
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hesham El-Seedi | + 6097 word(s) | 6097 | 2021-03-16 03:08:32 | | | |
| 2 | Bruce Ren | -1 word(s) | 6096 | 2021-03-18 09:07:13 | | | | |
| 3 | Bruce Ren | -1 word(s) | 6096 | 2021-03-18 09:08:34 | | | | |
| 4 | Bruce Ren | Meta information modification | 6096 | 2021-03-23 09:42:02 | | |
Video Upload Options
Wasps, members of the order Hymenoptera, are distributed in different parts of the world, including Brazil, Thailand, Japan, Korea, and Argentina. The lifestyles of the wasps are solitary and social. Social wasps use venom as a defensive measure to protect their colonies, whereas solitary wasps use their venom to capture prey. Chemically, wasp venom possesses a wide variety of enzymes, proteins, peptides, volatile compounds, and bioactive constituents, which include phospholipase A2, antigen 5, mastoparan, and decoralin. The bioactive constituents have anticancer, antimicrobial, and anti-inflammatory effects. However, the limited quantities of wasp venom and the scarcity of advanced strategies for the synthesis of wasp venom’s bioactive compounds remain a challenge facing the effective usage of wasp venom. Solid-phase peptide synthesis is currently used to prepare wasp venom peptides and their analogs such as mastoparan, anoplin, decoralin, polybia-CP, and polydim-I.
1. Introduction
Vespid wasps (Family: Vespidae) are distributed worldwide and comprise more than 5000 species. Wasp venom has a wide variety of chemical constituents, which includes proteins, peptides (e.g., mastoparan, eumenitin, eumenitin-R, rumenitin-F, EpVP, decoralin, and anoplin), enzymes (hyaluronidase, α-glucosidase, phosphatase phospholipase A2, and phospholipase B), and small molecules [1][2][3]. The isolated compounds from wasp venom have shown several beneficial activities such as antimicrobial [4][5], anticancer [6], and anti-inflammatory effects [7]. However, their peptides have been presented in trace quantities. Solid phase peptides synthesis (SPPS) was attributed to the design and development of these molecules [8]. Successfully, several peptides and their analogues were synthesized via SPPS technology such as mastoparan [9], anoplin [10], decoralin [11], polybia-MP-I [12], polybia-CP [13][14], polydim-I [15], and agelaia-MP [16]. The synthetic peptides have antimicrobal, and anticancer properties [17][18].The nests and venoms of wasps have been their role in the synthesis of nanoparticles of gold and silver tested. These nanoparticles were proven effective as antimicrobial and anticancer entities against a variety of microorganisms and cancer cells [19][20][21].Despite preliminary medicinal outcomes, the interaction between wasp venom and human organs is still under debate. Wasp venom impacts the physiological aspects of the human body and could also lead to an allergic reaction [22].aAllergic reaction to wasp venom is a devastating problem due to the progressing immune responses of different systems. For instance, Vespa velutina venom administration lead to the failure of multi-organisms and even death among the Chinese population; and that was mostly due to toxins that are usually known to cause pain, inflammation, kidney and liver failure, cardiac arrhythmia, and sometimes neurotoxicity. Thus, many efforts are being invested into combating the allergic reactions and improving life quality using venom immunotherapy (VIT) [23]. VIT is the most effective method known so far for the avoidance of the systemic sting reactions even after discontinuation of the therapy [24].
2. Biological Properties of Wasp Venom, and Their Isolated and Synthesized Bioactive Peptides
2.1. Biological Properties
Studies have been conducted on venomous wasp structures, and their mode of action dating back to over 50 years ago. However, the therapeutic value of these toxins remains relatively unexplored. Further experiments are needed to fill the gap, and implementat quality control to elucidate wasp venom biological properties. As shown below, wasp venom exhibits biological properties, including antimicrobial, anticoagulant, genotoxic, and anti-inflammatory properties (Figure 1) [25][26][27][28].
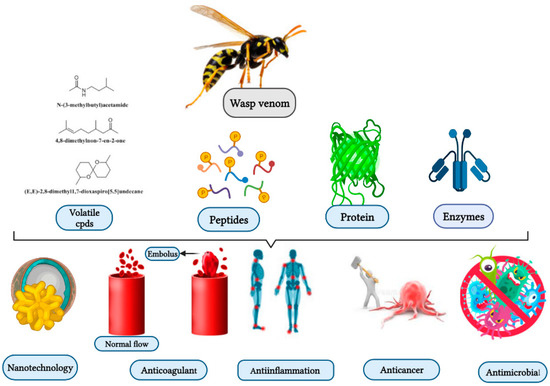
Figure 1. Wasp venom as a source of bioactive compounds and its biological activities and application.
2.1.1. Antimicrobial Activities
Today, microbial infections are a significant human concern globally. The emergence of infectious diseases and the scarcity of vaccines pose a significant danger to human health; thus, there is an immediate need to develop new antimicrobial agents [29]. Vespa orientalis’s crude venom contains peptides and proteins. The venom has antimicrobial activity against Gram-positive and Gram-negative bacteria at very low concentrations relative to tetracycline (positive control). The inhibition zones were 10.2, 12.6, 22.4, and 22.7 mm for Klebsiella pneumonia, Staphylococcus aureus, Escherichia coli, and Bacillus subtilis, respectively, while MIC values were 128, 64, 64, and 8 μg/mL, respectively. The MIC50 and MIC90 values were 74.4 and 119.2 μg/mL for K. pneumonia, 63.6 and 107 μg/mL for S. aureus, 45.3 and 65.7 μg/mL for E. coli, and 4.3 and 7.0 μg/mL for B. subtilis, respectively [30]. Previous studies have determined that the venom from Parischnogaster, Liostenogaster, Eustenogaster, and Metischnogaster wasps inhibited the development of Gram-positive B. subtilis, Gram-negative E. coli, and Saccharomyces cerevisiae yeast [31]. The peptide mastoparan-c, derived from Vespa crabro venom, triggered antimicrobial action toward resistant strains of S. aureus (Gram-positive) bacteria [25].
2.1.2. Anti-Inflammatory Activities
Inflammation is an underlying cause of several destructive disorders such as arthritis, cancer, and asthma. Anti-inflammatory medications are currently used to suppress short- and long-term body responses, and thus, it is vital to recognize new molecules with similar properties [32]. Vespa tropica venom effectively reduced oxidative stress and stimulated microglia via lipopolysaccharides (LPS) release. Wasp venom treatment (5 and 10 μg/mL) greatly attenuated LPS induced activation of NF-kB phosphorylation [33]. Bracon hebetor venom (BHV) affected LPS-induced nitric oxide (NO) in RAW 264.7 cells and septic shock in mouse models. BHV strongly mediated LPS-induced inflammation without any cytotoxicity at a concentration of 0.1–0.4 μg/mL [34]. Moreover, Nasonia vitripennis venom contains at least 80 proteins, and it exerts anti-inflammatory impacts via down-regulation of the proinflammatory cytokine IL-1β [26].
2.1.3. Genotoxicity
Polybia paulista wasp venom concentrations below 0.01–10 μg/mL did not cause cytotoxicity and showed genotoxic and mutagenic potential in HepG2 cells. The genotoxic and mutagenic behavior of P. paulista venom could be explained by the action of phospholipase, mastoparan, and hyaluronidase, leading to cell membrane disruption and genetic material alterations or even DNA mutations [28].
2.1.4. Anticoagulant
The venom of Polybia occidentalis, a social wasp, has anticoagulant, and fibrinogen-degrading pharmacological properties. Anticoagulation occurs at different stages of the clotting process (intrinsic, extrinsic, and specific pathway). Venom can inhibit platelet aggregation and destroy plasma fibrinogens [27].
2.2. Isolated and Synthesized Bioactive Peptides from Wasp Venoms
Wasp venoms are cocktails of peptides, proteins, and small organic molecules like volatiles compounds (Figure 1 and Figure 2), where peptides are the most abundant compounds, as mentioned in Table 1 [35][36]. The minute quantity of extracted venom stands as a hindrance to the analysis and understanding of the pharmacological, biological, and ecological aspects of the venom constituents. Here, we discuss the isolated peptides from wasp venom and their chemical design via SPPS [8].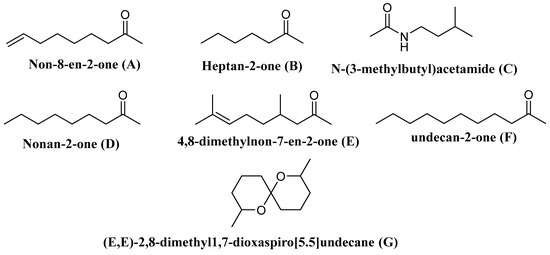
Figure 2. Some of the volatile compounds identified from wasp venom.Table 1. Isolated constitutes from Wasp-Venom and their biological activity.
| Wasp-Scientific Name | Isolated Compounds | Biological Activity | Reference |
|---|---|---|---|
| Peptides | |||
| Vespa xanthoptera Vespula lewisii |
Mastoparan (MPX) (INWKGIAAMAKKLL-NH2) | Cytotoxic against Glioblastoma multiforme (T98G) cell, 60% inhibition at 20 μmol/L (in vitro) Anti-Escherichia coli and anti-Lactococcus lactis at MIC 8, and 2.5 µM, respectively (in vitro). |
[37][38][39] |
| Anterhynchium flavomarginatum micado | Mastoparan-AF (EMP-AF) (INLLKIAKGIIKSL-NH2) |
Blocked lobster neuromuscular transmission. Mediated depolarization of the muscle membrane, often leading to a weak contraction of the muscle at 0.1 ± 1 mM (in vitro). |
[1][40] |
| V. lewisii, Vespa tropica and Polybia paulista | Mastoparan (INLKALAALAKKIL) | Induces apoptosis in B16F10-Nex2 melanoma cells treated with 165 µM. Potent anti-inflammatory. Shows activity against colistin-susceptible Acinetobacter baumannii and colistin-resistant Acinetobacter baumannii at MIC50 value of 4, and 8 mg/l, respectively. Antimicrobial activity on the epimastigote, trypomastigote and amastigote forms of Trypanosoma cruzi Y strain via dose-dependent growth inhibition (in vitro). |
[41][42][43] |
| Vespa basalis | Mastoparan B (LKLKSIVSWAKKVL) | Anti-Enterococcus faecalis and anti-Bucillus subtilis at MIC of 3.13 mg/mL (in vitro). | [44] |
| V. basalis | Mastoparan-I1 (INLKAIAALVKKVL) | ND | [44] |
| V. basalis | Mastoparan-A (IKWKAILDAVKKVI) | ND | [44] |
| V. basalis | Mastoparan-T (INLKAIAAFAKKLL) | ND | [44] |
| Vespula vulgaris | Mastoparan V1 (INWKKIKSIIKAAMN) | Potent antimicrobial activity against Streptococcus mutans and Salmonella enterica at 50 µM (in vitro). | [4] |
| Vespa orientalis L. | Mastoparan (HRI) (INLKAIAALVKKVL-NH2) |
Cytotoxic towards T98G cells and give 80% inhibition at 20 μmol/L (in vitro). | [37] |
| Vespa crabro | Mastoparan-C (MP-C) (LNLKALLAVAKKIL-NH2) | Inhibition of the biofilm formation by Staphylococcus Aureus and Pseudomonas aeruginosa at 32 μM MBIC (in vitro). | [25] |
| V. tropica | Mastoparan-VT1 (INLKAIAALAKKLL) | Anti-E. faecalis at 2.5 µg/mL (in vitro). | [29] |
