| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francisca Dias | + 2299 word(s) | 2299 | 2021-02-25 04:09:18 |
Video Upload Options
The development and progression of colorectal cancer (CRC) have been associated with genetic and epigenetic alterations and more recently with changes in cell metabolism. Amino acid trans-porters are key players in tumor development, and it is described that tumor cells upregulate some AA transporters in order to support the increased amino acid (AA) intake to sustain the tumor additional needs for tumor growth and proliferation through the activation of several signaling pathways. LAT1 and ASCT2 are two AA transporters involved in the regulation of the mTOR pathway that has been reported as upregulated in CRC. Some attempts have been made in order to develop therapeutic approaches to target these AA transporters, however none have reached the clinical setting so far. MiRNA-based therapies have been gaining increasing attention from pharmaceutical companies and now several miRNA-based drugs are currently in clinical trials with promising results.
1. Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide, with 1,849,518 new cases in 2018, being the third most common cancer [1]. Currently, CRC accounts for approximately 10% of all diagnosed cancers and it is the world ́s second most deadly cancer [2]. CRC is the second most common neoplasia diagnosed in women, and the third in men, being the incidence and mortality approximately 25% lower in woman [2]. CRC development can be modulated by several factors, being the high alcohol consumption, overweigh, physical inactivity, tobacco smoking, diabetes mellitus, age, personal or family history of CRC well established risk factors [3][4]. Although the mortality rates have declined due to the improvement in diagnosis and treatment, CRC still represents one of the most lethal cancer types [3]. Furthermore, metastasis is also found in, approximately, 15–25% of CRC cases at the diagnosis, and increase to 50% during the course of the disease [2][5]. The advances in the pathophysiological and molecular CRC knowledge allowed the increase of the treatment options, but these new therapeutic approaches were proven to be more effective in patients with non-metastatic disease [2]. Thus, it is imperative to clarify the mechanisms involved in disease progression, aggressiveness and metastasis formation in order to improve the patients’ follow up and to identify new therapeutic approaches.
2. Amino Acid Transporters Deregulation in CRC: the Impact of LAT1 and ASCT2
It has been nearly a century since the discovery that normal and tumor cells differ in energy metabolism, with tumor cells presenting a higher need of nutrients, being the AA bioavailability crucial to support cell proliferation and growth [6]. Amino acids can be classified into three groups: (1) essential AA (EAA), if the organism is not able to synthesize them and needs to acquire them from the diet; (2) non-essential AA, if they are synthesized in sufficient quantities by the organism or (3) conditional AA, if are usually nonessential, except in times of illness, trauma or stress were they become conditionally essential [7][8].
In addition to their need in protein synthesis, several amino acids have other roles in supporting cancer development. One example is glutamine, the most abundant AA that participates in energy production, redox homeostasis, macromolecular synthesis and cell signaling [9]. In fact, the commitment of glutamine in the these cell processes makes this AA conditionally essential in conditions characterized by a high proliferation rate, such as cancer, in which endogenous glutamine synthesis is not sufficient to satisfy the cell need [8].
Since AAs are hydrophilic, they need selective transport proteins in order to cross the plasma membrane of the cells. There are approximately two-dozen amino acid transporters in humans, and cancer cells must regulate one or more of these transporters to satisfy their nutrient demand [10]. LAT1 (SLC7A5) is a transmembrane transporter involved in the import of large and neutral AA such as leucine and phenylalanine, in exchange for intracellular AA, such as glutamine [11][12][13]. According to various studies, LAT1 is highly upregulated in multiple human cancers, including gastrointestinal cancers [11][13][14][15]. In fact, Hayase and coworkers found a higher expression of LAT1 in 72.4% of CRC cases when compared to colonic adenoma cases, concluding that LAT1 could be a marker for malignant lesions [11]. Furthermore, Zhang and colleagues also found an association of higher LAT1 expression levels to poorer outcomes and shorter survival in several types of cancer, including CRC [16]. The higher LAT1 expression in cancer cells shows the importance of this AA transporter in the maintenance of AA nutrition in cancer cells [10]. Studies conducted by Elorza and coworkers show that the upregulation of LAT1 is involved in the increase of mTORC1 activity through HIF2α activation, showing a relationship between the hypoxic microenvironment, HIF2α and LAT1 [17]. Furthermore, LAT1 mediates leucine uptake with high affinity, which is a key AA activator of the mTOR signaling pathway [18]. However, for mTOR activation, the functional LAT1 is coupled to ASCT2, another AA transporter involved in glutamine uptake [7].
The ASCT2 (SLC1A5) is expressed in most human tissues including the large intestine and CRC tumor cells, and is essentially responsible for the influx of glutamine inside the cells, inducing asparagine, serine and threonine efflux [19][20][21]. According to Liu and colleagues, ASCT2 expression levels can modulate the migration capacity of CRC cells, being the overexpression of this AA transporters associated with a poorer patients’ prognosis [1][22]. In fact, ASCT2 is upregulated in several cancers, including triple-negative breast cancer, CRC, lung cancer, melanoma, neuroblastoma, glioblastoma and prostate cancer [23]. Some studies in glioblastomas and neuroblastoma support the involvement of the activation of c-Myc, n-Myc oncogenes in the inducing of ASCT2 expression [24][25].
