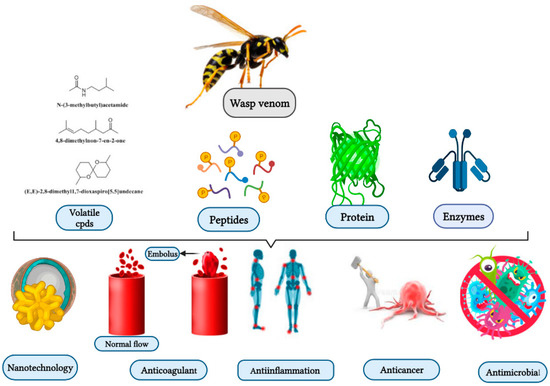
Wasps, members of the order Hymenoptera, are distributed in different parts of the world, including Brazil, Thailand, Japan, Korea, and Argentina. The lifestyles of the wasps are solitary and social. Social wasps use venom as a defensive measure to protect their colonies, whereas solitary wasps use their venom to capture prey. Chemically, wasp venom possesses a wide variety of enzymes, proteins, peptides, volatile compounds, and bioactive constituents, which include phospholipase A2, antigen 5, mastoparan, and decoralin. The bioactive constituents have anticancer, antimicrobial, and anti-inflammatory effects. However, the limited quantities of wasp venom and the scarcity of advanced strategies for the synthesis of wasp venom’s bioactive compounds remain a challenge facing the effective usage of wasp venom. Solid-phase peptide synthesis is currently used to prepare wasp venom peptides and their analogs such as mastoparan, anoplin, decoralin, polybia-CP, and polydim-I.
- wasp’s venom
- biomedical properties
- bioactive compounds
- nanotechnology applications
- allergy
1. Introduction
2. Biological Properties of Wasp Venom, and Their Isolated and Synthesized Bioactive Peptides
2.1. Biological Properties
2.1.1. Antimicrobial Activities
2.1.2. Anti-Inflammatory Activities
2.1.3. Genotoxicity
2.1.4. Anticoagulant
2.2. Isolated and Synthesized Bioactive Peptides from Wasp Venoms
| Wasp-Scientific Name | Isolated Compounds | Biological Activity | Reference |
|---|---|---|---|
| Peptides | |||
| Vespa xanthoptera Vespula lewisii |
Mastoparan (MPX) (INWKGIAAMAKKLL-NH2) | Cytotoxic against Glioblastoma multiforme (T98G) cell, 60% inhibition at 20 μmol/L (in vitro) Anti-Escherichia coli and anti-Lactococcus lactis at MIC 8, and 2.5 µM, respectively (in vitro). |
[40,79,80] |
| Anterhynchium flavomarginatum micado | Mastoparan-AF (EMP-AF) (INLLKIAKGIIKSL-NH2) |
Blocked lobster neuromuscular transmission. Mediated depolarization of the muscle membrane, often leading to a weak contraction of the muscle at 0.1 ± 1 mM (in vitro). |
[1,81] |
| V. lewisii, Vespa tropica and Polybia paulista | Mastoparan (INLKALAALAKKIL) | Induces apoptosis in B16F10-Nex2 melanoma cells treated with 165 µM. Potent anti-inflammatory. Shows activity against colistin-susceptible Acinetobacter baumannii and colistin-resistant Acinetobacter baumannii at MIC50 value of 4, and 8 mg/l, respectively. Antimicrobial activity on the epimastigote, trypomastigote and amastigote forms of Trypanosoma cruzi Y strain via dose-dependent growth inhibition (in vitro). |
[38,41,82] |
| Vespa basalis | Mastoparan B (LKLKSIVSWAKKVL) | Anti-Enterococcus faecalis and anti-Bucillus subtilis at MIC of 3.13 mg/mL (in vitro). | [51] |
| V. basalis | Mastoparan-I1 (INLKAIAALVKKVL) | ND | [51] |
| V. basalis | Mastoparan-A (IKWKAILDAVKKVI) | ND | [51] |
| V. basalis | Mastoparan-T (INLKAIAAFAKKLL) | ND | [51] |