| V. tropica | Mastoparan-VT2 (NLKAIAALAKKLL) | Anti-E. faecalis, anti-E.coli and anti-S.aureus at 5 µg/mL (in vitro). | [29] |
| V. tropica | Mastoparan-VT3 (INLKAITALAKKLL) | Anti-S. aureus and anti-Candida parapsilosis at 2.5 µg/mL (in vitro). | [29] |
| V. tropica | Mastoparan-VT4 (INLKAIAPLAKKLL) | Anti-Bacillus pyocyaneus, anti-P. aeruginosa, and anti-Bacillus dysenteriae at 10 µg/mL (in vitro). | [29] |
| V. tropica | Mastoparan-VT5 (VIVKAIATLASKLL) | Anti-Candida albicans at 40 µg/mL (in vitro). | [29] |
| V.tropica | Mastoparan-VT6 (INLKAIAALVKKLL) | Anti-S. aureus and anti-B. dysenteriae at 20 µg/mL (in vitro). | [29] |
| V. tropica | Mastoparan-VT7 (INLKAIAALARNY) | Anti-E. faecalis at 5 µg/mL (in vitro). | [29] |
| Polistes rothneyi iwatai | Polistes-mastoparan-R1 (Pm-R1) (INWLKLGKKILGAI-NH2) | Has histamine-releasing activities from rat mast cells (EC50 = 0.09 µM) (in vitro). | [39] |
| P. rothneyi iwatai. | Polistes-mastoparan-R3 (Pm-R3) (INWLKLGKQILGAL-NH2) |
Has histamine-releasing activities from rat mast cells (EC50 = 0.19 mM) (in vitro). | [39] |
| Vespa magnifica | Peptide 5e (FLPIIAKLLGLL) | Anti-S. aureus, MIC = 5 µg/mL (in vitro). | [45] |
| V. magnifica | Peptide 5f (FLPIPRPILLGLL) | Anti-S. aureus, MIC = 10 µg/mL (in vitro). | [45] |
| V. magnifica | Peptide 5g (FLIIRRPIVLGLL) | Anti-S. aureus MIC = 10 µg/mL (in vitro). | [45] |
| V. magnifica | Peptide 12a (INWKGIAAMAKKLL) | Anti-S. Aureus, and anti-C. albicans at MIC = 3.7 µg/mL (in vitro). | [45] |
| V. magnifica | Peptide 12b (INWKGIAAMKKLL) | Anti-S. aureus MIC = 3.7 µg/mL (in vitro). | [45] |
| P. dimorpha | Polydim-I (AVAGEKLWLLPHLLKMLLTPTP) | Antimycobacterial activity at 7.6 μg/mL (in vitro). Anti-S. aureus at MIC50 4.1 µg/mL (in vitro). |
[15][46] |
| Anoplus samariensis | As-126 (EDPPVVKMK-NH2) | ND | [47] |
| Batozonellus maculifrons | Bm-10 (ETAPVPKAISK-NH2) | ND | [47] |
| A. samariensis | Anoplin (GLLKRIKTLL-NH2) |
Cytotoxic for T98G cells, gives 10% inhibition at 20 μmol/L (in vitro). | [37][48] |
| P. hypochondriaca | Pimplin (KRKPPRPNPKPKPIP) | Effective against Musca domestica at dose of 40 ng (in vitro). | [49] |
| A. flavomarginatum micado | Af-113 (INLLKIAKGIIKSLNH2) | ND | [50] |
| Agelaia vicina | Agelaiatoxin-8 (AVTx8) (INWKLGKALNALLNH2) | Inhibits gamma-aminobutyric acid (GABA) neurotransmission uptake at EC50 value of 0.09 ± 0.04 µM and maximum inhibition of 97 ± 5% (in vitro). | [51] |
| Agelaia pallipes pallipes | AgelaiaMP-I (INWLKLGKAIIDAL-NH2) |
Has hemolytic activity at ED50 = 60 µM. | [27] |
| A. pallipes pallipes | AgelaiaMP-II (INWKAILQRIKKML-NH2) | Has hemolytic activity at ED50 = 240 µM (in vitro). | [52] |
| Anoplius samariensis, and Batozonellus maculifrons |
Pompilidotoxins (α-PMTXs) (RIKIGLFDQLSKL-NH2) | Facilitates synaptic transfer in the motor neuron of the lobster and delays downregulation of the sodium channel (in vitro). | [53] |
| A. samariensis, and B. maculifrons |
β-PMTXs (RIKIGLFDQRSKL-NH2) |
Facilitates synaptic transfer in the neuromuscular junction of the lobster, and slows the sodium channel inactivation (in vitro). | [53] |
| A. flavomarginatum micado | Eumenine mastoparan-AF (EMP-AF) (INLLKIAKGIIKSL-NH2) |
Effective hemolytic response in human erythrocytes. Enhancing degranulation of rat peritoneal mast cells and RBL-2H3 cells (in vitro). |
[40] |
| Agelaia pallipes pallipes, and Protonectarina sylveirae | Protonectin (ILGTILGLLKGL-NH2) |
Antibacterial activity towards Gram-positive and Gram-negative bacteria. Releasing Lactate dehydrogenase (LDH) from mast cells. Chemotaxis against polymorphonuclear leukocytes (PMNL) (in vitro). |
([54] |
| A. pallipes pallipes, and P. sylveirae | Protonectin (1–6) (ILGTIL-NH2) |
ND | [54] |
| A. pallipes pallipes | Protonectin (1–4)-OH (ILGT-OH) |
Has poor hemolytic activity at ED50 = 1 mM (in vitro). | [52] |
| A. pallipes pallipes | Protonectin (7–12) (GLLKGL-NH2) |
ND | [52] |
| A. pallipes pallipes | Protonectin (1–5)-OH (ILGTI-OH) |
Has weak hemolytic activity at ED50 = 1 mM (in vitro). | [52] |
| A. pallipes pallipes | Protonectin (1–6)-OH (ILGTIL-OH) |
Has poor hemolytic activity at ED50 = 1 mM (in vitro). | [52] |
| Orancistrocerus drewseni | Orancis-protonectin (ILGIITSLLKSL-NH2) | Has hemolytic activity of the sheep blood cells at 50 µM (in vitro). | [55] |
| A. pallipes pallipes | Pallipine-I (GIIDDQQCKKKPGQSSPVCS-OH) | ND | [52] |
| A. pallipes pallipes | Pallipine-II (SIKHKICKLLERTLKLTT PFC-NH2) | ND | [52] |
| A. pallipes pallipes | Pallipine-III (SIKKHKCIALLERRGGSKLPFC-NH2) |
ND | [52] |
| P. paulista | Paulistine (SIKDKICKIIQCGKKLPFT-NH2) (oxidized form) |
Causes mast cells degranulation or hemolysis (in vitro). | [56] |
| Vespa mandarinia | Ves-CP-M (FLPILGKLLSGL-NH2) | ND | [57] |
| V. xanthoptera | Ves-CP-X (FLPIIAKLLGGLL) | ND | [57] |
| Paravespula lewisi | Ves-CP-P (FLPIIAKLVSGLL) | ND | [57] |
| V. tropica | Ves-CP-T (FLPILGKILGGLL) | ND | [57] |
| V. crabro | Crabrolin (FLPLILRKIVTAL-NH2) | Releases histamine from rat peritoneal mast cells at ED50 of 11.8 µg/mL (in vitro). | [58][59] |
| Eumenes rubronotatus | Eumenitin (LNLKGIFKKVASLLT) | Shows antimicrobial activity against S. aureus, Staphylococcus saprophytius, E. coli at MIC = 6 µM (in vitro). | [60] |
| E. rubrofemoratus | Eumenine mastoparan-ER (EMP-ER) (FDIMGLIKKVAGAL-NH2) | Anti-C. albicans at MIC 7.5 µM. Has Leishmanicidal activity at IC50 20 µM (in vitro). |
[61] |
| Eumenes micado | Eumenine mastoparan-EM1 (LKLMGIVKKVLGAL-NH2) | Anti-S. aureus and anti-E. coli at MIC 7 µM (in vitro). Has Leishmanicidal activity with an IC50 of 36 µM (in vitro). |
[62] |
| E. micado | Eumenine mastoparan-EM2 (LKLLGIVKKVLGAI-NH2) | Anti-S. aureus and anti-E. coli at MIC of 3 µM (in vitro). Has Leishmanicidal activity with an IC50 of 36 µM (in vitro). |
[62] |
| Eumenes fraterculus | Eumenine mastoparan-EF (EMP-EF) (FDVMGIIKKIASALNH2 | Anti-C. albicans at MIC of 7.5 µM. Has Leishmanicidal behavior at IC50 of 40 µM (in vitro). |
[61] |
| O. drewseni | Eumenine mastoparan-OD (EMP-OD) (GRILSFIKGLAEHL-NH2) |
Induces hemolysis of the sheep blood cells at 50 µM (in vitro). | [55] |
| E. rubrofemoratus | Eumenitin-R (LNLKGLIKKVASLLN) | Anti-Sreptococcus pyogenes, anti-Micrococcus luteus, and anti-Stenotrophomonas maltophilia at MIC of 15 µM. Anti-B. subtilis at MIC 7.5 µM (in vitro). |
[61] |
| E. fraterculus | Eumenitin-F (LNLKGLFKKVASLLT) | Anti-C. albicans at MIC of 7.5 µM. Has Leishmanicidal activity at IC50 of 52 µM (in vitro). Anti-S. maltophilia at MIC of 15 µM (in vitro). |
[61] |
| P. paulista. | Polybia-CP (ILGTILGLLKSL-NH2) |
Anti-microbial against S. aureus and B. subtilis at 15 µg/mL compared with 0.5 and 18 µg/mL of tetracycline (in vitro). | [14][57] |
| P. paulista | Polybia-CP 2 (ILGTILGKIL-OH) | Has chemotaxis, mast cell degranulation, and hemolytic activities (in vivo). | [63] |
| Polybia-CP 3 (ILGTILGTFKSL-NH2) | Has chemotaxis, mast cell degranulation, and hemolytic activities (in vivo). Antiplasmodial and anticancer properties (in vitro). |
[8][63] | |
| P. paulista | Polybia-MP1 (IDWKKLLDAAKQIL-NH2) |
Antitumor against bladder and prostate cancer cells. Exhibits potent activity against S. aureus, MIC of 9 µΜ (in vitro). Anti-C. albicans (EC50 = 12.9 μM) and C. neoformans (EC50 = 11 μM) (in vitro). Fungicidal activity against Candida glabrata (EC50 = 8 μM) and C. albicans (EC50 = 16 μM) (in vitro). Anti-E. coli, P. aeruginosa, B. subtilis, and S. aureus at MIC of 8, 8, 4, and 15 μg/mL compared to 2, 18, 18, and 0.5 of tetracycline (in vitro). |
[64][49] |
| V. orientalis L. | HR-1 (INLKAIAALVKKVL-NH2 | ND | [65] |
| V. orientalis L. | HR-2 (FLPLILGKLVKGLL-NH2) | ND | [65] |
| Polistes jadwigae | Polisteskinin-J (RRRPPGFSPFR-OH) | ND | [63] |
| Pollistes chiensis | Polisteskinin-C (SKRPPGFSPFR-OH) | ND | [63] |
| P. rothney | Polisteskinin-R (ARRPPGFTPFR-OH) | Exerts potent anxiolytic effects at 6, 3, and 1.5 ηmol compared to positive control Diazepam (in vivo) | [63][66] |
| Vespa analis | Vespakinin-A (GRPPGFSPFRVI-OH) | ND | [63] |
| Vespa mandarínia | Vespakinin-X (ARPPGFSPFR-OH) | ND | [63] |
| V. magnifica, Parapolybia varia, V. tropica | Vespid Chemotactic Peptides (VCP) | Anti-tumor activities towards NIH-OVCAR-3 and SK-OV-3 ovarian cancer cell lines at concentrations higher than 10 μM (in vitro). | [33][67] |
| V. magnifica (Smith) | VCP-5h (FLPIIGKLLSGLL-NH2) | MICs of 5, 25, and 30, µg/mL for S. aureus, C. albicans and E. coli, respectively (in vitro). | [68] |
| Parapolybia varia | Vespakinin (Vespk) | Antitumor activity to SK-OV-3 at 24 h post-treatment (in vitro). | [67] |
| V. magnifica | Vespakinin-M GRPPGFSPFRID | ND | [69] |
| Batozonellus maculifrons | Pompilidotoxins (β-PMTXs) (RIKIGLFDQLSRL-NH2) | Inactivation of the Na+ channel, and the Nav1.6 channel was more selective (in vitro). | [1] |
| O. drewseni | OdVP1 (GRILSFIKGLAEHL-NH2) | Anti-E. coli, and anti-C. albicans at MIC of 6 µM (in vitro). | [70][71] |
| O. drewseni | OdVP2 (ILGIITSLLKSL-NH2) | Anti-S. aureus at MIC of 25 µg/mL. Anti-gray mold Botrytis cinerea at MIC of 0.4 µM (in vitro). |
[70][71] |
| O. drewseni | OdVP3 (KDLHTVVSAILQAL-NH2) | Anti-gray mold B. cinerea at MIC of 5 µM (in vitro). | [70][71] |
| O. drewseni | OdVP4 (LDPKVVQSLL-NH2) |
ND | [70] |
| Nasonia vitripennis | Defensin-NV (VTCELLMFGGVVGDSACAANCLSMGKAGGSCNGGLCDCRKTTFKELWDKRFG) | Anti-S. aureus, and Anti-B. cereus at MIC of 0.93 µM (in vitro). Anti-B. dysenteriae at MIC of 0.46 µM (in vitro). Anti-E. coli, and anti-C. albicans at MIC of 1.86 µM (in vitro). Anti-P. aeruginosa at MIC of 9.3 µM (in vitro). |
[72] |
| Chartergellus communis | Communis (INWKAILGKIGK-COOH) |
ND | [73] |
| C. communis | Communis-AAAA (INWKAILGKIGKAAAAVNH2) | Hemolytic activity at EC50 = 142.6 μM (in vitro). Hyperalgesic effect at 2 nmol/animal (in vivo). |
[73] |
| Cyphononyx Fulvognathus |
Bradykinin (RPPGFSPFR) |
Acts as a chemoattractant directing glioma cells into blood vessels in the brain of rats (in vivo). | [74] |
| Megascolia flavifrons, and Colpa interrupta |
Megascoliakinin = Thr6BK-Lys-Ala (BK = bradykinin) (RPPGFTPFRKA) | Prevents the synaptic transmission of the nicotinic acetylcholine receptor (nAChR) in the central nervous system of insect (in vitro). | [75] |