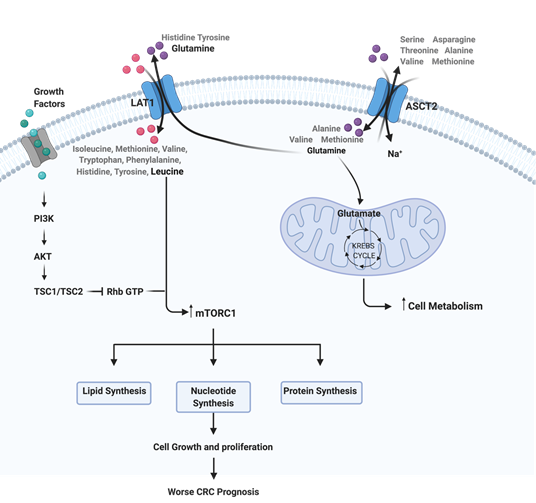
Metabolic reprogramming is a well-known hallmark of cancer that has been gaining increasing attention in the last few years due to its importance in cancer cells viability and growth [26]. Cancer associated metabolic reprogramming influences intracellular and extracellular availability of metabolites that will result in alterations in gene expression, cellular differentiation and also in the tumor microenvironment [27]. Glutamine is considered to be a crucial nutrient for cancer proliferation due to its ability to donate its nitrogen and carbon to several growth-promoting pathways [28]. In 2012, Mootha and colleagues reported that tumor cells have a high necessity of glutamine uptake compared to other AA and, consequently, a glutamine starvation can interfere with tumor metabolism inhibiting tumor proliferation and progression [28]. More recently, Varshavi and colleagues, described a molecular association between CRC that present oncogenic KRAS mutation and glutamine metabolism, since these cells exhibit special metabolic phenotypes, including differences in glycolysis, glutamine utilization and AA metabolism [29]. Furthermore, glutamine is described as a signaling factor in the uptake of AA for the activation of mTORC1 [30]. Thus, the upregulation of AA transporters have an important role in the support of the high-level protein synthesis for continuous cancer growth and proliferation [11][31]. The mTOR pathway is well described as deregulated in CRC, and the availability of AA functions as a regulator of this pathway, since a high AA microenvironmental bioavailability induces mTOR activity and consequent biological processes, such as protein translation [32]. Some studies report a relationship between LAT1 and ASCT2, with a two-step mechanism of these AAT being able to regulate mTOR pathway [33][34][35]. Firstly, ASCT2 regulates the intracellular concentration of glutamine, and in turn LAT1 uses this intracellular glutamine as an efflux substrate, in order to regulate the uptake of extracellular leucine, which will lead to an activation of mTOR signaling and consequent induction of cell growth and proliferation [36] (Figure 1). Furthermore, according to Rajasinghe and coworkers, the inhibition of glutamine uptake in proliferating cells, through the inhibition of glutamine transporters LAT1 and ASCT2, results in the inhibition of cell proliferation and induces apoptosis, through the downregulation of the mTOR pathway [34]. Thus, the inhibition of LAT1 and ASCT2 expression levels could represent a promising therapeutic approach for CRC since it would reduce the AA intake, consequently causing mTOR pathway inhibition and compromising cancer cell proliferation.
Figure 1. Representation of the interplay between ASCT2, LAT1 and mTOR pathway in colorectal cancer (CRC). This image was created using BioRender.
The use of pharmacologic approaches against LAT1 and ASCT2 in cancers with overexpression of these two AA transporters seems be a promising strategy. In fact, over the last few years there was investment in the development of drugs against LAT1 and ASCT2 [21][34][37][38]. The design of drugs against these two AA transporters usually follows an approach based on substrate analogues, which act as competitive inhibitors [21]. In the case of ASCT2 there are also been developed monoclonal antibodies against its cell surface domains [39]. However, it is imperative to keep in mind that the block of AA transporters could be associated with the upregulation of compensatory and redundant pathways, being crucial an accurate overview of all network involved in the process [40]. In addition to that, there are some limitations in the use of pharmacological inhibitors due to the low affinity for the transporter and low selective capacity observed to cancer cells. Thus, these data highlight the need for a deeper understanding of other therapeutic approaches for the selective inhibition of LAT1 and ASCT2 in CRC.
3. Applicability of microRNAs as Therapeutic Agents
MiRNAs are a family of short non-coding RNAs with a length of approximately 19–25 nucleotides that post-transcriptionally regulate gene expression, with an important role in several biological pathways, including cell proliferation and differentiation [41][42]. MiRNAs can regulate the expression of more than 50% of protein-coding genes by binding to their target mRNA transcript and causing its degradation or translation repression [43]. Regarding their applicability in the clinical setting, a growing number of evidence suggests a significant utility of miRNAs as biomarkers for pathogenic conditions, modulators of drug resistance and as therapeutic agents for medical intervention in almost all human health-related conditions [44][45][46][47]. The pleiotropic nature of miRNAs makes them particularly attractive, both as drugs or drug targets, for diseases with a multifactorial origin and no current effective treatments [48][49]. Overall, the current evidence suggests a viable future for miRNA drugs in diseases with no current effective treatments, such as CRC.
4. miRNAs that target both LAT1 and ASCT2 and their Impact on CRC
From the 33 known miRNA that target both LAT1 and ASCT2, only 16 have already been described in CRC (Table 2). However, in terms of the miRNA:mRNA target interaction with LAT1 and ASCT2, none of the miRNAs have been yet validated for CRC.