| Vespula vulgaris | Mastoparan V1 (INWKKIKSIIKAAMN) | Potent antimicrobial activity against Streptococcus mutans and Salmonella enterica at 50 µM (in vitro). | [4] |
| Vespa orientalis L. | Mastoparan (HRI) (INLKAIAALVKKVL-NH2) |
Cytotoxic towards T98G cells and give 80% inhibition at 20 μmol/L (in vitro). | [40] |
| Vespa crabro | Mastoparan-C (MP-C) (LNLKALLAVAKKIL-NH2) | Inhibition of the biofilm formation by Staphylococcus Aureus and Pseudomonas aeruginosa at 32 μM MBIC (in vitro). | [26] |
| V. tropica | Mastoparan-VT1 (INLKAIAALAKKLL) | Anti-E. faecalis at 2.5 µg/mL (in vitro). | [30] |
| V. tropica | Mastoparan-VT2 (NLKAIAALAKKLL) | Anti-E. faecalis, anti-E.coli and anti-S.aureus at 5 µg/mL (in vitro). | [30] |
| V. tropica | Mastoparan-VT3 (INLKAITALAKKLL) | Anti-S. aureus and anti-Candida parapsilosis at 2.5 µg/mL (in vitro). | [30] |
| V. tropica | Mastoparan-VT4 (INLKAIAPLAKKLL) | Anti-Bacillus pyocyaneus, anti-P. aeruginosa, and anti-Bacillus dysenteriae at 10 µg/mL (in vitro). | [30] |
| V. tropica | Mastoparan-VT5 (VIVKAIATLASKLL) | Anti-Candida albicans at 40 µg/mL (in vitro). | [30] |
| V.tropica | Mastoparan-VT6 (INLKAIAALVKKLL) | Anti-S. aureus and anti-B. dysenteriae at 20 µg/mL (in vitro). | [30] |
| V. tropica | Mastoparan-VT7 (INLKAIAALARNY) | Anti-E. faecalis at 5 µg/mL (in vitro). | [30] |
| Polistes rothneyi iwatai | Polistes-mastoparan-R1 (Pm-R1) (INWLKLGKKILGAI-NH2) | Has histamine-releasing activities from rat mast cells (EC50 = 0.09 µM) (in vitro). | [80] |
| P. rothneyi iwatai. | Polistes-mastoparan-R3 (Pm-R3) (INWLKLGKQILGAL-NH2) |
Has histamine-releasing activities from rat mast cells (EC50 = 0.19 mM) (in vitro). | [80] |
| Vespa magnifica | Peptide 5e (FLPIIAKLLGLL) | Anti-S. aureus, MIC = 5 µg/mL (in vitro). | [83] |
| V. magnifica | Peptide 5f (FLPIPRPILLGLL) | Anti-S. aureus, MIC = 10 µg/mL (in vitro). | [83] |
| V. magnifica | Peptide 5g (FLIIRRPIVLGLL) | Anti-S. aureus MIC = 10 µg/mL (in vitro). | [83] |
| V. magnifica | Peptide 12a (INWKGIAAMAKKLL) | Anti-S. Aureus, and anti-C. albicans at MIC = 3.7 µg/mL (in vitro). | [83] |
| V. magnifica | Peptide 12b (INWKGIAAMKKLL) | Anti-S. aureus MIC = 3.7 µg/mL (in vitro). | [83] |
| P. dimorpha | Polydim-I (AVAGEKLWLLPHLLKMLLTPTP) | Antimycobacterial activity at 7.6 μg/mL (in vitro). Anti-S. aureus at MIC50 4.1 µg/mL (in vitro). |
[15,73] |
| Anoplus samariensis | As-126 (EDPPVVKMK-NH2) | ND | [84] |
| Batozonellus maculifrons | Bm-10 (ETAPVPKAISK-NH2) | ND | [84] |
| A. samariensis | Anoplin (GLLKRIKTLL-NH2) |
Cytotoxic for T98G cells, gives 10% inhibition at 20 μmol/L (in vitro). | [40,55] |
| P. hypochondriaca | Pimplin (KRKPPRPNPKPKPIP) | Effective against Musca domestica at dose of 40 ng (in vitro). | [85] |
| A. flavomarginatum micado | Af-113 (INLLKIAKGIIKSLNH2) | ND | [86] |
| Agelaia vicina | Agelaiatoxin-8 (AVTx8) (INWKLGKALNALLNH2) | Inhibits gamma-aminobutyric acid (GABA) neurotransmission uptake at EC50 value of 0.09 ± 0.04 µM and maximum inhibition of 97 ± 5% (in vitro). | [87] |
| Agelaia pallipes pallipes | AgelaiaMP-I (INWLKLGKAIIDAL-NH2) |
