.; Latham, K.; Khoshmanesh, K.; Mitchell, A.; O’Mullane, A.P.; Kalantar-zadeh, K. Liquid Metal/Metal Oxide Frameworks. Adv. Funct. Mater. 2014, 24, 3799–3807.
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169.
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. Engl. 2010, 49, 6288–6308.
- Sivan, V.; Tang, S.-Y.; O’Mullane, A.P.; Petersen, P.; Eshtiaghi, N.; Kalantar-zadeh, K.; Mitchell, A. Liquid Metal Marbles. Adv. Funct. Mater. 2013, 23, 144–152.
- Lu, Y.; Hu, Q.; Lin, Y.; Pacardo, D.B.; Wang, C.; Sun, W.; Ligler, F.S.; Dickey, M.D.; Gu, Z. Transformable liquid-metal nanomedicine. Nat. Commun. 2015, 6, 10066.
- Zhang, M.K.; Yao, S.Y.; Rao, W.; Liu, J. Transformable soft liquid metal micro/nanomaterials. Mater. Sci. Eng. R Rep. 2019, 138, 1–35.
- Sun, X.; Guo, R.; Yuan, B.; Chen, S.; Wang, H.; Dou, M.; Liu, J.; He, Z.Z. Low-Temperature Triggered Shape Transformation of Liquid Metal Microdroplets. ACS Appl. Mater. Interfaces 2020, 12, 38386–38396.
- Gook, D.A.; Edgar, D.; Stern, C. Cryopreservation of human ovarian tissue. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, S41–S44.
- Soto, E.R.; O’Connell, O.; Dikengil, F.; Peters, P.J.; Clapham, P.R.; Ostroff, G.R. Targeted Delivery of Glucan Particle Encapsulated Gallium Nanoparticles Inhibits HIV Growth in Human Macrophages. J. Drug Deliv. 2016, 2016, 8520629.