Table 2. Selected miRNAs’ impact on CRC.
|
miRNA |
Expression |
Sample Type |
Effect |
Reference |
|
Hsa-miR-122-5p |
Down |
CRC Tissue and cells |
Increase in cell proliferation, migration and invasion through the upregulation of CDC25A |
Yin 2020 [50] |
|
Down |
CRC Tissues |
Upregulation of the PI3K/Akt pathway through upregulation of TRIM29 |
Asadi 2019 [51] |
|
|
Up
|
CRC liver metastatic tissues |
Not described |
Liu 2019 [52] |
|
|
Up |
Serum and HT-29 and SW480 cell lines |
Lymph node metastasis biomarker and cell migration inducer |
Qu 2018 [53] |
|
|
Up |
CRC Plasma |
Worse prognosis in metastatic patients and shorter RFS and OS in non-metastatic patients |
Maiertheler 2017 [54] |
|
|
Hsa-miR-1224-3p |
Up |
CRC Tissues |
Upregulated in E cadherin positive tissues |
Lin 2017 [55] |
|
Hsa-miR-1260a |
Down |
CRC Serum |
Not described |
Wang 2017 [56] |
|
Hsa-miR-1260b |
Up |
HCT116 cells |
Chemoresistance to 5-FU through upregulation of PDCD4 |
Zhao 2018 [57] |
|
Down |
SW480 cells |
Downregulated by STAT3-siRNA |
Zhang 2014 [58] |
|
|
Up |
Carcinoma vs adenoma (tissue) |
Not described |
Slattery 2016 [59] |
|
|
Down |
CRC Serum |
Not described |
Zhang 2017 [60] |
|
|
Up |
DKO-1 cells |
Enriched in KRAS mutant cells |
Cha 2015 [61] |
|
|
Hsa-miR-1273g-3p |
Up |
LoVo cells |
Proliferation, migration and invasion through activation of ERBB4/PIK3R3/mTOR/S6K2 pathway |
Li 2018 [62] |
|
Hsa-miR-1273h-5p |
Up |
CRC tissues |
Not described |
Du 2018 [63] |
|
Hsa-miR-149-3p |
Down |
HCT-8 and HCT-116 cells |
Chemoresistance to 5-FU through upregulation of PDK2 |
Liang 2020 [64] |
|
Hsa-miR-15b-5p |
Down |
CRC tissues and cell lines |
Chemoresistance to 5-FU through upregulation of XIAP |
Zhao 2017 [65] |
|
Up |
HT-29 cell line |
Cell growth and inhibition of the proapoptotic pathway |
Gasparello 2020 [66] |
|
|
Down |
KRAS mutated CRC tissues vs wild type CRC tissues |
Not described |
Milanesi 2020 [67] |
|
|
Hsa-miR-16-5p |
Down |
CRC tissues and cell lines |
Upregulation of VEGFA |
Wu 2020 [29] |
|
Hsa-miR-193b-3p |
Down |
CRC tissues vs adjacent normal tissues |
Shorter OS of CRC patients and upregulation of STMN1 |
Guo 2016 [68] |
|
Up |
CRC tissues |
Downregulation of RAD51 |
Kara 2015 [69] |
|
|
Hsa-miR-3199 |
Down |
SW620 cell line |
Upregulation of SMAD4 |
Yan 2018 [70] |
|
Hsa-miR-383-3p |
Down |
CRC tissues and HT-29 and LoVo cell lines |
Upregulation of APRIL |
Cui 2018 [71] |
|
Hsa-miR-4690-5p |
Down |
CRC Stool |
Not described |
Ghanbari 2015 [72] |
|
Up |
CRC tissues |
Upregulated in CIMP high/MSI CRC tissues |
Mullany 2016 [73] |
|
|
Hsa-miR-619-5p |
Down |
CRC tissues vs adjacent normal tissues |
Upregulation of MALAT1, lymphovascular invasion perineural invasion, shorter DFS and shorter OS |
Qiu 2016 [74] |
|
Hsa-miR-6821-5p |
Down |
SW480 CSCs vs SW480 wild-type |
Not described |
Zhou 2019 [75] |
|
Up |
CRC tissues |
Not described |
Du 2018 [63] |
|
|
Hsa-miR-6883-5p |
Down |
TCGA dataset and Cell lines |
Upregulation of CDK4 and CDK6 and cell growth stimulus |
Lulla 2017 [76] |
In table 2 are listed the miRNAs Through the analysis of Table 2 we can observe that some of the miRNAs present opposite results regarding their expression levels, which may be related with the type of biological sample from which their expression levels are analyzed. Regarding their effects on CRC, the deregulation of miR-122-5p, miR-1273g-3p, miR-16-5p, miR-3199, miR-383-3p, miR-619-5p and miR-6883-5p was associated with the upregulation of important players of oncogenic pathways, such as TRIM29, CDC25A, PI3K/Akt, mTOR, VEGFA, MALAT1, SMAD4, STMN1, APRIL and CDK4, with an impact on cell proliferation, invasion and migration. In addition to that, miR-1260b, miR-149-3p and miR-15b-5p were reported as associated with resistance to 50-FU treatment through the upregulation of PDCD4, PDK2 and XIAP, respectively. Moreover, only three miRNAs were associated with clinical endpoints. Higher plasmatic levels of hsa-miR-122-5p were associated with worse prognosis in metastatic patients and shorter RFS and OS in non-metastatic patients, while lower levels of CRC tissue hsa-miR-193b-3p and hsa-miR-619-5p were associated with shorter OS. Moreover, lower levels of CRC tissue hsa-miR-619-5p were also associated with shorter DFS, lymphovascular invasion and perineural invasion.
Taking this information into consideration, we can conclude that miRNAs that target both LAT1 and ASCT2 play an important role on CRC development and aggressiveness and could be used as potential new therapeutic approaches for this neoplasia, but further studies are needed.