Has hemolytic activity at ED50 = 60 µM. | [28] |
| A. pallipes pallipes | AgelaiaMP-II (INWKAILQRIKKML-NH2) | Has hemolytic activity at ED50 = 240 µM (in vitro). | [88] |
| Anoplius samariensis, and Batozonellus maculifrons |
Pompilidotoxins (α-PMTXs) (RIKIGLFDQLSKL-NH2) | Facilitates synaptic transfer in the motor neuron of the lobster and delays downregulation of the sodium channel (in vitro). | [89] |
| A. samariensis, and B. maculifrons |
β-PMTXs (RIKIGLFDQRSKL-NH2) |
Facilitates synaptic transfer in the neuromuscular junction of the lobster, and slows the sodium channel inactivation (in vitro). | [89] |
| A. flavomarginatum micado | Eumenine mastoparan-AF (EMP-AF) (INLLKIAKGIIKSL-NH2) |
Effective hemolytic response in human erythrocytes. Enhancing degranulation of rat peritoneal mast cells and RBL-2H3 cells (in vitro). |
[81] |
| Agelaia pallipes pallipes, and Protonectarina sylveirae | Protonectin (ILGTILGLLKGL-NH2) |
Antibacterial activity towards Gram-positive and Gram-negative bacteria. Releasing Lactate dehydrogenase (LDH) from mast cells. Chemotaxis against polymorphonuclear leukocytes (PMNL) (in vitro). |
([90] |
| A. pallipes pallipes, and P. sylveirae | Protonectin (1–6) (ILGTIL-NH2) |
ND | [90] |
| A. pallipes pallipes | Protonectin (1–4)-OH (ILGT-OH) |
Has poor hemolytic activity at ED50 = 1 mM (in vitro). | [88] |
| A. pallipes pallipes | Protonectin (7–12) (GLLKGL-NH2) |
ND | [88] |
| A. pallipes pallipes | Protonectin (1–5)-OH (ILGTI-OH) |
Has weak hemolytic activity at ED50 = 1 mM (in vitro). | [88] |
| A. pallipes pallipes | Protonectin (1–6)-OH (ILGTIL-OH) |
Has poor hemolytic activity at ED50 = 1 mM (in vitro). | [88] |
| Orancistrocerus drewseni | Orancis-protonectin (ILGIITSLLKSL-NH2) | Has hemolytic activity of the sheep blood cells at 50 µM (in vitro). | [91] |
| A. pallipes pallipes | Pallipine-I (GIIDDQQCKKKPGQSSPVCS-OH) | ND | [88] |
| A. pallipes pallipes | Pallipine-II (SIKHKICKLLERTLKLTT PFC-NH2) | ND | [88] |
| A. pallipes pallipes | Pallipine-III (SIKKHKCIALLERRGGSKLPFC-NH2) |
ND | [88] |
| P. paulista | Paulistine (SIKDKICKIIQCGKKLPFT-NH2) (oxidized form) |
Causes mast cells degranulation or hemolysis (in vitro). | [92] |
| Vespa mandarinia | Ves-CP-M (FLPILGKLLSGL-NH2) | ND | [65] |
| V. xanthoptera | Ves-CP-X (FLPIIAKLLGGLL) | ND | [65] |
| Paravespula lewisi | Ves-CP-P (FLPIIAKLVSGLL) | ND | [65] |
| V. tropica | Ves-CP-T (FLPILGKILGGLL) | ND | [65] |
| V. crabro | Crabrolin (FLPLILRKIVTAL-NH2) | Releases histamine from rat peritoneal mast cells at ED50 of 11.8 µg/mL (in vitro). | [93,94] |
| Eumenes rubronotatus | Eumenitin (LNLKGIFKKVASLLT) | Shows antimicrobial activity against S. aureus, Staphylococcus saprophytius, E. coli at MIC = 6 µM (in vitro). | [95] |
| E. rubrofemoratus | Eumenine mastoparan-ER (EMP-ER) (FDIMGLIKKVAGAL-NH2) | Anti-C. albicans at MIC 7.5 µM. Has Leishmanicidal activity at IC50 20 µM (in vitro). |
[96] |