| C. fulvognathus and P. paulista |
RA-Thr6 -Bradykinin (RARPPGFTPFR-OH) | ND | [63] |
| Polybia occidentalis, M. flavifrons, C. interrupta, and P. paulista | Threonine6-bradykinin (Thr6-BK) RPPGFTPFR-OH |
Anti-nociceptive effects with approximately two-fold higher than bradykinin and morphine (in vivo). | [63][76] |
| P. paulista | RA-Thr6 -Bradykinin-DT (RARPPGFTPFRDT-OH) | ND | [63] |
| C. fulvognathus | Fulvonin (SIVLRGKAPFR) |
Displays hyperalgesic impact after intraplantar injection in the rat paw pressure test (in vivo). | [77] |
| C. fulvognathus (Japan) |
Cyphokinin (DTRPPGFTPFR) | Demonstrates hyperalgesic impact after intraplantar injection in the rat paw pressure test (in vivo). | [77] |
| C. fulvognathus (Japan) |
Cd-146 (SETGNTVTVKGFSPLR) | Shows hyperalgesic effect in the rat paw pressure test after intraplantar injection (in vivo). | [77] |
| C. fulvognathus | Cd-125 (DTARLKWH) | ND | [77] |
| P. paulista | Mastoparan (MPI) (IDWKKLLDAAKQIL-NH2) |
Cytotoxic towards T98G cells, gives 30% inhibition at 20 μmol/L (in vitro). | [37] |
| Pseudopolybia vespiceps | Mastoparan Polybia-MPII (INWLKLGKMVIDAL-NH2) | Anti-staphylococcal activity with an EC50 of 1.83 μM and EC90 of 2.90 μM (in vitro). Mice treated with 5 mg/kg showed a decline in bacterial load from 108 to ca. 106 CFUs (in vitro). Potent hemolytic activity against mouse cells (EC50 = 24.18 Μm, EC90 = 58.12 μM) (in vitro). Inhibits the growth of C. neoformans (EC50 = 11 μM) and C. albicans (EC50 = 12.9 μM) (in vitro). Anti-A. baumannii AB 0 at MIC of 12.5 µM while MIC against A. baumannii AB 53 and AB 72 was 6.25 µM (in vitro). Adhesion inhibition for A. baumannii AB 02 and AB 72 at 25 µM while A. baumannii AB 53 was inhibited at a concentration of 12.5 µM (in vitro). |
[27][78] |
| P. paulista | Polybia-MPIII (INWLKLGKAVIDAL) | Anti-S. aureus, MIC of 19 μM (in vitro). | [57] |
| P. paulista | Polybia-MP IV (IDWLKLRVISVIDL-NH2) | Shows strong mast cell degranulation. Has weak haemolytic activity, hypernociception and edema formation (in vitro). |
[63] |
| P. paulista | Polybia-MP V (INWHDIAIKNIDAL-NH2) | Medium mast cell degranulation, haemolytic activity and hypernociception (in vitro). | [63] |
| P. paulista | Polybia-MP VI (IDWLKLGKMVM-OH) | Medium haemolytic activity and hypernociception (in vitro). | [63] |
| P. paulista | unk-1 (IPAGWAIVKV-NH2) | Shows weak mast cell degranulation and haemolytic activity (in vitro). | [63] |
| P. paulista | unk-2 (TGDSPDVR-OH) | Shows weak mast cell degranulation and haemolytic activity, weak chemotaxis for PMNLs, and a range of weak to strong hypernociception and oedema formation (in vitro). | [63] |
| V. orientalis L. | Orientotoxin (Neurotoxin) |
Has lysophospholipase activity and inhibits both mediated and spontaneous release of the neurotransmitter from the presynaptic nerve membrane (in vivo). | [79][80] |
| V. orientalis L. | Peptide I (AGVILFGR-NH2) | Histamine release from mast cells ED50 = 5.10−7 (in vivo). | [81] |
| V. orientalis L. | Peptide II (AGVIFRSP-NH2) | Histamine release from mast cells ED50 = 3.10−6 (in vivo). | [81] |
| Oreumenes decoratus |
Decoralin (De-NH2) (SLLSLIRKLIT-NH2) |
Has hemolytic activity at EC50 of 80 µM (in vitro). Anti-S. aureus, MIC = 4 µM (in vitro). Anti-B. Subtilis, MIC = 8 µM (in vitro). Anti-C. albicans, MIC = 20 µM (in vitro). Has leishmanicidal activity, IC50 =11 µM (in vitro). |
[82] |
| V. ducalis | VACP1 (AQKWLKYWKADKVKGFGRKIKKIWFG) |
Potently inhibits cell proliferation and promotes the cell apoptosis of osteosarcoma (OS) cells, and this was concomitant with the activation of the JNK and p38 MAPK signaling pathway (in vitro). | [6] |
| Emerald Jewel, and Ampulex compressa | Ampulexin-1 (axn1) (CKDDYVNPKEQLGYDILEKLRQKP) | ND | [83] |
| Ampulexin -2 (axn2) (CQNDYVNPKLQFACDLLQKAKERQ) | ND | [83] | |
| Ampulexin -3 axn3 SFSMLLQKAKERQ | ND | [83] | |
| V. orientalis | AuNPs+ peptide (INLKAIAALVKKV) | Antibacterial using AuNPs against K. pneumoniae, B. cereus, S. mutans, S. typhimuriu, E. coli, and S. aureus, and with the inhibition zones of 9.21, 14.32, 14.71,19.21, 15.24 and 15.33 mm, respectively (in vitro). | [19] |
| Vespa bicolor Fabricius | V. chemotatic peptide (VESP-VBs) (FMPIIGRLMSGSL) | Anti-S. aureus, MIC = 1 µg/mL (in vitro). | [5] |
| V. bicolor Fabricius | V. mastoparan (MP-VBs) (INMKASAAVAKKLL) | Anti-S. aureus, MIC = 1.9 µg/mL (in vitro). | [5] |
| Polistes dominulus | Dominulin A (INWKKIAEVGGKILSSL) | Anti-B. Subtilis, and E. coli at MIC = 2 and 8 µg/mL, respectively (in vitro). | [84] |
| P. dominulus | Dominulin B (INWKKIAEIGKQVLSAL) | Anti-B. Subtilis, and E. coli at MIC = 2 and 8 µg/mL, respectively (in vitro). | [17] |
| Protonectarina sylveirae | Protonectarina-MP (INWKALLDAAKKVL) | Anti-B. subtilis and anti-S. Aureus MIC = 3.9 µg/mL (in vitro). | [85] |
| Parapolybia indica | Parapolybia-MP (INWKKMAATALKMI-NH2) | Anti-S. aureus, MIC = 3.9 µg/mL (in vitro). | [85] |
| P. jadwigae | Polistes mastoparan (VDWKKIGQHIKSVL) | Degranulation of mast cells at 5 nM/mL. | [86] |
| V. magnifica (Smith) | Vespid chemotactic peptide (VCP) | MICs for S. aureus, C. albicans, and E. coli were 5, 25, and 30, µg/mL, respectively (in vitro). | [68] |
| V. bicolor Fabricius | VESP-VB1 (FMPIIGRLMSGSL) | Anti-E. coli, MIC = 7.5 µg/mL (in vitro). Anti-S. aureus, MIC = 1.9 µg/mL (in vitro). Anti-P. aeruginosa, MIC = 3.75 µg/mL (in vitro). Anti-C. albicans, MIC = 30 µg/mL (in vitro). |
[5] |
| V. bicolor Fabricius | MP-VB1 (INMKASAAVAKKLL) | Anti-E. coli, MIC = 15 µg/mL (in vitro). Anti-S. aureus, MIC = 3.75 µg/mL (in vitro). Anti-P. aeruginosa, MIC = 15 µg/mL (in vitro). Anti-C. albicans, MIC = 15 µg/mL (in vitro). |
[5] |
| V. tropica | VCP-VT1 | Anti-E. coli, Enterobacter cloacae, and C. parapsilosis at 2.5 µg/mL and Anti-S. aureus at 1.2 µg/mL (in vitro). | [29] |
| V. tropica | VCP-VT2 FLPIIGKLLSG |
Antimicrobial against S. aureus, E. cloacae at 2.5 µg/mL (in vitro). | [29] |
| Protopolybia exigua (Kinins) | Protopolybiakinin-I (DKNKKPIRVGGRRPPGFTR-OH) |
Caused degranulation of 35% of the mast cells (in vitro). | [87] |
| P. exigua | Protopolybiakinin-II (Kinins) (DKNKKPIWMAGFPGFTPIR-OH) | Caused degranulation of 52 % of the mast cells (in vitro). | [87] |
| V. mandarinia | VESCP-M2 (FLPILAKILGGLL) | Induces pain and severe tissue injury, oedema, cutaneous necrosis, and blister. | [88] |
| Polistes lanio lanio | PllTkP-I (QPPTPPEHRFPGLM) | ND | [89] |
| P. lanio lanio | PllTkP-II (ASEPTALGLPRIFPGLM) | ND | [89] |
| V. magnifica (Smith) | 5-Hydroxytryptamine | ND | [90] |
| V. magnifica (Smith) | Vespakinin-M (GRPPGFSPFRID-NH2) | ND | [90] |
| V. magnifica (Smith) | Mastoparan M (INLKAIAALAKKLL-NH2) | ND | [90] |
| V. magnifica (Smith) | Vespid chemotactic peptide M (FLPIIGKLLSGLL-NH2) | ND | [90] |
| Sphex argentatus argentatus | Sa12b (EDVDHVFLRF) | Inhibits acid-sensing ion channels (ASIC) of rat dorsal root ganglion (DRG) neurons at IC50 of 81 nM while inhibiting it completely at 1 μM (in vivo). | [91] |
| Isodontia harmandi | Sh5b(DVDHVFLRF-NH2) | ND | [91] |
| P. paulista | Neuropolybin | Antiseizure | [36] |
| Synoeca surinama | Synoeca-MP I/LNWI/LKI/LGKKI/LI/LASL/NH2 |
Antimicrobial activity, MIC50 values were 1.9, 2, 8.3, 5.2, and 3.5 μM for methicillin-resistant S. aureus—MRSA, E. coli ESBL, vancomycin-resistant E. Faecalis, P. aeruginosa metallo-ß-lactamase, and Klebsiella pneumoniae KPC, respectively (in vitro). Anti-Candida species, with MICs varying from 10–40 μM (in vitro). |
[92] |
| Enzymes and proteins | |||
| V. magnifica | Magnifin (PLA1) | Activates platelet aggregation and induces thrombosis at 18 nM with causes 85% washed platelets aggregation in 60 s (in vivo). | [93] |
| P. paulista (southeast Brazil) |
Phospholipase A1(Ves v 1) | Catalyzes the ester bonds hydrolysis of 1,2-diacyl-3 snglycerophospholipids at the sn-1 and sn-2 positions, respectively. | [94] |
| P.paulista | Phospholipase A1 | Hydrolyzes phospholipids and produces 2-acyl-lysophospholipids and fatty acids. | [94][95] |
| P. Occidentalis and P. paulista | Phospholipase A2 (PLA2) | Potent hemolytic actions in washed red cells (in vitro). Hydrolyzes natural phospholipids, catalysing the deacylation of 1,2-diacyl-sn-3-phosphoglycerides at position 2 and thus releases free fatty acids and lysophospholipids (in vitro). |
[96][97] |
| P. paulista, Vespula maculate, Vespula arenaria, V. crabro, V. orientalis, Paravespula germanica, Paravespula vulgaris, Dolichovespula saxonica, Dolichovespula media, and Polistes Gallicus |
Hyaluronidase (Polyp2) | Hydrolyses hyaluronic acid which facilitates the diffusion of toxin into the tissue and blood circulation of the prey. | [98][99][100] |
| Polistes comanchus | Polistin (protein) | Responsible for the cytotoxic effect of the whole venom. | [101] |
| P. paulista | Antigen5 (Polyp5) | Major allergen could be used for allergy diagnostics and treatment. | [102] |
| Cyphononyx dorsalis | Arginine kinase-like protein | Exhibits paralytic activity in spiders with the same characteristic symptoms as the crude venom. | [103] |
| Pteromalus puparum | Vn.11 (protein) |
ND | [104] |
| Cotesia rubecula | Vn 4.6 | ND | [105] |
| V. magnifica | Magnvesin | Exerts anti-coagulant properties via hydrolyzing coagulant factors VII, VIII, TF, IX and X. | [106] |
| Some volatile compounds | |||
| Vespa velutina | Undecan-2-one | Elicits the defense behavior | [107] |
| V. velutina | Non-8-en-2-one | ||
| V. velutina | Nonan-2-one | ||
| V. velutina | Heptan-2-one | ||
| V. velutina | 4,8-Dimethylnon-7-en-2-one | ||
| Polistes metricus Say, Polistes bellicosus Cresson, and Polistes dorsalis (F.), as well as workers of Polistes aurifer (Saussure), P. bellicosus, P. metricus, and P. dorsalis | N-(3-Methylbutyl)acetamide | ND | [108] |
| P. occidentalis | (E,E)-2,8-Dimethyl1,7-dioxaspiro[5.5]undecane | Elicit the defense behavior | [109] |
2.2.1. Mastoparans
The mastoparans are comprised of a class of peptides isolated from Vespula lewisii [41], V. crabro [25], Vespula vulgaris [4], and Polistes jadwigae [86]. Mastoparans are characterized by their antitumor activity against melanoma cells (B16F10-Nex2) [41].