References
- Inés Mármol; Cristina Sánchez-De-Diego; Alberto Pradilla Dieste; Elena Cerrada; María Jesús Rodriguez Yoldi; Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. International Journal of Molecular Sciences 2017, 18, 197, 10.3390/ijms18010197.
- Evelien Dekker; Pieter J Tanis; Jasper L A Vleugels; Pashtoon M Kasi; Michael B Wallace; Colorectal cancer. The Lancet 2019, 394, 1467-1480, 10.1016/s0140-6736(19)32319-0.
- Monjur Ahmed; Colon Cancer: A Clinician’s Perspective in 2019. Gastroenterology Research 2020, 13, 1-10, 10.14740/gr1239.
- Nasibeh Karimi; Mohammad Ali Hosseinpour Feizi; Reza Safaralizadeh; Shahryar Hashemzadeh; Behzad Baradaran; Behrooz Shokouhi; Shahram Teimourian; Serum overexpression of miR-301a and miR-23a in patients with colorectal cancer. Journal of the Chinese Medical Association 2019, 82, 215-220, 10.1097/jcma.0000000000000031.
- Bo Young Oh; Hye Kyung Hong; Woo Yong Lee; Yong Beom Cho; Animal models of colorectal cancer with liver metastasis. Cancer Letters 2017, 387, 114-120, 10.1016/j.canlet.2016.01.048.
- Vadivel Ganapathy; Muthusamy Thangaraju; Puttur D. Prasad; Nutrient transporters in cancer: Relevance to Warburg hypothesis and beyond. Pharmacology & Therapeutics 2009, 121, 29-40, 10.1016/j.pharmthera.2008.09.005.
- Bo-Hyun Choi; Jonathan L. Coloff; The Diverse Functions of Non-Essential Amino Acids in Cancer. Cancers 2019, 11, 675, 10.3390/cancers11050675.
- MariaFrancesca Scalise; Lara Console; Filomena Rovella; Michele Galluccio; Lorena Pochini; Cesare Indiveri; Membrane Transporters for Amino Acids as Players of Cancer Metabolic Rewiring. Cells 2020, 9, 2028, 10.3390/cells9092028.
- Christopher T. Hensley; Ajla T. Wasti; Ralph J. DeBerardinis; Glutamine and cancer: cell biology, physiology, and clinical opportunities. Journal of Clinical Investigation 2013, 123, 3678-3684, 10.1172/jci69600.
- Yangzom D. Bhutia; Ellappan Babu; Sabarish Ramachandran; Vadivel Ganapathy; Amino Acid Transporters in Cancer and Their Relevance to “Glutamine Addiction”: Novel Targets for the Design of a New Class of Anticancer Drugs. Cancer Research 2015, 75, 1782-1788, 10.1158/0008-5472.can-14-3745.
- Suguru Hayase; Kensuke Kumamoto; Katsuharu Saito; Yasuhide Kofunato; Yu Sato; Hirokazu Okayama; Kotaro Miyamoto; Shinji Ohki; Seiichi Takenoshita; L‑type amino acid transporter 1 expression is upregulated and associated with cellular proliferation in colorectal cancer. Oncology Letters 2017, 14, 7410-7416, 10.3892/ol.2017.7148.
- MariaFrancesca Scalise; Lorena Pochini; Lara Console; Maria A. Losso; Cesare Indiveri; The Human SLC1A5 (ASCT2) Amino Acid Transporter: From Function to Structure and Role in Cell Biology. Frontiers in Cell and Developmental Biology 2018, 6, 96, 10.3389/fcell.2018.00096.
- Natesh Singh; Gerhard F. Ecker; Insights into the Structure, Function, and Ligand Discovery of the Large Neutral Amino Acid Transporter 1, LAT1. International Journal of Molecular Sciences 2018, 19, 1278, 10.3390/ijms19051278.
- Pascal Häfliger; Roch-Philippe Charles; The L-Type Amino Acid Transporter LAT1—An Emerging Target in Cancer. International Journal of Molecular Sciences 2019, 20, 2428, 10.3390/ijms20102428.
- Hiroomi Ogawa; Kyoichi Kaira; Yoko Motegi; Takehiko Yokobori; Takahiro Takada; Ryuji Katoh; Katsuya Osone; Ryo Takahashi; Chika Katayama; Tetsunari Oyama; et al.Yoshikatsu KanaiTakashi YaoTakayuki AsaoHiroyuki KuwanoKen Shirabe Role of Amino Acid Transporter Expression as a Prognostic Marker in Patients With Surgically Resected Colorectal Cancer. Anticancer Research 2019, 39, 2535-2543, 10.21873/anticanres.13375.
- Chuanmeng Zhang; Jie Xu; Shanshan Xue; Jun Ye; Prognostic Value of L-Type Amino Acid Transporter 1 (LAT1) in Various Cancers: A Meta-Analysis. Molecular Diagnosis & Therapy 2020, 24, 523-536, 10.1007/s40291-020-00470-x.
- Ainara Elorza; Inés Soro-Arnáiz; Florinda Meléndez-Rodríguez; Victoria Rodríguez-Vaello; Glenn Marsboom; Guillermo de Cárcer; Bárbara Acosta-Iborra; Lucas Albacete-Albacete; Angel Ordóñez; Leticia Serrano-Oviedo; et al.Jose Miguel Giménez-BachsAlicia Vara-VegaAntonio SalinasRicardo Sánchez-PrietoRafael Martín del RíoFrancisco Sánchez-MadridMarcos MalumbresManuel O. LandázuriJulián Aragonés HIF2α Acts as an mTORC1 Activator through the Amino Acid Carrier SLC7A5. Molecular Cell 2012, 48, 681-691, 10.1016/j.molcel.2012.09.017.