| Eumenes micado | Eumenine mastoparan-EM1 (LKLMGIVKKVLGAL-NH2) | Anti-S. aureus and anti-E. coli at MIC 7 µM (in vitro). Has Leishmanicidal activity with an IC50 of 36 µM (in vitro). |
[97] |
| E. micado | Eumenine mastoparan-EM2 (LKLLGIVKKVLGAI-NH2) | Anti-S. aureus and anti-E. coli at MIC of 3 µM (in vitro). Has Leishmanicidal activity with an IC50 of 36 µM (in vitro). |
[97] |
| Eumenes fraterculus | Eumenine mastoparan-EF (EMP-EF) (FDVMGIIKKIASALNH2 | Anti-C. albicans at MIC of 7.5 µM. Has Leishmanicidal behavior at IC50 of 40 µM (in vitro). |
[96] |
| O. drewseni | Eumenine mastoparan-OD (EMP-OD) (GRILSFIKGLAEHL-NH2) |
Induces hemolysis of the sheep blood cells at 50 µM (in vitro). | [91] |
| E. rubrofemoratus | Eumenitin-R (LNLKGLIKKVASLLN) | Anti-Sreptococcus pyogenes, anti-Micrococcus luteus, and anti-Stenotrophomonas maltophilia at MIC of 15 µM. Anti-B. subtilis at MIC 7.5 µM (in vitro). |
[96] |
| E. fraterculus | Eumenitin-F (LNLKGLFKKVASLLT) | Anti-C. albicans at MIC of 7.5 µM. Has Leishmanicidal activity at IC50 of 52 µM (in vitro). Anti-S. maltophilia at MIC of 15 µM (in vitro). |
[96] |
| P. paulista. | Polybia-CP (ILGTILGLLKSL-NH2) |
Anti-microbial against S. aureus and B. subtilis at 15 µg/mL compared with 0.5 and 18 µg/mL of tetracycline (in vitro). | [14,65] |
| P. paulista | Polybia-CP 2 (ILGTILGKIL-OH) | Has chemotaxis, mast cell degranulation, and hemolytic activities (in vivo). | [98] |
| Polybia-CP 3 (ILGTILGTFKSL-NH2) | Has chemotaxis, mast cell degranulation, and hemolytic activities (in vivo). Antiplasmodial and anticancer properties (in vitro). |
[8,98] | |
| P. paulista | Polybia-MP1 (IDWKKLLDAAKQIL-NH2) |
Antitumor against bladder and prostate cancer cells. Exhibits potent activity against S. aureus, MIC of 9 µΜ (in vitro). Anti-C. albicans (EC50 = 12.9 μM) and C. neoformans (EC50 = 11 μM) (in vitro). Fungicidal activity against Candida glabrata (EC50 = 8 μM) and C. albicans (EC50 = 16 μM) (in vitro). Anti-E. coli, P. aeruginosa, B. subtilis, and S. aureus at MIC of 8, 8, 4, and 15 μg/mL compared to 2, 18, 18, and 0.5 of tetracycline (in vitro). |
[64,85] |
| V. orientalis L. | HR-1 (INLKAIAALVKKVL-NH2 | ND | [99] |
| V. orientalis L. | HR-2 (FLPLILGKLVKGLL-NH2) | ND | [99] |
| Polistes jadwigae | Polisteskinin-J (RRRPPGFSPFR-OH) | ND | [98] |
| Pollistes chiensis | Polisteskinin-C (SKRPPGFSPFR-OH) | ND | [98] |
| P. rothney | Polisteskinin-R (ARRPPGFTPFR-OH) | Exerts potent anxiolytic effects at 6, 3, and 1.5 ηmol compared to positive control Diazepam (in vivo) | [98,100] |
| Vespa analis | Vespakinin-A (GRPPGFSPFRVI-OH) | ND | [98] |
| Vespa mandarínia | Vespakinin-X (ARPPGFSPFR-OH) | ND | [98] |
| V. magnifica, Parapolybia varia, V. tropica | Vespid Chemotactic Peptides (VCP) | Anti-tumor activities towards NIH-OVCAR-3 and SK-OV-3 ovarian cancer cell lines at concentrations higher than 10 μM (in vitro). | [34,101] |
| V. magnifica (Smith) | VCP-5h (FLPIIGKLLSGLL-NH2) | MICs of 5, 25, and 30, µg/mL for S. aureus, C. albicans and E. coli, respectively (in vitro). | [102] |