Mastoparan (MP)Mastoparan (MP), a major component of P. jadwigae wasp venom, is a basic amphiphilic α-helical peptide that consists of 14 amino acid residues, hydrophobic and essential amino acids, and an amino acid C-terminus, as shown in Table 1 [86]. These characters are specific for the cationic amphiphilic peptide (CAP) class and favour the α-helix conformation while in contact with bilayer phospholipids [37]. MP has several biological effects and has shown antimicrobial properties [42], increased histamine release from mast cells [110], and cytotoxicity effect on tumor cells [18]. MP-induced mitochondrial permeability and powerful transition of mitochondrial permeability (PT) in a range of 25 μM in a homogeneous K562 cell are reported [111]. Moreover, MP exerts anticancer activities toward leukemia, myeloma, and breast cancer cells. In a mouse model of mammary carcinoma, MP and gemcitabine (drug) worked synergistically [18]. MP was active on a dose-dependent basis with doses ranging from 77.9 to 432.5 μM against human cancer cells (A2058 (melanoma), SiHa (cervical carcinoma), Jurkat (T cell leukemia), MCF-7, MDA-MB-231, and SK-BR-3 (breast cancer). The IC50 of B16F10 murine melanoma was 165 μM. MP-induced apoptosis involves activation of caspase −9, −12, and −3, PARP cleavage, upregulation of pro-apoptotic Bax, and Bim, down-regulation of anti-apoptotic Bcl-XL; furthermore, cell apoptosis induced mitochondrial membrane disruption [41].MP inhibited bradykinin-induced phosphoinositide hydrolysis within 5 min of administration at a concentration of 30 μM and induced the release of prostaglandin E2 (PGE 2) in rabbit astrocytes within 10 min [112].The synthetic peptide derived from MP is called mastoparan ([I5, R8] MP) and has a wide range of antimicrobial activities against bacteria and fungi at MIC values of 3–25 µM with no hemolytic or cytotoxic properties to the human embryonic kidney cell line (HEK-293 cells). The synthesis does not appear to change the α-helical conformation but enhances the biological activity [113]. Ten MP derivatives have been synthesized via SPPS strategies and evolved against Acinetobacter baumannii. MP analogs (H-INIKALAALAKKII-NH2, H-INLKALAALAKKIL-CH2CH2NH2, and Gu-INLKALAALAKKIL-NH2) demonstrated the same behavior against A. baumannii as the original peptide (2.7 µM) and retained its consistency in the presence of human serum for more than 24 h [9]. Three MP analogs, MK4589 (INWKKIAKKVAGML-NH2), MK45789 (INWKKIKKKVAGM), and MK4578911 (INWKKIKKKVKGML-NH2), were synthesized, and exhibited strong antibacterial properties against Gram-negative bacteria compared to the reference antibiotic, chloramphenicol [114]. Mastoparan-V1 (MP-V1), a de novo type of V. vulgaris venom mastoparan, has higher anti-Salmonella activity than other mastoparans [4]. MP analog peptides showed activity against Candida albicans, with low cytotoxicity and non-teratogenicity using cell cultures and zebrafish models [115]. Synthetic MP-V1 has antimicrobial properties at MICsvalues of 106.95, 56.86, and 123 µg/mL against Salmonella Gallinarum, S. typhimurium, and S. enteritidis, respectively [116].
Mastoparan-B (MP-B)Mastoparan-B (MP-B), the mastoparan homolog of Vespa basalis venom, has a less hydrophobic amino acid sequence with four lysines (LKLKSIVSWAKKVL-NH2) [117] and is approved as a cardiovascular depressor [118] and antibacterial agent [44]. MP-B shows powerful hemolytic activity secondary to the stimulation of histamine release from rat peritoneal mast cells [117]. A synthetic MP-B analog (LDLKSIVSWAKKVL-NH2), in which lysine was replaced by asparagine at position 2, showed a remarkable decline of cardiovascular depressors; in contrast, the analog with leucine replacing lysine at position 4, 11, or 12 (LKLLSIVSWALLVL-NH2) did not display the same effect [118].
Mastoparan-MMastoparan-M is an amphipathic tetradecapeptide toxin and a vespid venom mastoparan counterpart isolated from the Vespa mandarinia hornet in Japan. Mastoparan-M has the (INLKAIAALAKKLL) sequence. At a minimum concentration (MIC) of 0.5 nmol/mL, the peptide degranulated rat peritoneal mast cells [119]. Mast cell degranulation induced the release of inflammatory mediators, such as TNF-α, IL-1β, and nitrite, from cultured mouse spleen macrophages [120].SPPS was used to synthesize D-mastoparan M (INLKAIALAKKLL) and L-mastoparan M (INLKAIAALAKKLL). D-mastoparan M showed MIC of 6.25 mg/L against E. coli and Pseudomonas aeruginosa and 3.12 mg/L against S. aureus. The antibacterial impact of D-mastoparan was twice as effective as L-mastoparan M. After the supplementation of D-mastoparan M, bacterial lysis was observed at 1 h and was completed after 4 h [121].
2.2.2. Anoplin
Anoplin (ANP) is the smallest antimicrobial, naturally occurring peptide isolated from the solitary spider wasp Anoplius samariensis (Hymenoptera: Pompillidae) and contains ten amino acids (Table 1), making it an ideal research template [48]. The peptide causes mast cell degranulation and has antimicrobial activity [48]. The presence of four-polar residues makes ANP water-soluble. Its interaction with amphipathic environments, such as trifluoroacetic acid (TFE)/water mixtures, or with anisotropic media, such as sodium dodecyl sulfate (SDS) micelles or anionic vesicles, induces α-helical conformations and amphiphilic properties as indicated by the circular dichroism (CD) spectra [48]. ANP inhibited the proliferation of murine erythroleukemia (MEL) cells in a time- and dose-dependent manner. The IC50 values were 161.49, 121.03, and 114.88 μM at 24, 48, and 72 h, respectively. Disrupting the cell membrane integrity was the primary mechanism behind anoplin’s cytotoxicity [122].Synthetic ANP peptides have a broad spectrum of antimicrobial activity against Gram-positive and Gram-negative bacteria. ANP antimicrobial activity is susceptible to salt. Gram-negative bacteria were entirely immune to ANP in high-salt media (150 mM NaCl); however, Gram-positive bacteria’s efficacy was greatly diminished [48]. Equally interesting, it stimulates rat peritoneal mast cell degranulation, and ANP’s hemolytic activity was relatively low or virtually inactive on human erythrocytes [48].ANP’s activity is highly sensitive to minor changes of the primary structure, such as single amino acid mutations in certain positions. For example, 37 anoplin analogs have been synthesized by replacing single and multiple residues leading to a change in amphipathicity and charge. Accordingly, the effects against S. aureus and E. coli varied considerably depending on the hydrophobicity and position of the various replaced amines. Residue replacement at positions 5, and 8 with phenylalanine or tryptophan caused by an increase in antibacterial, and hemolytic activity owed to the role of these aromatic residues in the membrane anchoring. Lysine placement in position 8 improved peptide selectivity for prokaryotic cells due to the higher charge [123], whereas C-and N-terminal truncation and C-terminus deamidation drastically decreased peptide antibacterial properties [124]. Antimicrobial activities were measured against E. coli and B. subtilis for all three derivatives of ANP (ANP-NH2, D-ANP-NH2, and ANP-OH). Both amidated ANP derivatives display 50 μg/mL, MIC values for B. subtilis, and 100 μg/mL for E. coli. Alternatively, the deamidated form showed significantly lower bactericidal activity with MIC values of 200 μg/mL. The LD50 values for both amidated ANP forms were identical and approximately 10- to 30-fold lower than those of ANP-OH. ANP loses its biological activity after deamidation. Both amidated and carboxylated forms have secondary structures similar to those of the lytic ANP [125]. The natural cationic ANP was modified by substituting residues Gly1 for Lys or Arg, Arg5 for Phe, and Thr8 for Lys. The antimicrobial properties change dramatically, and high activity against Gram-negative bacterium Zymomonas mobilis at MIC 7 μg/mL was observed, compared to native peptide MIC 200 μg/mL; additionally, it was non-toxic to erythrocytes and resistant to proteolysis [10]. Interestingly, the antimicrobial activities of ANP and analogs ANP-2 (WLLKRWKLL-NH2), and ANP-4 (KLLKWKKLL-NH2) were significantly higher than ANP-1 (WLLKRWKKLL-NH2) and ANP-3 (KLLKWWKKLL-NH2). The highest antimicrobial activity against B. subtili was shown by ANP-2 and ANP-4 (MIC value: 4 μM) compared to the parent peptide (MIC value: 32 µM). ANP-4 treatment significantly reduced the mortality rate of mice infected with E. coli compared to ANP. ANP-4 is a novel analog of ANP with high antimicrobial activity and enzyme stability that represent it as a successful agent for infections treatment [126].
2.2.3. Decoralin
Decoralin (Dec-NH2) is a peptide derived from the solitary Eumenine wasp (Oreumenes decoratus) [82] and was synthesized by solid-phase synthesis [127]. Equally important, a natural antimicrobial peptide, Dec-NH2, was isolated from wasp venom, and its synthetic derivatives were manufactured using peptide design. Dec-NH2 exhibits potent activity toward cancer cells at doses of 12.5 μmol/L and specific inhibition of MCF-7 breast cancer cells [127]. In a biological assessment, synthetic Dec-NH2 demonstrated strong broad-spectrum antimicrobial activity, slight mast cell degranulation, and leishmanicidal activity. The peptide displayed low hemolytic function against mouse erythrocytes, EC50 lower 300 µM [82]. A synthetic Dec-NH2 analog with C-terminal amidation demonstrated much more efficient activity against Gram-positive and Gram-negative bacteria and yeast. When isoleucine was substituted by phenylalanine residue at position 6, the peptide increased its resistance to degradation in bovine fetal serum. Besides that, lower hemolytic activity was obtained for [Pro]4-decoralin-NH2 and [Phe]6-Des[Thr]11-decoralin-NH2. The antimicrobial effect was increased in the case of [Phe]9-[Phe]10-Dec-NH2 (MIC = 0.39 vs. native peptide of 0.78 µmol/L) against Micrococcus luteus A 270 [128]. Torres et al. synthesized two leucine-substituted Dec-NH2 analogs; [Leu]8-Dec-NH2, and [Leu]10-Dec-NH2 using SPPS. [Leu]10-Dec-NH2 analog showed similar activity against E. coli, and P. aeruginosa (MIC 1.6 μmol/L), and higher activities against M. luteus and C. albicans. The same helical structure of the [Leu]8-Dec-NH2 analog exhibited evidential low activities against M. luteus, E. coli, Salmonella arizonae, B. subtilis, P. aeruginosa, and C. albicans [11]. The natural sequence of amidated Dec-NH2 and eight synthesized analogs, along with their biological activity toward Plasmodium, were reviewed. The Dec-NH2 template compound did not display antiplasmodial properties; on the other hand, it’s designed analogs showed significant antiplasmodial activity (>95%). The highest antiplasmodial behavior was achieved by mutations made to the N-terminus of Dec [64].