- Peter M Taylor; Role of amino acid transporters in amino acid sensing. The American Journal of Clinical Nutrition 2013, 99, 223S-230S, 10.3945/ajcn.113.070086.
- Yang Liu; Tingli Zhao; ZhengZheng Li; Lai Wang; Shengtao Yuan; Li Sun; The role of ASCT2 in cancer: A review. European Journal of Pharmacology 2018, 837, 81-87, 10.1016/j.ejphar.2018.07.007.
- Yann Cormerais; Pierre André Massard; Milica Vucetic; Sandy Giuliano; Eric Tambutté; Jerome Durivault; Valérie Vial; Hitoshi Endou; Michael F. Wempe; Scott K. Parks; et al.Jacques Pouyssegur The glutamine transporter ASCT2 (SLC1A5) promotes tumor growth independently of the amino acid transporter LAT1 (SLC7A5). Journal of Biological Chemistry 2018, 293, 2877-2887, 10.1074/jbc.ra117.001342.
- Hongli Jiang; Ning Zhang; Tongzhong Tang; Feng Feng; HaoPeng Sun; Wei Qu; Target the human Alanine/Serine/Cysteine Transporter 2(ASCT2): Achievement and Future for Novel Cancer Therapy. Pharmacological Research 2020, 158, 104844, 10.1016/j.phrs.2020.104844.
- MariaFrancesca Scalise; Lorena Pochini; Michele Galluccio; Lara Console; Cesare Indiveri; Glutamine Transport and Mitochondrial Metabolism in Cancer Cell Growth. Frontiers in Oncology 2017, 7, -, 10.3389/fonc.2017.00306.
- Yangzom D. Bhutia; Vadivel Ganapathy; Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 2016, 1863, 2531-2539, 10.1016/j.bbamcr.2015.12.017.
- David R. Wise; Ralph J. DeBerardinis; Anthony Mancuso; Nabil Sayed; Xiao-Yong Zhang; Harla K. Pfeiffer; Ilana Nissim; Evgueni Daikhin; Marc Yudkoff; Steven B. McMahon; et al.Craig B. Thompson Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proceedings of the National Academy of Sciences 2008, 105, 18782-18787, 10.1073/pnas.0810199105.
- Ping Ren; Ming Yue; Daibiao Xiao; Ruijuan Xiu; Lei Gan; Hudan Liu; Guoliang Qing; ATF4 and N-Myc coordinate glutamine metabolism inMYCN-amplified neuroblastoma cells through ASCT2 activation. The Journal of Pathology 2014, 235, 90-100, 10.1002/path.4429.
- Douglas Hanahan; Robert A. Weinberg; Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646-674, 10.1016/j.cell.2011.02.013.
- Natalya N. Pavlova; Craig B. Thompson; The Emerging Hallmarks of Cancer Metabolism. Cell Metabolism 2016, 23, 27-47, 10.1016/j.cmet.2015.12.006.
- Maria V. Liberti; Jason W. Locasale; The Warburg Effect: How Does it Benefit Cancer Cells?. Trends in Biochemical Sciences 2016, 41, 211-218, 10.1016/j.tibs.2015.12.001.
- Hailu Wu; Ming Wei; Xinglu Jiang; Jiacheng Tan; Wei Xu; Xiaobo Fan; Rui Zhang; Chenbo Ding; Fengfeng Zhao; Xiangyu Shao; et al.Zhigang ZhangRuihua ShiWeijia ZhangGuoqiu Wu lncRNA PVT1 Promotes Tumorigenesis of Colorectal Cancer by Stabilizing miR-16-5p and Interacting with the VEGFA/VEGFR1/AKT Axis. Molecular Therapy - Nucleic Acids 2020, 20, 438-450, 10.1016/j.omtn.2020.03.006.
- Patrick S. Ward; Craig B. Thompson; Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell 2012, 21, 297-308, 10.1016/j.ccr.2012.02.014.
- Osamu Yanagida; Yoshikatsu Kanai; Arthit Chairoungdua; Do Kyung Kim; Hiroko Segawa; Tomoko Nii; Seok Ho Cha; Hirotaka Matsuo; Jun-Ichi Fukushima; Yoshiki Fukasawa; et al.Yoshiko TaniYutaka TaketaniHiroshi UchinoJu Young KimJun InatomiIsao OkayasuKen-Ichi MiyamotoEiji TakedaTomoyuki GoyaHitoshi Endou Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochimica et Biophysica Acta (BBA) - Biomembranes 2001, 1514, 291-302, 10.1016/s0005-2736(01)00384-4.
- Liron Bar-Peled; David M. Sabatini; Regulation of mTORC1 by amino acids. Trends in Cell Biology 2014, 24, 400-406, 10.1016/j.tcb.2014.03.003.
- Kosuke Toda; Gen Nishikawa; Masayoshi Iwamoto; Yoshiro Itatani; Ryo Takahashi; Yoshiharu Sakai; Kenji Kawada; Clinical Role of ASCT2 (SLC1A5) in KRAS-Mutated Colorectal Cancer. International Journal of Molecular Sciences 2017, 18, 1632, 10.3390/ijms18081632.
- Lichchavi Dhananjaya Rajasinghe; Melanie Hutchings; Smiti Vaid Gupta; Delta-Tocotrienol Modulates Glutamine Dependence by Inhibiting ASCT2 and LAT1 Transporters in Non-Small Cell Lung Cancer (NSCLC) Cells: A Metabolomic Approach. Metabolites 2019, 9, 50, 10.3390/metabo9030050.