| Parapolybia varia | Vespakinin (Vespk) | Antitumor activity to SK-OV-3 at 24 h post-treatment (in vitro). | [101] |
| V. magnifica | Vespakinin-M GRPPGFSPFRID | ND | [103] |
| Batozonellus maculifrons | Pompilidotoxins (β-PMTXs) (RIKIGLFDQLSRL-NH2) | Inactivation of the Na+ channel, and the Nav1.6 channel was more selective (in vitro). | [1] |
| O. drewseni | OdVP1 (GRILSFIKGLAEHL-NH2) | Anti-E. coli, and anti-C. albicans at MIC of 6 µM (in vitro). | [104,105] |
| O. drewseni | OdVP2 (ILGIITSLLKSL-NH2) | Anti-S. aureus at MIC of 25 µg/mL. Anti-gray mold Botrytis cinerea at MIC of 0.4 µM (in vitro). |
[104,105] |
| O. drewseni | OdVP3 (KDLHTVVSAILQAL-NH2) | Anti-gray mold B. cinerea at MIC of 5 µM (in vitro). | [104,105] |
| O. drewseni | OdVP4 (LDPKVVQSLL-NH2) |
ND | [104] |
| Nasonia vitripennis | Defensin-NV (VTCELLMFGGVVGDSACAANCLSMGKAGGSCNGGLCDCRKTTFKELWDKRFG) | Anti-S. aureus, and Anti-B. cereus at MIC of 0.93 µM (in vitro). Anti-B. dysenteriae at MIC of 0.46 µM (in vitro). Anti-E. coli, and anti-C. albicans at MIC of 1.86 µM (in vitro). Anti-P. aeruginosa at MIC of 9.3 µM (in vitro). |
[106] |
| Chartergellus communis | Communis (INWKAILGKIGK-COOH) |
ND | [107] |
| C. communis | Communis-AAAA (INWKAILGKIGKAAAAVNH2) | Hemolytic activity at EC50 = 142.6 μM (in vitro). Hyperalgesic effect at 2 nmol/animal (in vivo). |
[107] |
| Cyphononyx Fulvognathus |
Bradykinin (RPPGFSPFR) |
Acts as a chemoattractant directing glioma cells into blood vessels in the brain of rats (in vivo). | [108] |
| Megascolia flavifrons, and Colpa interrupta |
Megascoliakinin = Thr6BK-Lys-Ala (BK = bradykinin) (RPPGFTPFRKA) | Prevents the synaptic transmission of the nicotinic acetylcholine receptor (nAChR) in the central nervous system of insect (in vitro). | [109] |
| C. fulvognathus and P. paulista |
RA-Thr6 -Bradykinin (RARPPGFTPFR-OH) | ND | [98] |
| Polybia occidentalis, M. flavifrons, C. interrupta, and P. paulista | Threonine6-bradykinin (Thr6-BK) RPPGFTPFR-OH |
Anti-nociceptive effects with approximately two-fold higher than bradykinin and morphine (in vivo). | [98,110] |
| P. paulista | RA-Thr6 -Bradykinin-DT (RARPPGFTPFRDT-OH) | ND | [98] |
| C. fulvognathus | Fulvonin (SIVLRGKAPFR) |
Displays hyperalgesic impact after intraplantar injection in the rat paw pressure test (in vivo). | [111] |
| C. fulvognathus (Japan) |
Cyphokinin (DTRPPGFTPFR) | Demonstrates hyperalgesic impact after intraplantar injection in the rat paw pressure test (in vivo). | [111] |
| C. fulvognathus (Japan) |
Cd-146 (SETGNTVTVKGFSPLR) | Shows hyperalgesic effect in the rat paw pressure test after intraplantar injection (in vivo). | [111] |
| C. fulvognathus | Cd-125 (DTARLKWH) | ND | [111] |
| P. paulista | Mastoparan (MPI) (IDWKKLLDAAKQIL-NH2) |
Cytotoxic towards T98G cells, gives 30% inhibition at 20 μmol/L (in vitro). | [40] |