2.2.4. Polybia-MP-I
Polybia-MP-I is one of the 14 amino acid residues (Table 1) of mastoparan peptides [57]. The peptide was derived from the venom of the social wasp P. paulista. It causes moderate mast cell degranulation, demonstrates chemotactic action for polymorphonuclear leukocytes, exhibits active antimicrobial activity, and is non-hemolytic to rat erythrocytes [129]. Polybia-MP-I is cytotoxic to leukemic T lymphocytes and strongly selective to these individual cells [130]. Polybia-MP-I has demonstrated antitumor action against bladder and prostate cancer [12]; however, this antitumor activity drastically decreased with the synthesized analogs (replacement of the amino acids at position 7, 8, or 9 with Pro residue). These substitutions influence the original helical structure and electrostatic equilibrium and increase the degree of peptide hydrophilic behavior (Pro7 and Pro9). Polybia-MP-I exerts pore formation and thus alters the intact cellular structure leading to a cytotoxic and antiproliferative outcome. It can selectively inhibit the proliferation of prostate cancer cell lines (PC-3), human bladder cancer cell lines (Biu87 and EJ), and human umbilical vein endothelial cell lines (HUVEC) at IC50 of 20.8, 25.32, and 36.97 μM, respectively [12].The peptides polybia-MP-I (IDWKKLLDAAKQIL-NH2) and Asn-2-polybia-MP-I (INWKKLLDAAKQIL-NH2) were manually synthesized in the solid phase. Polybia-MP-I and N-2-polybia-MP-I exhibited a significant reduction in the pain threshold at 30, and 50 μg/50 μL as detected at 2, and 8 h after peptide injection into the hind paw of mice [131][85]. The polybia-MP-I analogs (proline replacement) showed reduced antibacterial activity compared to the parent. MIC values of polybia-MP-I were 4, 16, 16, and 32 μM for B. subtilis, E. coli, S. epidermidis, and S. aureus, respectively. The MIC value was 8 µM for C. glabrata versus 16 µM against C. albicans. The fungicidal activity of polybia-MP-I versus both Candida glabrata and C. albicans was measured as an minimum fungicidal concentration (MFC) of 32 μM [132][133].
2.2.5. Polybia-CP
Polybia-CP has been isolated from P. paulista, and gradually synthesized by SPPS, and its effects on bacteria have been recorded [14]. Polybia-CP’s MICs for E. coli, P. aeruginosa, S. aureus, S. epidemic, and B. subtilis were 16, 128, 4, 16, and 4 μM, respectively, while the MBCs were 8, 16, 128, and 16 μM, for B. subtilis, S. aureus, and E. coli, respectively. The peptide was stable at different temperature ranges of 20–100 °C, and the temperature changes did not affect the MIC values [134]. Polybia-CP showed antimicrobial activities with MIC values of 4–64 μM in eight fungal strains, where the highest activity was noted against C. tropicalis at a MIC of 4 μM [13].Synthetic Polybia-CP has potent antitumor activity against Biu87 and PC-3 cell lines. Cell proliferation inhibition was observed at IC50 of 17.84 and 11.01 μM, respectively. The cytotoxicity of polybia-CP was explained by the disruption of cell membrane integrity [14].
2.2.6. Polydim-I
Polydim-I is a peptide derived from the venom of a neotropical wasp (Polybia dimorpha). The peptide contains 22 amino acid residues and is known for its amphipathic properties due to the presence of hydrophobic amino acid residues (e.g., methionine, leucine, valine, and proline) [15].Polydim-I was synthesized with high quality (>99%), and the relevant peptide sequence was tested and validated by MS analysis. The synthetic peptide is active against Mycobacterium abscessus subsp. massiliense infections as described in in vitro and in vivo studies. In vitro study, the inhibition was 55 to 68% of M. abscessus subsp massiliense strains growth at a concentration of 15.2 μg/mL in which the cell shape was expressively damaged. The peptide prevents bacterial growth through the inhibition of protein synthesis, did not result in visible morphological changes. Polydim-I treatment at 2 mg/kg/mLW showed significant reduction of the bacterial load in in the lungs, spleen, and liver [15], and the antimicrobial properties against S. aureus, E. coli, Enterococcus faecalis, Acinetobacter calcoaceticus-baumannii were displayed with MIC50 values of 4.1, 50.7, 73.2, and 84.0 μg/mL, respectively [46].
2.2.7. Protonectarina-MP and Agelaia-MP
Protonectarina-MP was isolated from Protonectarina syleirae venom and is a member of the 14 amino residue class of mastoparans [135]. The peptide protonectarina-MP-NH2 (INWKALLDAAKKVL-NH2) and its analogue protonectarina-MP-OH (INWKALLDAAKKVL-OH) were produced by step-by-step manual SPPS. Protonectarin-MP-NH2 is a powerful mast cell degranulating peptide with slightly higher degranulating activity (ED50 = 8 ×10−5 M) than the standard peptide (ED50 = 20 × 10−5 M). Protonectarina-MP-OH, and even at high concentrations, has reduced degranulation activity. Protonectarina-MP-NH2 has effective antimicrobial activity against both Gram-positive and Gram-negative bacteria, while protonectarina-MP-OH has much poorer antimicrobial activity [17].Agelaia-MP is a mastoparan peptide that contains 14 residues (INWLKLGKAIIDAL-NH2) and is isolated from the venom of the social wasp Agelaia pallipes. It was characterized by its poor antimicrobial action and the lack of chemotaxis toward mast cells [135]. Using the Fmoc strategy, agelaia-MP has been chemically and manually synthesized. At a concentration of 10 μM, the peptide enhances the insulin secretion from the mice pancreatic islets using different glucose doses (2.8, 11.1, and 22.2 mM). In mouse models, agelaia-MP-I has a dose-dependent anti-nociceptive effect. For example, nociception significantly declined when the highest dosage (6.4 nmol) was administered, while the maximal effect was observed 4 h after the peptide injection [16].Protonectin is derived from the venom of the neotropical social wasp (Agelaia pallipes), with a sequence of ILGTILGLLKGL-NH2. The peptide exhibits poor hemolysis to rat erythrocytes [135]. Protonectin has some mast cell degranulating activity and potent antimicrobial action with E. coli, P. aeruginosa, B. subtilis, and S. aureus at MICs of 25, 1.7, 3.1, and 12.5 µg/mL, respectively [135].Protonectin and its three analogues were synthesized through a stepwise solid-phase assay by replacing L-proline. Proline is a unique amino acid among the 20 protein-forming amino acids because its amine nitrogen is linked to two groups of alkyls, making it a secondary amine. The insertion of proline inside the peptide considerably changes the secondary structure. Protonectin has demonstrated potent antibacterial action toward multidrug-resistant S. aureus, and E. coli at MICs of 8, and 32 μM, respectively. MBC values were 8, 8, 16, and 64 µM for B. subtilis, S. epidermidis, S. aureus, and E. coli, respectively, indicating potent bactericidal effect [136].
2.2.8. Philanthotoxin-433 (PhTX-433)
Philanthotoxin-433 (PhTX-433) is a polyamine-based toxin isolated from Egyptian digger wasp (Philanthus triangulum) venom. The venom induces prey paralysis by suppressing nicotinic acetylcholine receptors (nAChRs) and ionotropic glutamate receptors (iGluRs). PhTX-433 is an important lead compound in neuropharmacology [137][138]. The action of 17 analogs of PhTX-343 against ganglionic (α3β4) and brain (α4β2) nAChRs has been expressed in Xenopus oocytes. IC50 values for PhTX-343 inhibition of α3β4 and α4β2 receptors were 7.7 and 80 nM, respectively [139]. Their total synthesis achieved good yield (77%) and purity (80%) using a mild borane reduction protocol of polyamide precursors to access the polyamine chains. The synthesis of PhTX-433 isomers proved this strategy’s potential for the generation of branched analogs [137].
References
- Konno, K.; Kazuma, K.; Nihei, K. Peptide toxins in solitary wasp venoms. Toxins 2016, 8, 114.
- Lin, Z.; Cheng, Y.; Wang, R.J.; Du, J.; Volovych, O.; Li, J.C.; Hu, Y.; Lu, Z.Y.; Lu, Z.; Zou, Z. A metalloprotease homolog venom protein from a parasitoid wasp suppresses the toll pathway in host hemocytes. Front. Immunol. 2018, 9, 2301–2315.
- Gong, J.; Yuan, H.; Gao, Z.; Hu, F. Wasp venom and acute kidney injury: The mechanisms and therapeutic role of renal replacement therapy. Toxicon 2019, 163, 1–7.
- Kim, Y.; Son, M.; Noh, E.Y.; Kim, S.; Kim, C.; Yeo, J.H.; Park, C.; Lee, K.W.; Bang, W.Y. MP-V1 from the venom of social wasp Vespula vulgaris is a de novo type of mastoparan that displays superior antimicrobial activities. Molecules 2016, 21, 512.
- Chen, W.; Yang, X.; Yang, X.; Zhai, L.; Lu, Z.; Liu, J.; Yu, H. Antimicrobial peptides from the venoms of Vespa bicolor Fabricius. Peptides 2008, 29, 1887–1892.
- Wu, R.; Li, D.; Tang, Q.; Wang, W.; Xie, G.; Dou, P. A novel peptide from Vespa ducalis induces apoptosis in osteosarcoma cells by activating the p38 mapk and jnk signaling pathways. Biol. Pharm. Bull. 2018, 41, 458–464.
- Danneels, E.L.; Gerlo, S.; Heyninck, K.; Van Craenenbroeck, K.; De Bosscher, K.; Haegeman, G.; De Graaf, D.C. How the venom from the ectoparasitoid wasp Nasonia vitripennis exhibits anti-inflammatory properties on mammalian cell lines. PLoS ONE 2014, 9, e96825.
- Torres, M.D.T.; Silva, A.F.; Andrade, G.P.; Pedron, C.N.; Cerchiaro, G.; Ribeiro, A.O.; Oliveira, V.X.; de la Fuente-Nunez, C. The wasp venom antimicrobial peptide polybia-CP and its synthetic derivatives display antiplasmodial and anticancer properties. Bioeng. Transl. Med. 2020, 5, e10167.
- Vila-Farrés, X.; López-Rojas, R.; Pachón-Ibáñez, M.E.; Teixidó, M.; Pachón, J.; Vila, J.; Giralt, E. Sequence-activity relationship, and mechanism of action of mastoparan analogues against extended-drug resistant Acinetobacter baumannii. Eur. J. Med. Chem. 2015, 101, 34–40.
- Chionis, K.; Krikorian, D.; Koukkou, A.; Sakarellos-daitsiotis, M.; Panou-pomonis, E. Synthesis and biological activity of lipophilic analogs of the cationic antimicrobial active peptide anoplin. Pept. Sci. 2016, 22, 731–736.
- Torres, M.D.T.; Pedron, C.N.; da Silva Lima, J.A.; da Silva, P.I.; da Silva, F.D.; Oliveira, V.X. Antimicrobial activity of leucine-substituted decoralin analogs with lower hemolytic activity. J. Pept. Sci. 2017, 23, 818–823.
- Wang, K.; Zhang, B.; Zhang, W.; Yan, J.; Li, J.; Wang, R. Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides 2008, 29, 963–968.
- Wang, K.; Jia, F.; Dang, W.; Zhao, Y.; Zhu, R. Antifungal effect and action mechanism of antimicrobial peptide polybia-CP. Pept. Sci. 2016, 22, 28–35.
- Wang, K.; Yan, J.; Liu, X.; Zhang, J.; Chen, R.; Zhang, B.; Dang, W.; Zhang, W.; Kai, M.; Song, J.; et al. Novel cytotoxity exhibition mode of polybia-CP, a novel antimicrobial peptide from the venom of the social wasp Polybia paulista. Toxicology 2011, 288, 27–33.