- Maria José Ferreira Alves Marie; The expression of the aminoacid transporters ASCT2 (SLC1A5) and LAT1 (SLC7A5) in astrocytomas. Medical Express 2016, 3, -, 10.5935/MedicalExpress.2016.06.05.
- Paul Nicklin; Philip Bergman; Bailin Zhang; Ellen Triantafellow; Henry Wang; Beat Nyfeler; Haidi Yang; Marc Hild; Charles Kung; Christopher Wilson; et al.Vic E. MyerJeffrey P. MacKeiganJeffrey A. PorterY. Karen WangLewis C. CantleyPeter M. FinanLeon O. Murphy Bidirectional Transport of Amino Acids Regulates mTOR and Autophagy. Cell 2009, 136, 521-534, 10.1016/j.cell.2008.11.044.
- Alan C. Foster; Natalie Rangel-Diaz; Jia-Ying Yang; Mahmud Penjwini; Veena Viswanath; Yong-Xin Li; Ursula Staubli; Phenylglycine analogs are inhibitors of the neutral amino acid transporters ASCT1 and ASCT2 and enhance NMDA receptor-mediated LTP in rat visual cortex slices. Neuropharmacology 2017, 126, 70-83, 10.1016/j.neuropharm.2017.08.010.
- Keitaro Hayashi; Naohiko Anzai; Novel therapeutic approaches targeting L-type amino acid transporters for cancer treatment. World Journal of Gastrointestinal Oncology 2017, 9, 21-29, 10.4251/wjgo.v9.i1.21.
- Masayo Suzuki; Hiroe Toki; Akiko Furuya; Hiroshi Ando; Establishment of monoclonal antibodies against cell surface domains of ASCT2/SLC1A5 and their inhibition of glutamine-dependent tumor cell growth. Biochemical and Biophysical Research Communications 2017, 482, 651-657, 10.1016/j.bbrc.2016.11.089.
- Stefan Bröer; Amino Acid Transporters as Disease Modifiers and Drug Targets. SLAS DISCOVERY: Advancing the Science of Drug Discovery 2018, 23, 303-320, 10.1177/2472555218755629.
- Mario Acunzo; Giulia Romano; Dorothee Wernicke; Carlo M. Croce; MicroRNA and cancer – A brief overview. Advances in Biological Regulation 2015, 57, 1-9, 10.1016/j.jbior.2014.09.013.
- Sanna Khan; Humaira Ayub; Taous Khan; Fazli Wahid; MicroRNA biogenesis, gene silencing mechanisms and role in breast, ovarian and prostate cancer. Biochimie 2019, 167, 12-24, 10.1016/j.biochi.2019.09.001.
- Oscar A. Tovar-Camargo; Shusuke Toden; Ajay Goel; Exosomal microRNA Biomarkers: Emerging Frontiers in Colorectal and Other Human Cancers. Expert Review of Molecular Diagnostics 2016, 16, 553-567, 10.1586/14737159.2016.1156535.
- Johora Hanna; Gazi S. Hossain; Jannet Kocerha; The Potential for microRNA Therapeutics and Clinical Research. Frontiers in Genetics 2019, 10, 478, 10.3389/fgene.2019.00478.
- Francisca Dias; Ana Luísa Teixeira; Inês Nogueira; Mariana Morais; Joana Maia; Cristian Bodo; Marta Ferreira; Alexandra Silva; Manuela Vilhena; João Lobo; et al.José Pedro SequeiraJoaquina MaurícioJorge OliveiraKlaas KokBruno Costa-SilvaRui Medeiros Extracellular Vesicles Enriched in hsa-miR-301a-3p and hsa-miR-1293 Dynamics in Clear Cell Renal Cell Carcinoma Patients: Potential Biomarkers of Metastatic Disease. Cancers 2020, 12, 1450, 10.3390/cancers12061450.
- Bárbara Filipa Adem; Nuno Ricardo Alves Bastos; Francisca Dias; Ana Luísa Teixeira; Rui Medeiros; miRNAs: mediators of ErbB family targeted therapy resistance. Pharmacogenomics 2016, 17, 1175-1187, 10.2217/pgs-2016-0038.
- Homa Mollaei; Reza Safaralizadeh; Zeinab Rostami; MicroRNA replacement therapy in cancer. Journal of Cellular Physiology 2019, 234, 12369-12384, 10.1002/jcp.28058.
- Chiranjib Chakraborty; Ashish Ranjan Sharma; Garima Sharma; C. George Priya Doss; Sang-Soo Lee; Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Molecular Therapy - Nucleic Acids 2017, 8, 132-143, 10.1016/j.omtn.2017.06.005.
- Eva Van Rooij; Angela L. Purcell; Arthur A. Levin; Developing MicroRNA Therapeutics. Circulation Research 2012, 110, 496-507, 10.1161/circresaha.111.247916.
- Wenzhe Yin; Jun Xu; Chao Li; Xiankui Dai; Tong Wu; Jifeng Wen; Circular RNA circ_0007142 Facilitates Colorectal Cancer Progression by Modulating CDC25A Expression via miR-122-5p. OncoTargets and Therapy 2020, ume 13, 3689-3701, 10.2147/ott.s238338.
- Milad Asadi‡; Shoan Taheri Talesh‡; Morten Frier Gjerstorff; Dariush Shanehbandi; Behzad Baradaran; Shahriar Hashemzadeh; Venus Zafari; Identification of miRNAs correlating with stage and progression of colorectal cancer. Colorectal Cancer 2019, 8, CRC06, 10.2217/crc-2018-0014.