| Pseudopolybia vespiceps | Mastoparan Polybia-MPII (INWLKLGKMVIDAL-NH2) | Anti-staphylococcal activity with an EC50 of 1.83 μM and EC90 of 2.90 μM (in vitro). Mice treated with 5 mg/kg showed a decline in bacterial load from 108 to ca. 106 CFUs (in vitro). Potent hemolytic activity against mouse cells (EC50 = 24.18 Μm, EC90 = 58.12 μM) (in vitro). Inhibits the growth of C. neoformans (EC50 = 11 μM) and C. albicans (EC50 = 12.9 μM) (in vitro). Anti-A. baumannii AB 0 at MIC of 12.5 µM while MIC against A. baumannii AB 53 and AB 72 was 6.25 µM (in vitro). Adhesion inhibition for A. baumannii AB 02 and AB 72 at 25 µM while A. baumannii AB 53 was inhibited at a concentration of 12.5 µM (in vitro). |
[28,112] |
| P. paulista | Polybia-MPIII (INWLKLGKAVIDAL) | Anti-S. aureus, MIC of 19 μM (in vitro). | [65] |
| P. paulista | Polybia-MP IV (IDWLKLRVISVIDL-NH2) | Shows strong mast cell degranulation. Has weak haemolytic activity, hypernociception and edema formation (in vitro). |
[98] |
| P. paulista | Polybia-MP V (INWHDIAIKNIDAL-NH2) | Medium mast cell degranulation, haemolytic activity and hypernociception (in vitro). | [98] |
| P. paulista | Polybia-MP VI (IDWLKLGKMVM-OH) | Medium haemolytic activity and hypernociception (in vitro). | [98] |
| P. paulista | unk-1 (IPAGWAIVKV-NH2) | Shows weak mast cell degranulation and haemolytic activity (in vitro). | [98] |
| P. paulista | unk-2 (TGDSPDVR-OH) | Shows weak mast cell degranulation and haemolytic activity, weak chemotaxis for PMNLs, and a range of weak to strong hypernociception and oedema formation (in vitro). | [98] |
| V. orientalis L. | Orientotoxin (Neurotoxin) |
Has lysophospholipase activity and inhibits both mediated and spontaneous release of the neurotransmitter from the presynaptic nerve membrane (in vivo). | [113,114] |
| V. orientalis L. | Peptide I (AGVILFGR-NH2) | Histamine release from mast cells ED50 = 5.10−7 (in vivo). | [115] |
| V. orientalis L. | Peptide II (AGVIFRSP-NH2) | Histamine release from mast cells ED50 = 3.10−6 (in vivo). | [115] |
| Oreumenes decoratus |
Decoralin (De-NH2) (SLLSLIRKLIT-NH2) |
Has hemolytic activity at EC50 of 80 µM (in vitro). Anti-S. aureus, MIC = 4 µM (in vitro). Anti-B. Subtilis, MIC = 8 µM (in vitro). Anti-C. albicans, MIC = 20 µM (in vitro). Has leishmanicidal activity, IC50 =11 µM (in vitro). |
[61] |
| V. ducalis | VACP1 (AQKWLKYWKADKVKGFGRKIKKIWFG) |
Potently inhibits cell proliferation and promotes the cell apoptosis of osteosarcoma (OS) cells, and this was concomitant with the activation of the JNK and p38 MAPK signaling pathway (in vitro). | [6] |
| Emerald Jewel, and Ampulex compressa | Ampulexin-1 (axn1) (CKDDYVNPKEQLGYDILEKLRQKP) | ND | [116] |
| Ampulexin -2 (axn2) (CQNDYVNPKLQFACDLLQKAKERQ) | ND | [116] | |
| Ampulexin -3 axn3 SFSMLLQKAKERQ | ND | [116] | |
| V. orientalis | AuNPs+ peptide (INLKAIAALVKKV) | Antibacterial using AuNPs against K. pneumoniae, B. cereus, S. mutans, S. typhimuriu, E. coli, and S. aureus, and with the inhibition zones of 9.21, 14.32, 14.71,19.21, 15.24 and 15.33 mm, respectively (in vitro). | [19] |
| Vespa bicolor Fabricius | V. chemotatic peptide (VESP-VBs) (FMPIIGRLMSGSL) | Anti-S. aureus, MIC = 1 µg/mL (in vitro). | [5] |