- Coutinho, R.; Trentini, M.M.; De Castro, J.; Simon, K.S.; Bocca, A.L.; Silva, L.P.; Mortari, R.; Kipnis, A.; Junqueira-Kipnis, A.P. Antimycobacterial activity of a new peptide polydim-I isolated from Neotropical social wasp Polybia dimorpha. PLoS ONE 2016, 11, e0149729.
- Gonçalves, J.; Rangel, M.; Biolchi, A.; Alves, E.; Moreira, K.; Silva, L.; Mortari, M. Antinociceptive properties of the mastoparan peptide Agelaia-MPI isolated from social wasps. Toxicon 2016, 120, 15–21.
- Da Silva, A.V.R.; De Souza, B.M.; Dos Santos Cabrera, M.P.; Dias, N.B.; Gomes, P.C.; Neto, J.R.; Stabeli, R.G.; Palma, M.S. The effects of the C-terminal amidation of mastoparans on their biological actions and interactions with membrane-mimetic systems. Biochim. Biophys. Acta—Biomembr. 2014, 1838, 2357–2368.
- Hilchie, A.L.; Sharon, A.J.; Haney, E.F.; Hoskin, D.W.; Bally, M.B.; Franco, O.L.; Corcoran, J.A.; Hancock, R.E.W. Mastoparan is a membranolytic anti-cancer peptide that works synergistically with gemcitabine in a mouse model of mammary carcinoma. Biochim. Biophys. Acta—Biomembr. 2016, 1858, 3195–3204.
- Jalaei, J.; Layeghi-Ghalehsoukhteh, S.; Hosseini, A.; Fazeli, M. Antibacterial effects of gold nanoparticles functionalized with the extracted peptide from Vespa orientalis wasp venom. J. Pept. Sci. 2018, 24, e3124–e3133.
- Kalansuriya, P.; Quezada, M.; Espósito, B.P.; Capon, R.J. Talarazines A–E: Noncytotoxic iron (III) chelators from an Australian mud dauber wasp-associated fungus, talaromyces sp. (CMB-W045). J. Nat. Prod. 2017, 80, 609–615.
- Pepe, D.; Carvalho, V.F.M.; McCall, M.; de Lemos, D.P.; Lopes, L.B. Transportan in nanocarriers improves skin localization and antitumor activity of paclitaxel. Int. J. Nanomed. 2016, 11, 2009–2019.
- Perkins, J.B.; Yates, A.B. Allergy to stinging insects: Diagnosis and management. EMJ Allergy Immunol. 2018, 3, 99–105.
- Pospischil, I.M.; Kagerer, M.; Cozzio, A.; Angelova-Fischer, I.; Guenova, E.; Ballmer-Weber, B.; Hoetzenecker, W. Comparison of the safety profiles of 3 different Hymenoptera venom immunotherapy protocols: A retrospective 2-center study of 143 patients. Int. Arch. Allergy Immunol. 2020, 181, 783–789.
- Sahiner, U.M.; Durham, S.R. Hymenoptera venom allergy: How does venom immunotherapy prevent anaphylaxis from bee and wasp stings? Front. Immunol. 2019, 10, 1959–1966.
- Chen, X.; Zhang, L.; Wu, Y.; Wang, L.; Ma, C.; Xi, X.; Bininda-Emonds, O.R.P.; Shaw, C.; Chen, T.; Zhou, M. Evaluation of the bioactivity of a mastoparan peptide from wasp venom and of its analogues designed through targeted engineering. Int. J. Biol. Sci. 2018, 14, 599–607.
- Danneels, E.L.; Formesyn, E.M.; de Graaf, D.C. Exploring the potential of venom from Nasonia vitripennis as therapeutic agent with high-throughput screening tools. Toxins 2015, 7, 2051–2070.
- Czaikoski, P.G.; Menaldo, D.L.; Marcussi, S.; Baseggio, A.L.; Fuly, A.L.; Paula, R.C.; Quadros, A.U.; Romao, P.R.; Buschini, M.L.; Cunha, F.Q.; et al. Anticoagulant and fibrinogenolytic properties of the venom of Polybia occidentalis social wasp. Blood Coagul. Fibrinolysis 2010, 21, 653–659.
- Hoshina, M.M.; Santos, L.D.; Palma, M.S.; Marin-morales, M.A. Cytotoxic, genotoxic/antigenotoxic and mutagenic/antimutagenic effects of the venom of the wasp Polybia paulista. Toxicon 2013, 72, 64–70.
- Yang, X.; Wang, Y.; Lee, W.H.; Zhang, Y. Antimicrobial peptides from the venom gland of the social wasp Vespa tropica. Toxicon 2013, 74, 151–157.
- Jalaei, J.; Fazeli, M.; Rajaian, H.; Shekarforoush, S.S. In vitro antibacterial effect of wasp (Vespa orientalis) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 22–27.
- Baracchi, D.; Mazza, G.; Turillazzi, S. From individual to collective immunity: The role of the venom as antimicrobial agent in the Stenogastrinae wasp societies. J. Insect Physiol. 2011, 58, 188–193.
- Pålsson-McDermott, E.M.; O’Neill, L.A.J. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020, 30, 300–314.
- Kaushik, D.K.; Thounaojam, M.C.; Mitra, A.; Basu, A. Vespa tropica venom suppresses lipopolysaccharide-mediated secretion of pro-inflammatory cyto-chemokines by abrogating nuclear factor-κ B activation in microglia. Inflamm. Res. 2014, 63, 657–665.
- Saba, E.; Shafeeq, T.; Irfan, M.; Lee, Y.Y.; Kwon, H.W.; Seo, M.G.; Park, S.J.; Lee, K.Y.; Rhee, M.H. Anti-inflammatory activity of crude venom isolated from parasitoid wasp, Bracon hebetor Say. Mediat. Inflamm. 2017, 2017, 1–11.
- Danneels, E.L.; Rivers, D.B.; de Graaf, D.C. Venom proteins of the parasitoid wasp Nasonia vitripennis: Recent discovery of an untapped pharmacopee. Toxins 2010, 2, 494–516.
- De Castro e Silva, J.; Lopes do Couto, L.; de Oliveira Amaral, H.; Maria Medeiros Gomes, F.; Avohay Alves Campos, G.; Paulino Silva, L.; Renata Mortari, M. Neuropolybin: A new antiseizure peptide obtained from wasp venom. Biochem. Pharmacol. 2020, 181, 114119.
- Da Silva, A.M.B.; Silva-Gonçalves, L.C.; Oliveira, F.A.; Arcisio-Miranda, M. Pronecrotic activity of cationic mastoparan peptides in human glioblastoma multiforme cells via membranolytic action. Mol. Neurobiol. 2018, 55, 5490–5504.
- Crandall, Y.M.; Bruch, M.D. Characterization of the structure and dynamics of mastoparan-X during folding in a TFE by CD and NMR spectroscopy. Biopolymers 2007, 89, 197–209.
- Henriksen, J.R.; Etzerodt, T.; Gjetting, T.; Andresen, T.L. Side chain hydrophobicity modulates therapeutic activity and membrane selectivity of antimicrobial peptide mastoparan-X. PLoS ONE 2014, 9, e91007.
- Konno, K.; Hisada, M.; Naoki, H.; Itagaki, Y.; Kawai, N.; Miwa, A.; Yasuhara, T.; Morimoto, Y.; Nakata, Y. Structure and biological activities of eumenine mastoparan-AF (EMP-AF), a new mast cell degranulating peptide in the venom of the solitary wasp (Anterhynchium flavomarginatum micado). Toxicon 2000, 38, 1505–1515.
- De Azevedo, R.A.; Figueiredo, C.R.; Ferreira, A.K.; Matsuo, A.L.; Massaoka, M.H.; Girola, N.; Auada, A.V.; Farias, C.F.; Pasqualoto, K.F.; Rodrigues, C.P.; et al. Mastoparan induces apoptosis in B16F10-Nex2 melanoma cells via the intrinsic mitochondrial pathway and displays antitumor activity in vivo. Peptides 2015, 68, 113–119.
- Vila-Farres, X.; Garcia de la Maria, C.; López-Rojas, R.; Pachón, J.; Giralt, E.; Vila, J. In vitro activity of several antimicrobial peptides against colistin-susceptible and colistin-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 2012, 18, 383–387.
- Ramon, R.; De Souza, B.M.; Havt, A.; Mario, S.; Pires, R.; Lins, E.; Albuquerque, D.; Nogueira, V.; Maria, A.; Martins, C. Trypanocidal activity of mastoparan from Polybia paulista wasp venom by interaction with TcGAPDH. Toxicon 2017, 137, 168–172.
- Park, N.G.; Yamato, Y.; Lee, S.; Sugihara, G. Interaction of mastoparan-B from venom of a hornet in taiwan with phospholipid bilayers and its antimicrobial activity. Biopolymers 1995, 36, 793–801.
- Xu, X.; Li, J.; Lu, Q.; Yang, H.; Zhang, Y.; Lai, R. Two families of antimicrobial peptides from wasp (Vespa magnifica) venom. Toxicon 2006, 47, 249–253.
- Rangel, M.; Dos Santos Castro, F.F.; Mota-Lima, L.D.; Clissa, P.B.; Martins, D.B.; Dos Santos Cabrera, M.P.; Mortari, M.R. Polydim-I antimicrobial activity against MDR bacteria and its model membrane interaction. PLoS ONE 2017, 12, e0178785.
- Hisada, M.; Konno, K.; Itagaki, Y.; Naoki, H.; Nakajima, T. Sequencing wasp venom peptides by endopeptidase digestion and nested collision-induced dissociation/post-source decay methods. Rapid Commun. Mass Spectrom. 2002, 16, 1040–1048.
- Konno, K.; Hisada, M.; Fontana, R.; Lorenzi, C.C.B.; Naoki, H.; Itagaki, Y.; Miwa, A.; Kawai, N.; Nakata, Y.; Yasuhara, T.; et al. Anoplin, a novel antimicrobial peptide from the venom of the solitary wasp Anoplius samariensis. Biochim. Biophys. Acta—Protein Struct. Mol. Enzymol. 2001, 1550, 70–80.
- Parkinson, N.; Smith, I.; Audsley, N.; Edwards, J. Purification of pimplin, a paralytic heterodimeric polypeptide from venom of the parasitoid wasp Pimpla hypochondriaca, and cloning of the cDNA encoding one of the subunits. Insect Biochem. Mol. Biol. 2002, 32, 1769–1773.
- Hisada, M.; Konno, K.; Itagaki, Y.; Naoki, H.; Nakajima, T. Advantages of using nested collision induced dissociation/post-source decay with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: Sequencing of novel peptides from wasp venom. Rapid Commun. Mass Spectrom. 2000, 14, 1828–1834.
- Pizzo, A.B.; Beleboni, R.O.; Gomes Carolino, R.O.; de Oliveira, L.; Miranda, A.; Coutinho-Netto, J.; Fontana, A.C.K.; dos Santos, W.F. Isolation and chemical characterization of agelaiatoxin8 (AvTx8) from Agelaia vicina wasp venom and its biological effects on GABA neurotransmission. J. Biochem. Mol. Toxicol. 2017, 31, e21941.
- Baptista-Saidemberg, N.B.; Saidemberg, D.M.; Palma, M.S. Profiling the peptidome of the venom from the social wasp Agelaia pallipes pallipes. J. Proteom. 2011, 74, 2123–2137.
- Konno, K.; Hisada, M.; Itagaki, Y.; Naoki, H.; Kawai, N.; Miwa, A.; Yasuhara, T.; Takayama, H. Isolation and structure of pompilidotoxins, novel peptide neurotoxins in solitary wasp venoms. Biochem. Biophys. Res. Commun. 1998, 250, 612–616.
- Baptista-Saidemberg, N.B.; Saidemberg, D.M.; de Souza, B.M.; César-Tognoli, L.M.; Ferreira, V.M.; Mendes, M.A.; dos Santos Cabrera, M.P.; Neto, J.R.; Palma, M.S. Protonectin (1–6): A novel chemotactic peptide from the venom of the social wasp Agelaia pallipes pallipes. Toxicon 2010, 56, 880–889.
- Murata, K.; Shinada, T.; Ohfune, Y.; Hisada, M.; Yasuda, A.; Naoki, H.; Nakajima, T. Novel mastoparan and protonectin analogs isolated from a solitary wasp, Orancistrocerus drewseni drewseni. Amino Acids 2009, 37, 389–394.
- Gomes, P.C.; de Souza, B.M.; Dias, N.B.; Brigatte, P.; Mourelle, D.; Arcuri, H.A.; dos Santos Cabrera, M.P.; Stabeli, R.G.; Neto, J.R.; Palma, M.S. Structure–function relationships of the peptide paulistine: A novel toxin from the venom of the social wasp Polybia paulista. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2014, 1840, 170–183.