- Jingwei Liu; Hao Li; Liping Sun; Shixuan Shen; Quan Zhou; Yuan Yuan; Chengzhong Xing; Epigenetic Alternations of MicroRNAs and DNA Methylation Contribute to Liver Metastasis of Colorectal Cancer. Digestive Diseases and Sciences 2019, 64, 1523-1534, 10.1007/s10620-018-5424-6.
- Ailin Qu; YongMei Yang; Xin Zhang; Wenfei Wang; Yingjie Liu; Guixi Zheng; Lutao Du; Chuanxin Wang; Development of a preoperative prediction nomogram for lymph node metastasis in colorectal cancer based on a novel serum miRNA signature and CT scans. EBioMedicine 2018, 37, 125-133, 10.1016/j.ebiom.2018.09.052.
- Melanie Maierthaler; Axel Benner; Michael Hoffmeister; Harald Surowy; Lina Jansen; Phillip Knebel; Jenny Chang-Claude; Hermann Brenner; Barbara Burwinkel; Plasma miR-122 and miR-200 family are prognostic markers in colorectal cancer. International Journal of Cancer 2016, 140, 176-187, 10.1002/ijc.30433.
- Maosong Lin; Bensong Duan; Jiangfeng Hu; Hong Yu; Haihui Sheng; Hengjun Gao; Junxing Huang; Decreased expression of miR-193a-3p is associated with poor prognosis in colorectal cancer. Oncology Letters 2017, 14, 1061-1067, 10.3892/ol.2017.6266.
- Ya-Nan Wang; Zhao-Hua Chen; Wei-Chang Chen; Novel circulating microRNAs expression profile in colon cancer: a pilot study. European Journal of Medical Research 2017, 22, 1-11, 10.1186/s40001-017-0294-5.
- Jun Zhao; Jingjie Cao; Lurong Zhou; Yunyi Du; Xiaoling Zhang; Bo Yang; Yangjun Gao; Yu Wang; Ning Ma; Wei Yang; et al. MiR‑1260b inhibitor enhances the chemosensitivity of colorectal cancer cells to fluorouracil by targeting PDCD4/IGF1. Oncology Letters 2018, 16, 5131-5139, 10.3892/ol.2018.9307.
- Jufeng Zhang; Xia Luo; Huiming Li; Ling Deng; Ying Wang; Genome-Wide Uncovering of STAT3-Mediated miRNA Expression Profiles in Colorectal Cancer Cell Lines. BioMed Research International 2014, 2014, 1-11, 10.1155/2014/187105.
- Martha L. Slattery; Jennifer S. Herrick; Daniel F. Pellatt; John R. Stevens; Lila E. Mullany; Erica Wolff; Michael D. Hoffman; Wade S. Samowitz; Roger K. Wolff; MicroRNA profiles in colorectal carcinomas, adenomas and normal colonic mucosa: variations in miRNA expression and disease progression. Carcinogenesis 2016, 37, 245-261, 10.1093/carcin/bgv249.
- Yajie Zhang; Min Li; Yijiang Ding; Zhimin Fan; Jinchun Zhang; Hongying Zhang; Bin Jiang; Yong Zhu; Serum MicroRNA profile in patients with colon adenomas or cancer.. BMC Medical Genomics 2017, 10, 23, 10.1186/s12920-017-0260-7.
- Diana J Cha; Jeffrey L Franklin; Yongchao Dou; Qi Liu; James N Higginbotham; Michelle Demory Beckler; Alissa M Weaver; Kasey C Vickers; Nirpesh Prasad; Shawn Levy; et al.Bing ZhangRobert J CoffeyJames G Patton KRAS-dependent sorting of miRNA to exosomes. eLife 2015, 4, e07197, 10.7554/elife.07197.
- Min Li; Xiaoping Qian; Mingzhen Zhu; Aiyi Li; Mingzhi Fang; Yong Zhu; Jingyu Zhang; miR‑1273g‑3p promotes proliferation, migration and invasion of LoVo cells via cannabinoid receptor 1 through activation of ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Molecular Medicine Reports 2018, 17, 4619-4626, 10.3892/mmr.2018.8397.
- Binbin Du; Dewang Wu; Xiongfei Yang; Tao Wang; Xinlong Shi; Yaochun Lv; Zhuolong Zhou; Qing Liu; Weisheng Zhang; The expression and significance of microRNA in different stages of colorectal cancer. Medicine 2018, 97, e9635, 10.1097/md.0000000000009635.
- Yu Liang; Lidan Hou; Linjing Li; Lei Li; Liming Zhu; Yu Wang; Xin Huang; Yichao Hou; Danxi Zhu; Huimin Zou; et al.Yan GuXiaoling WengYingying WangYue LiTianqi WuMengfei YaoIsabelle GrossChristian GaiddonMeng LuoJianhua WangXiangjun Meng Dichloroacetate restores colorectal cancer chemosensitivity through the p53/miR-149-3p/PDK2-mediated glucose metabolic pathway. Oncogene 2019, 39, 469-485, 10.1038/s41388-019-1035-8.
- Ci Zhao; Qi Zhao; Chunhui Zhang; Guangyu Wang; Yuanfei Yao; Xiaoyi Huang; Fei Zhan; Yuanyuan Zhu; Jiaqi Shi; Jianan Chen; et al.Feihu YanYanqiao Zhang miR-15b-5p resensitizes colon cancer cells to 5-fluorouracil by promoting apoptosis via the NF-κB/XIAP axis. Scientific Reports 2017, 7, 1-12, 10.1038/s41598-017-04172-z.