| V. bicolor Fabricius | V. mastoparan (MP-VBs) (INMKASAAVAKKLL) | Anti-S. aureus, MIC = 1.9 µg/mL (in vitro). | [5] |
| Polistes dominulus | Dominulin A (INWKKIAEVGGKILSSL) | Anti-B. Subtilis, and E. coli at MIC = 2 and 8 µg/mL, respectively (in vitro). | [117] |
| P. dominulus | Dominulin B (INWKKIAEIGKQVLSAL) | Anti-B. Subtilis, and E. coli at MIC = 2 and 8 µg/mL, respectively (in vitro). | [17] |
| Protonectarina sylveirae | Protonectarina-MP (INWKALLDAAKKVL) | Anti-B. subtilis and anti-S. Aureus MIC = 3.9 µg/mL (in vitro). | [69] |
| Parapolybia indica | Parapolybia-MP (INWKKMAATALKMI-NH2) | Anti-S. aureus, MIC = 3.9 µg/mL (in vitro). | [69] |
| P. jadwigae | Polistes mastoparan (VDWKKIGQHIKSVL) | Degranulation of mast cells at 5 nM/mL. | [39] |
| V. magnifica (Smith) | Vespid chemotactic peptide (VCP) | MICs for S. aureus, C. albicans, and E. coli were 5, 25, and 30, µg/mL, respectively (in vitro). | [102] |
| V. bicolor Fabricius | VESP-VB1 (FMPIIGRLMSGSL) | Anti-E. coli, MIC = 7.5 µg/mL (in vitro). Anti-S. aureus, MIC = 1.9 µg/mL (in vitro). Anti-P. aeruginosa, MIC = 3.75 µg/mL (in vitro). Anti-C. albicans, MIC = 30 µg/mL (in vitro). |
[5] |
| V. bicolor Fabricius | MP-VB1 (INMKASAAVAKKLL) | Anti-E. coli, MIC = 15 µg/mL (in vitro). Anti-S. aureus, MIC = 3.75 µg/mL (in vitro). Anti-P. aeruginosa, MIC = 15 µg/mL (in vitro). Anti-C. albicans, MIC = 15 µg/mL (in vitro). |
[5] |
| V. tropica | VCP-VT1 | Anti-E. coli, Enterobacter cloacae, and C. parapsilosis at 2.5 µg/mL and Anti-S. aureus at 1.2 µg/mL (in vitro). | [30] |
| V. tropica | VCP-VT2 FLPIIGKLLSG |
Antimicrobial against S. aureus, E. cloacae at 2.5 µg/mL (in vitro). | [30] |
| Protopolybia exigua (Kinins) | Protopolybiakinin-I (DKNKKPIRVGGRRPPGFTR-OH) |
Caused degranulation of 35% of the mast cells (in vitro). | [118] |
| P. exigua | Protopolybiakinin-II (Kinins) (DKNKKPIWMAGFPGFTPIR-OH) | Caused degranulation of 52 % of the mast cells (in vitro). | [118] |
| V. mandarinia | VESCP-M2 (FLPILAKILGGLL) | Induces pain and severe tissue injury, oedema, cutaneous necrosis, and blister. | [119] |
| Polistes lanio lanio | PllTkP-I (QPPTPPEHRFPGLM) | ND | [120] |
| P. lanio lanio | PllTkP-II (ASEPTALGLPRIFPGLM) | ND | [120] |
| V. magnifica (Smith) | 5-Hydroxytryptamine | ND | [121] |
| V. magnifica (Smith) | Vespakinin-M (GRPPGFSPFRID-NH2) | ND | [121] |
| V. magnifica (Smith) | Mastoparan M (INLKAIAALAKKLL-NH2) | ND | [121] |
| V. magnifica (Smith) | Vespid chemotactic peptide M (FLPIIGKLLSGLL-NH2) | ND | [121] |
| Sphex argentatus argentatus | Sa12b (EDVDHVFLRF) | Inhibits acid-sensing ion channels (ASIC) of rat dorsal root ganglion (DRG) neurons at IC50 of 81 nM while inhibiting it completely at 1 μM (in vivo). | [122] |
| Isodontia harmandi | Sh5b(DVDHVFLRF-NH2) | ND | [122] |
| P. paulista | Neuropolybin | Antiseizure | [37] |
| Synoeca surinama | Synoeca-MP I/LNWI/LKI/LGKKI/LI/LASL/NH2 |