- Ribeiro, S.P.; Mendes, M.A.; Dos Santos, L.D.; De Souza, B.M.; Marques, M.R.; De Azevedo, W.F.; Palma, M.S. Structural and functional characterization of N-terminally blocked peptides isolated from the venom of the social wasp Polybia paulista. Peptides 2004, 25, 2069–2078.
- Argiolas, A.; Pisano, J.J. Isolation and characterization of two new peptides, mastoparan C and crabrolin, from the venom of the European hornet, Vespa crabro. J. Biol. Chem. 1984, 259, 10106–10111.
- Aschi, M.; Bozzi, A.; Luzi, C.; Bouchemal, N.; Sette, M. Crabrolin, a natural antimicrobial peptide: Structural properties. J. Pept. Sci. 2017, 23, 693–700.
- Konno, K.; Hisada, M.; Naoki, H.; Itagaki, Y.; Fontana, R.; Rangel, M.; Oliveira, J.S.; dos Santos Cabrera, M.P.; Neto, J.R.; Hide, I.; et al. Eumenitin, a novel antimicrobial peptide from the venom of the solitary eumenine wasp Eumenes rubronotatus. Peptides 2006, 27, 2624–2631.
- Rangel, M.; dos Santos Cabrera, M.P.; Kazuma, K.; Ando, K.; Wang, X.; Kato, M.; Nihei, K.; Hirata, I.Y.; Cross, T.J.; Garcia, A.N.; et al. Chemical and biological characterization of four new linear cationic α-helical peptides from the venoms of two solitary eumenine wasps. Toxicon 2011, 57, 1081–1092.
- Konno, K.; Kazuma, K.; Rangel, M.; Stolarz-de-Oliveira, J.; Fontana, R.; Kawano, M.; Fuchino, H.; Hide, I.; Yasuhara, T.; Nakata, Y. New mastoparan peptides in the venom of the solitary eumenine wasp Eumenes micado. Toxins 2019, 11, 155.
- Dias, N.B.; De Souza, B.M.; Gomes, P.C.; Brigatte, P.; Palma, M.S. Peptidome profiling of venom from the social wasp Polybia paulista. Toxicon 2015, 107, 290–303.
- Torres, M.D.T.; Silva, A.F.; Pedron, C.N.; Capurro, M.L. Peptide design enables reengineering of an inactive wasp venom peptide into synthetic antiplasmodial agents. ChemistrySelect 2018, 3, 5859–5863.
- Klochkov, S.G.; Pikhtelev, A.R.; Kozlovskii, V.I. A new peptide from venom of the East-European hornet Vespa orientalis. Mass spectrometric de novo sequence. Chem. Nat. Compd. 2008, 44, 63–66.
- Dos Anjos, L.C.; Gomes, F.M.M.; Do Couto, L.L.; Mourão, C.A.; Moreira, K.G.; Silva, L.P.; Mortari, M.R. Anxiolytic activity and evaluation of potentially adverse effects of a bradykinin-related peptide isolated from a social wasp venom. Life Sci. 2016, 149, 153–159.
- Andrew, K.; Kim, K.; Kim, A.; Han, Y.; Young, W. Selective anti-tumor activities of venom peptides from the lesser paper wasp Parapolybia varia. J. Asia. Pac. Entomol. 2016, 19, 821–828.
- Yu, H.; Yang, H.; Ma, D.; Lv, Y.; Liu, T.; Zhang, K.; Lai, R.; Liu, J. Vespid chemotactic peptide precursor from the wasp, Vespa magnifica (Smith). Toxicon 2007, 50, 377–382.
- Zhou, Z. The first report of kininogen from invertebrates. Biochem. Biophys. Res. Commun. 2006, 347, 1099–1102.
- Baek, J.H.; Lee, S.H. Isolation and molecular cloning of venom peptides from Orancistrocerus drewseni (Hymenoptera: Eumenidae). Toxicon 2010, 55, 711–718.
- Baek, J.H.; Ji, Y.; Shin, J.S.; Lee, S.; Lee, S.H. Venom peptides from solitary hunting wasps induce feeding disorder in lepidopteran larvae. Peptides 2011, 32, 568–572.
- Ye, J.; Zhao, H.; Wang, H.; Bian, J.; Zheng, R. A defensin antimicrobial peptide from the venoms of Nasonia vitripennis. Toxicon 2010, 56, 101–106.
- Lopes, K.S.; Campos, G.A.A.; Camargo, L.C.; de Souza, A.C.B.; Ibituruna, B.V.; Magalhães, A.C.M.; da Rocha, L.F.; Garcia, A.B.; Rodrigues, M.C.; Ribeiro, D.M.; et al. Characterization of two peptides isolated from the venom of social wasp Chartergellus communis (Hymenoptera: Vespidae): Influence of multiple alanine residues and C-terminal amidation on biological effects. Peptides 2017, 95, 84–93.
- Montana, V.; Sontheimer, H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J. Neurosci. 2011, 31, 4858–4867.
- Yasuhara, T.; Mantel, P.; Nakajima, T.; Piek, T. Two kinins isolated from an extract of the venom reservoirs of the solitary wasp Megascolia flavifrons. Toxicon 1987, 25, 527–535.
- Mortari, M.R.; Cunha, A.O.S.; Carolino, R.O.G.; Coutinho-Netto, J.; Tomaz, J.C.; Lopes, N.P.; Coimbra, N.C.; Dos Santos, W.F. Inhibition of acute nociceptive responses in rats after i.c.v. injection of Thr 6-bradykinin, isolated from the venom of the social wasp, Polybia occidentalis. Br. J. Pharmacol. 2007, 151, 860–869.
- Picolo, G.; Hisada, M.; Moura, A.B.; Machado, M.F.M.; Sciani, J.M.; Conceição, I.M.; Melo, R.L.; Oliveira, V.; Lima-Landman, M.T.R.; Cury, Y.; et al. Bradykinin-related peptides in the venom of the solitary wasp Cyphononyx fulvognathus. Biochem. Pharmacol. 2010, 79, 478–486.
- Silva, J.C.; Neto, L.M.; Neves, R.C.; Gonçalves, J.C.; Trentini, M.M.; Mucury-filho, R.; Smidt, K.S.; Fensterseifer, I.C.; Silva, O.N.; Lima, L.D.; et al. Evaluation of the antimicrobial activity of the mastoparan Polybia-MPII isolated from venom of the social wasp Pseudopolybia vespiceps testacea (Vespidae, Hymenoptera). Int. J. Antimicrob. Agents 2017, 49, 167–175.
- Tuichibaev, M.U.; Tashmukhamedov, B.A. Lysophospholipase activity of orientotoxin from the venom of the hornet Vespa orientalis. Chem. Nat. Compd. 1996, 20, 765–766.
- Tuĭchibaev, M.U.; Tashmukhamedov, B.A.; Gotgil’f, I.G.; Mazannik, L.G. Orientotoxin--a new presynaptic neurotoxin from the venom of the giant hornet Vespa orientalis. Bioorg. Khim. 1984, 10, 318–322.
- L’vov, V.M.; Kolmakova, A.A.; Akhunov, A.A.; Mukhamedov, I.F. Vasoactive peptides from venom of the hornet Vespa orientalis physiochemical and functional characterization. Chem. Nat. Compd. 1988, 24, 218–221.
- Konno, K.; Rangel, M.; Oliveira, J.S.; dos Santos Cabrera, M.P.; Fontana, R.; Hirata, I.Y.; Hide, I.; Nakata, Y.; Mori, K.; Kawano, M.; et al. Decoralin, a novel linear cationic α-helical peptide from the venom of the solitary eumenine wasp Oreumenes decoratus. Peptides 2007, 28, 2320–2327.
- Moore, E.L.; Arvidson, R.; Banks, C.; Urenda, J.P.; Duong, E.; Mohammed, H.; Adams, M.E. Ampulexins: A new family of peptides in venom of the emerald jewel wasp, Ampulex compressa. Biochemistry 2018, 57, 1907–1916.
- Turillazzi, S.; Mastrobuoni, G.; Dani, F.R.; Moneti, G.; Pieraccini, G.; La Marca, G.; Bartolucci, G.; Perito, B.; Lambardi, D.; Cavallini, V.; et al. Dominulin A and B: Two new antibacterial peptides identified on the cuticle and in the venom of the social paper wasp Polistes dominulus using MALDI-TOF, MALDI-TOF/TOF, and ESI-ion trap. J. Am. Soc. Mass Spectrom. 2006, 17, 376–383.
- De Souza, B.M.; Dos Santos Cabrera, M.P.; Neto, J.R.; Palma, M.S. Investigating the effect of different positioning of lysine residues along the peptide chain of mastoparans for their secondary structures and biological activities. Amino Acids 2011, 40, 77–90.
- Hirai, Y.; Ueno, Y.; Yasuhara, T.; Yoshida, H.; Nakajima, T. A new mast cell degranulating peptide, polistes mastoparan, in the venom of Polistes jadwigae. Biomed. Res. 1980, 1, 185–187.
- Mendes, M.A.; Palma, M.S. Two new bradykinin-related peptides from the venom of the social wasp Protopolybia exigua (Saussure). Peptides 2006, 27, 2632–2639.
- Ombati, R.; Wang, Y.; Du, C.; Lu, X.; Li, B.; Nyachieo, A.; Li, Y.; Yang, S.; Lai, R. A membrane disrupting toxin from wasp venom underlies the molecular mechanism of tissue damage. Toxicon 2018, 148, 56–63.
- Yshii, L.M.; Souza, G.H.M.F.; Camargo, E.A.; Eberlin, M.N.; Ribela, M.T.C.P.; Muscará, M.N.; Hyslop, S.; Costa, S.K.P. Characterization of the mechanisms underlying the inflammatory response to Polistes lanio lanio (paper wasp) venom in mouse dorsal skin. Toxicon 2009, 53, 42–52.
- Zhou, S.T.; Luan, K.; Ni, L.L.; Wang, Y.; Yuan, S.M.; Che, Y.H.; Yang, Z.Z.; Zhang, C.G.; Yang, Z. Bin A strategy for quality control of vespa magnifica (Smith) venom based on HPLC fingerprint analysis and multi-component separation combined with quantitative analysis. Molecules 2019, 24, 2920.
- Hernández, C.; Konno, K.; Salceda, E.; Vega, R.; Zaharenko, A.J.; Soto, E. Sa12b peptide from solitary wasp inhibits ASIC currents in rat dorsal root ganglion neurons. Toxins 2019, 11, 585.
- Freire, D.O.; da Cunha, N.B.; Leite, M.L.; Kostopoulos, A.G.C.; da Silva, S.N.B.; de Souza, A.C.B.; Nolasco, D.O.; Franco, O.L.; Mortari, M.R.; Dias, S.C. Wasp venom peptide, synoeca-MP, from Synoeca surinama shows antimicrobial activity against human and animal pathogenic microorganisms. Pept. Sci. 2020, 112, e24141.
- Yang, H.; Xu, X.; Ma, D.; Zhang, K.; Lai, R. A phospholipase A1 platelet activator from the wasp venom of Vespa magnifica (Smith). Toxicon 2008, 51, 289–296.
- Santos, L.D.; Santos, K.S.; de Souza, B.M.; Arcuri, H.A.; Cunha-Neto, E.; Castro, F.M.; Kalil, J.E.; Palma, M.S. Purification, sequencing and structural characterization of the phospholipase A1 from the venom of the social wasp Polybia paulista (Hymenoptera, Vespidae). Toxicon 2007, 50, 923–937.
- Aoki, J.; Inoue, A.; Makide, K.; Saiki, N.; Arai, H. Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie 2007, 89, 197–204.
- Liu, N.-Y.; Xu, Z.-W.; Yan, W.; Ren, X.-M.; Zhang, Z.-Q.; Zhu, J.-Y. Venomics reveals novel ion transport peptide-likes (ITPLs) from the parasitoid wasp Tetrastichus brontispae. Toxicon 2018, 141, 88–93.
- De Oliveira, M.R.; Palma, M.S. Polybitoxins: A group of phospholipases A2 from the venom of the neotropical social wasp paulistinha (Polybia paulista). Toxicon 1998, 36, 189–199.
- Mueller, U.; Elliott, W.; Reisman, R.; Ishay, J.; Walsh, S.; Steger, R.; Wypych, J.; Arbesman, C. Comparison of biochemical and immunologic properties of venoms from four hornet species. J. Allergy Clin. Immunol. 1981, 67, 290–298.