- Jessica Gasparello; Laura Gambari; Chiara Papi; Andrea Rozzi; Alex Manicardi; Roberto Corradini; Roberto Gambari; Alessia Finotti; High Levels of Apoptosis Are Induced in the Human Colon Cancer HT-29 Cell Line by Co-Administration of Sulforaphane and a Peptide Nucleic Acid Targeting miR-15b-5p. Nucleic Acid Therapeutics 2020, 30, 164-174, 10.1089/nat.2019.0825.
- Elena Milanesi; Maria Dobre; Alina Ioana Bucuroiu; Vlad Herlea; Teodora Ecaterina Manuc; Alessandro Salvi; Giuseppina De Petro; Mircea Manuc; Gabriel Becheanu; miRNAs-Based Molecular Signature for KRAS Mutated and Wild Type Colorectal Cancer: An Explorative Study. Journal of Immunology Research 2020, 2020, 1-9, 10.1155/2020/4927120.
- Feng Guo; Yang Luo; Yi-Fei Mu; Shao-Lan Qin; Yang Qi; Yi-Er Qiu; Ming Zhong; miR-193b directly targets STMN1 and inhibits the malignant phenotype in colorectal cancer. American journal of cancer research 2016, 6, 2463-2475.
- Murat Kara; Onder Yumrutas; Onder Ozcan; Ozgur Ilhan Celik; Esra Bozgeyik; Ibrahim Bozgeyik; Sener Tasdemir; Differential expressions of cancer-associated genes and their regulatory miRNAs in colorectal carcinoma. Gene 2015, 567, 81-86, 10.1016/j.gene.2015.04.065.
- Wei Yan; Zhongcai Liu; Wenchao Yang; Guoyang Wu; miRNA expression profiles in Smad4-positive and Smad4-negative SW620 human colon cancer cells detected by next-generation small RNA sequencing. Cancer Management and Research 2018, ume 10, 5479-5490, 10.2147/cmar.s178261.
- Ying Cui; Le‑Gao Chen; Hai‑Bo Yao; Jun Zhang; Ke‑Feng Ding; Upregulation of microRNA‑383 inhibits the proliferation, migration and invasion of colon cancer cells. Oncology Letters 2017, 15, 1184-1190, 10.3892/ol.2017.7409.
- Reza Ghanbari; Neda Mosakhani; Virinder K. Sarhadi; Gemma Armengol; Nazila Nouraee; Ashraf Mohammadkhani; Samaneh Khorrami; Ehsan Arefian; Mahdi Paryan; Reza Malekzadeh; et al.Sakari Knuutila Simultaneous Underexpression of let-7a-5p and let-7f-5p microRNAs in Plasma and Stool Samples from Early Stage Colorectal Carcinoma. Biomarkers in Cancer 2015, 7s1, BIC.S25252, 10.4137/bic.s25252.
- Lila E. Mullany; Jennifer S. Herrick; Roger K. Wolff; John R. Stevens; Martha L. Slattery; Association of cigarette smoking and microRNA expression in rectal cancer: Insight into tumor phenotype. Cancer Epidemiology 2016, 45, 98-107, 10.1016/j.canep.2016.10.011.
- Gu Qiu; Xiu-Bing Zhang; Shu-Qing Zhang; Pei-Long Liu; Wei Wu; Jin-Ye Zhang; Shi-Rong Dai; Dysregulation of MALAT1 and miR-619-5p as a prognostic indicator in advanced colorectal carcinoma. Oncology Letters 2016, 12, 5036-5042, 10.3892/ol.2016.5312.
- Jun-Min Zhou; Shui-Qing Hu; Hang Jiang; Yi-Lin Chen; Ji-Hong Feng; Zheng-Quan Chen; Kun-Ming Wen; OCT4B1 Promoted EMT and Regulated the Self-Renewal of CSCs in CRC: Effects Associated with the Balance of miR-8064/PLK1. Molecular Therapy - Oncolytics 2019, 15, 7-20, 10.1016/j.omto.2019.08.004.
- Amriti R. Lulla; Michael J. Slifker; Yan Zhou; Avital Lev; Margret B. Einarson; David T. Dicker; Wafik S. El-Deiry; miR-6883 Family miRNAs Target CDK4/6 to Induce G1 Phase Cell-Cycle Arrest in Colon Cancer Cells. Cancer Research 2017, 77, 6902-6913, 10.1158/0008-5472.can-17-1767.
- Gu Qiu; Xiu-Bing Zhang; Shu-Qing Zhang; Pei-Long Liu; Wei Wu; Jin-Ye Zhang; Shi-Rong Dai; Dysregulation of MALAT1 and miR-619-5p as a prognostic indicator in advanced colorectal carcinoma. Oncology Letters 2016, 12, 5036-5042, 10.3892/ol.2016.5312.
- Jun-Min Zhou; Shui-Qing Hu; Hang Jiang; Yi-Lin Chen; Ji-Hong Feng; Zheng-Quan Chen; Kun-Ming Wen; OCT4B1 Promoted EMT and Regulated the Self-Renewal of CSCs in CRC: Effects Associated with the Balance of miR-8064/PLK1. Molecular Therapy - Oncolytics 2019, 15, 7-20, 10.1016/j.omto.2019.08.004.
- Amriti R. Lulla; Michael J. Slifker; Yan Zhou; Avital Lev; Margret B. Einarson; David T. Dicker; Wafik S. El-Deiry; miR-6883 Family miRNAs Target CDK4/6 to Induce G1 Phase Cell-Cycle Arrest in Colon Cancer Cells. Cancer Research 2017, 77, 6902-6913, 10.1158/0008-5472.can-17-1767.