Antimicrobial activity, MIC50 values were 1.9, 2, 8.3, 5.2, and 3.5 μM for methicillin-resistant S. aureus—MRSA, E. coli ESBL, vancomycin-resistant E. Faecalis, P. aeruginosa metallo-ß-lactamase, and Klebsiella pneumoniae KPC, respectively (in vitro). Anti-Candida species, with MICs varying from 10–40 μM (in vitro). |
[123] |
| Enzymes and proteins | |||
| V. magnifica | Magnifin (PLA1) | Activates platelet aggregation and induces thrombosis at 18 nM with causes 85% washed platelets aggregation in 60 s (in vivo). | [124] |
| P. paulista (southeast Brazil) |
Phospholipase A1(Ves v 1) | Catalyzes the ester bonds hydrolysis of 1,2-diacyl-3 snglycerophospholipids at the sn-1 and sn-2 positions, respectively. | [125] |
| P.paulista | Phospholipase A1 | Hydrolyzes phospholipids and produces 2-acyl-lysophospholipids and fatty acids. | [125,126] |
| P. Occidentalis and P. paulista | Phospholipase A2 (PLA2) | Potent hemolytic actions in washed red cells (in vitro). Hydrolyzes natural phospholipids, catalysing the deacylation of 1,2-diacyl-sn-3-phosphoglycerides at position 2 and thus releases free fatty acids and lysophospholipids (in vitro). |
[127,128] |
| P. paulista, Vespula maculate, Vespula arenaria, V. crabro, V. orientalis, Paravespula germanica, Paravespula vulgaris, Dolichovespula saxonica, Dolichovespula media, and Polistes Gallicus |
Hyaluronidase (Polyp2) | Hydrolyses hyaluronic acid which facilitates the diffusion of toxin into the tissue and blood circulation of the prey. | [129,130,131] |
| Polistes comanchus | Polistin (protein) | Responsible for the cytotoxic effect of the whole venom. | [132] |
| P. paulista | Antigen5 (Polyp5) | Major allergen could be used for allergy diagnostics and treatment. | [133] |
| Cyphononyx dorsalis | Arginine kinase-like protein | Exhibits paralytic activity in spiders with the same characteristic symptoms as the crude venom. | [134] |
| Pteromalus puparum | Vn.11 (protein) |
ND | [135] |
| Cotesia rubecula | Vn 4.6 | ND | [136] |
| V. magnifica | Magnvesin | Exerts anti-coagulant properties via hydrolyzing coagulant factors VII, VIII, TF, IX and X. | [137] |
| Some volatile compounds | |||
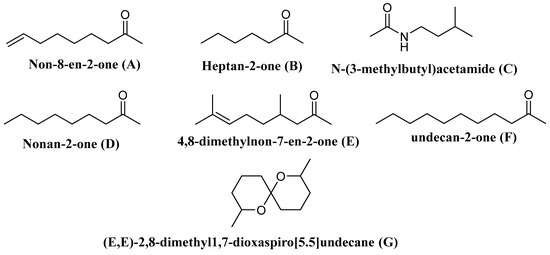
| Vespa velutina | Undecan-2-one | Elicits the defense behavior | [138] |
| V. velutina | Non-8-en-2-one | ||
| V. velutina | Nonan-2-one | ||
| V. velutina | Heptan-2-one | ||
| V. velutina | 4,8-Dimethylnon-7-en-2-one | ||
| Polistes metricus Say, Polistes bellicosus Cresson, and Polistes dorsalis (F.), as well as workers of Polistes aurifer (Saussure), P. bellicosus, P. metricus, and P. dorsalis | N-(3-Methylbutyl)acetamide | ND | [139] |
| P. occidentalis | (E,E)-2,8-Dimethyl1,7-dioxaspiro[5.5]undecane | Elicit the defense behavior | [140] |
2.2.1. Mastoparans
Mastoparan (MP)
Mastoparan-B (MP-B)
Mastoparan-M
2.2.2. Anoplin
2.2.3. Decoralin
2.2.4. Polybia-MP-I
2.2.5. Polybia-CP
2.2.6. Polydim-I
2.2.7. Protonectarina-MP and Agelaia-MP
2.2.8. Philanthotoxin-433 (PhTX-433)
This entry is adapted from the peer-reviewed paper 10.3390/toxins13030206