- Allalouf, D.; Ber, A.; Ishay, J. Hyaluronidase activity of extracts of venom sacs of a number of vespinae (Hymenoptera). Comp. Biochem. Physiol.—Part B Biochem. 1972, 43, 119–123.
- Rungsa, P.; Incamnoi, P.; Sukprasert, S.; Uawonggul, N.; Klaynongsruang, S.; Daduang, J.; Patramanon, R.; Roytrakul, S.; Daduang, S. Cloning, structural modelling and characterization of VesT2s, a wasp venom hyaluronidase (HAase) from Vespa tropica. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 1–11.
- Bernheimer, A.W.; Avigad, L.S.; Schmidt, J.O.; Ishay, J.S. Proteins in venoms of two wasps, Polistes comanchus navajoe and Vespa orientalis. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1982, 71, 203–207.
- Arcuri, H.A.; Kalil, J.E.; Palma, M.S. Using proteomic strategies for sequencing and post-translational modifications assignment of antigen-5, a major allergen from the venom of the social wasp Polybia paulista. J. Proteome Res. 2013, 13, 855–865.
- Yamamoto, T.; Arimoto, H.; Kinumi, T.; Oba, Y.; Uemura, D. Identification of proteins from venom of the paralytic spider wasp, Cyphononyx dorsalis. Insect Biochem. Mol. Biol. 2007, 37, 278–286.
- Wu, M.; Ye, G.; Zhu, J.; Chen, X.; Hu, C. Isolation and characterization of an immunosuppressive protein from venom of the pupa-specific endoparasitoid Pteromalus puparum. J. Invertebr. Pathol. 2008, 99, 186–191.
- Asgari, S.; Zareie, R.; Zhang, G.; Schmidt, O. Isolation and characterization of a novel venom protein from an endoparasitoid, Cotesia rubecula (Hym: Braconidae). Arch. Insect Biochem. Physiol. 2003, 53, 92–100.
- Han, J.; You, D.; Xu, X.; Han, W.; Lu, Y.; Lai, R.; Meng, Q. An anticoagulant serine protease from the wasp venom of Vespa magnifica. Toxicon 2008, 51, 914–922.
- Thiéry, D.; Bonnard, O.; Riquier, L.; De Revel, G.; Monceau, K. An alarm pheromone in the venom gland of Vespa velutina: Evidence revisited from the European invasive population. Entomol. Gen. 2018, 38, 145–156.
- Elmquist, D.C.; Landolt, P.J.; Cooper, W.R.; Reed, H.; Foutz, J.; Clepper, T.; Kacprzyk, B.; Teig, D.; Zack, R.S. The venom compound N-(3-methylbutyl) acetamide attracts several Polistes (Fuscopolistes) Species (Hymenoptera: Vespidae). J. Econ. Entomol. 2020, 113, 1073–1079.
- Dani, F.R.; Jeanne, R.L.; Clarke, S.R.; Jones, G.R.; Morgan, E.D.; Francke, W.; Turillazzi, S. Chemical characterization of the alarm pheromone in the venom of Polybia occidentalis and of volatiles from the venom of P. sericea. Physiol. Entomol. 2000, 25, 363–369.
- Mizuno, K.; Nakahata, N.; Ohizumi, Y. Characterization of mastoparan-induced histamine release from RBL-2H3 cells. Toxicon 1998, 36, 447–456.
- Yamada, Y.; Shinohara, Y.; Kakudo, T.; Chaki, S.; Futaki, S.; Kamiya, H.; Harashima, H. Mitochondrial delivery of mastoparan with transferrin liposomes equipped with a pH-sensitive fusogenic peptide for selective cancer therapy. Int. J. Pharm. 2005, 303, 1–7.
- Nakahata, N.; Imata, K.; Okawa, T.; Watanabe, Y.; Ishimoto, H.; Ono, T.; Ohizumi, Y.; Nakanishi, H. Mastoparan elicits prostaglandin E 2 generation and inhibits inositol phosphate accumulation via different mechanisms in rabbit astrocytes Norimichi. Biochim. Biophys. Acta (BBA)—Molecular Cell Res. 1996, 1310, 60–66.
- Irazazabal, L.N.; Porto, W.F.; Ribeiro, S.M.; Casale, S.; Humblot, V.; Ladram, A.; Franco, O.L. Selective amino acid substitution reduces cytotoxicity of the antimicrobial peptide mastoparan. Biochim. Biophys. Acta—Biomembr. 2016, 1858, 2699–2708.
- De Souza, B.M.; Cabrera, M.P.D.S.; Gomes, P.C.; Dias, N.B.; Stabeli, R.G.; Leite, N.B.; Neto, J.R.; Palma, M.S. Structure-activity relationship of mastoparan analogs: Effects of the number and positioning of Lys residues on secondary structure, interaction with membrane-mimetic systems and biological activity. Peptides 2015, 72, 164–174.
- Galeane, M.C.; Gomes, P.C.; De L Singulani, J.; De Souza, B.M.; Palma, M.S.; Mendes-Giannini, M.J.S.; Almeida, A.M.F. Study of mastoparan analog peptides against Candida albicans and safety in zebrafish embryos (Danio rerio). Future Microbiol. 2019, 14, 1087–1097.
- Ha, Y.J.; Kim, S.W.; Lee, C.W.; Bae, C.H.; Yeo, J.H.; Kim, I.S.; Gal, S.W.; Hur, J.; Jung, H.K.; Kim, M.J.; et al. Anti-Salmonella activity modulation of mastoparan V1—A wasp venom toxin—Using protease inhibitors, and its efficient production via an Escherichia coli secretion system. Toxins 2017, 9, 321.
- Ho, C.L.; Hwang, L.L. Structure and biological activities of a new mastoparan isolated from the venom of the hornet Vespa basalis. Biochem. J. 1991, 274, 453–456.
- Ho, C.L.; Hwang, L.L.; Lin, Y.L.; Chen, C.T.; Yu, H.M.; Wang, K.T. Cardiovascular effects of mastoparan B and its structural requirements. Eur. J. Pharmacol. 1994, 259, 259–264.
- Hirai, Y.; Yasuhara, T.; Yoshida, H.; Nakajima, T. A new mast cell degranulating peptide, mastoparan-M, in the venom of the hornet Vespa mandarinia. Biomed. Res. 1981, 2, 447–449.
- Wu, T.M.; Chou, T.C.; Ding, Y.A.; Li, M.L. Stimulation of TNF-α, IL-1β and nitrite release from mouse cultured spleen cells and lavaged peritoneal cells by mastoparan M. Immunol. Cell Biol. 1999, 77, 476–482.
- Li, M.; Liao, R.; Qiu, J.; Wang, Z.; Wu, T. Antimicrobial activity of synthetic all- D mastoparan M. Int. J. Antimicrob. Agents 2000, 13, 203–208.
- Zhu, L.; Fu, C.; Zhang, S.; Chen, W.; Jin, Y. Novel cytotoxic exhibition mode of antimicrobial peptide anoplin in MEL cells, the cell line of murine Friend leukemia virus-induced leukemic cells. Pept. Sci. 2013, 19, 566–574.
- Ifrah, D.; Doisy, X.; Ryge, T.S.; Hansen, P.R. Structure-activity relationship study of anoplin. J. Pept. Sci. 2005, 11, 113–121.
- Perez, M.; Santos, D.O.S.; Arcisio-miranda, M.; Broggio, T.; Ruggiero, R.; Ao, J.O. Study of the mechanism of action of anoplin, a helical antimicrobial decapeptide with ion channel-like activity, and the role of the amidated C -terminus. J. Pept. Sci. 2008, 661–669.
- Won, A.; Khan, M.; Gustin, S.; Akpawu, A.; Seebun, D.; Avis, T.J.; Leung, B.O.; Hitchcock, A.P.; Ianoul, A. Investigating the effects of L- to D-amino acid substitution and deamidation on the activity and membrane interactions of antimicrobial peptide anoplin. Biochim. Biophys. Acta—Biomembr. 2011, 1808, 1592–1600.
- Wang, Y.; Chen, J.; Zheng, X.; Yang, X.; Ma, P.; Cai, Y.; Zhang, B.; Chen, Y. Design of novel analogues of short antimicrobial peptide anoplin with improved antimicrobial activity. J. Pept. Sci. 2014, 20, 945–951.
- Torres, M.D.T.; Andrade, G.P.; Sato, R.H.; Pedron, C.N.; Manieri, T.M.; Cerchiaro, G.; Ribeiro, A.O.; de la Fuente-Nunez, C.; Oliveira, V.X. Natural and redesigned wasp venom peptides with selective antitumoral activity. Beilstein J. Org. Chem. 2018, 14, 1693–1703.
- Torres, M.D.T.; Pedron, C.N.; Araújo, I.; Silva, P.I.; Silva, F.D.; Oliveira, V.X. Decoralin analogs with increased resistance to degradation and lower hemolytic activity. ChemistrySelect 2017, 2, 18–23.
- Luong, H.X.; Kim, D.H.; Lee, B.J.; Kim, Y.W. Antimicrobial activity and stability of stapled helices of polybia-MP1. Arch. Pharm. Res. 2017, 40, 1414–1419.
- Dos Santos Cabrera, M.P.; Arcisio-Miranda, M.; Gorjão, R.; Leite, N.B.; De Souza, B.M.; Curi, R.; Procopio, J.; Ruggiero Neto, J.; Palma, M.S. Influence of the bilayer composition on the binding and membrane disrupting effect of polybia-MP1, an antimicrobial mastoparan peptide with leukemic T-lymphocyte cell selectivity. Biochemistry 2012, 51, 4898–4908.
- Brigatte, P.; Cury, Y.; De Souza, B.M.; Baptista-Saidemberg, N.B.; Saidemberg, D.M.; Gutierrez, V.P.; Palma, M.S. Hyperalgesic and edematogenic effects of peptides isolated from the venoms of honeybee (Apis mellifera) and neotropical social wasps (Polybia paulista and Protonectarina sylveirae). Amino Acids 2011, 40, 101–111.
- Wang, K.; Yan, J.; Dang, W.; Liu, X.; Chen, R.; Zhang, J.; Zhang, B.; Zhang, W.; Kai, M.; Yan, W.; et al. Membrane active antimicrobial activity and molecular dynamics study of a novel cationic antimicrobial peptide polybia-MPI, from the venom of Polybia paulista. Peptides 2013, 39, 80–88.
- Wang, K.; Yan, J.; Dang, W.; Xie, J.; Yan, B.; Yan, W.; Sun, M.; Zhang, B.; Ma, M.; Zhao, Y.; et al. Dual antifungal properties of cationic antimicrobial peptides polybia-MPI: Membrane integrity disruption and inhibition of biofilm formation. Peptides 2014, 56, 22–29.
- Wang, K.; Yan, J.; Chen, R.; Dang, W.; Zhang, B.; Zhang, W.; Song, J.; Wang, R. Membrane-active action mode of polybia-CP, a novel antimicrobial peptide isolated from the venom of Polybia paulista. Antimicrob. Agents Chemother. 2012, 56, 3318–3323.
- Mendes, M.A.; De Souza, B.M.; Marques, M.R.; Palma, M.S. Structural and biological characterization of two novel peptides from the venom of the neotropical social wasp Agelaia pallipes pallipes. Toxicon 2004, 44, 67–74.
- Wang, K.; Yan, J.; Chen, R.; Dang, W.; Zhang, B.; Zhang, W.; Song, J.; Wang, R.; Yan, J.; Chen, R.; et al. Membrane perturbation action mode and structure-activity relationships of protonectin, a novel antimicrobial peptide from the venom of the neotropical social wasp Agelaia pallipes pallipes. Antimicrob. Agents Chemother. 2013, 56, 4632–4639.
- Wang, F.; Manku, S.; Hall, D.G. Solid phase syntheses of polyamine toxins HO-416b and PhTX-433. Use of an efficient polyamide reduction strategy that facilitates access to branched analogues. Org. Lett. 2000, 2, 1581–1583.
- Choi, S.K.; Kalivretenos, A.G.; Usherwood, P.N.R.; Nakanishi, K. Labeling studies of photolabile philanthotoxins with nicotinic acetylcholine receptors: Mode of interaction between toxin and receptor. Chem. Biol. 1995, 2, 23–32.
- Kachel, H.S.; Franzyk, H.; Mellor, I.R. Philanthotoxin analogues that selectively inhibit ganglionic nicotinic acetylcholine receptors with exceptional potency. J. Med. Chem. 2019, 62, 6214–6222.




