
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ricardo Portela | + 1466 word(s) | 1466 | 2021-03-11 07:35:31 | | | |
| 2 | Rita Xu | Meta information modification | 1466 | 2021-03-16 11:32:38 | | |
Video Upload Options
Silver nanoparticles (AgNPs) have been successfully applied in several areas due to their significant antimicrobial activity against several microorganisms. In dentistry, AgNP can be applied in disinfection, prophylaxis, and prevention of infections in the oral cavity.
1. Introduction
The use of silver in dentistry has been documented since 1840, mainly in the prevention and treatment of dental caries [1]. Initially, it was used as silver nitrate (AgNO3), and then in association with fluorine (AgF). In the 2000s, silver started to be also used in restorative materials such as silver amalgam. In the 20th century, the study of nanomaterials started a new field in health sciences, then named nanotechnology. The nanometric dimension of the particles used in this new field altered the usual properties of biomaterials, showing new characteristics, processability, and capabilities [2].
Among metallic nanoparticles, silver nanoparticles (AgNP) have stood out in scientific research for presenting antimicrobial properties and biological activity against bacteria, fungi, and enveloped viruses [3][4]. The mechanism of action of AgNPs is mainly associated with the release of cationic silver and its oxidative potential [5]. Particle size and shape can also influence the mechanism of action of AgNPs, as well as their synthesis.
Therefore, silver nanoparticles emerged as a promising compound to be used in dentistry, since the incorporation of antimicrobial substances in dental biomaterials has been a strategy adopted by some researchers [6][7]. Silver nanoparticles have already proved to be effective against several multi-drug-resistant microorganisms [8][9]. However, the commercial use of silver nanoparticles (NP) in dentistry is incipient, with only three products with AgNPs in their composition being commercially available: dental adhesive (NanoCare Gold DNT™) [5][10]; Novaron AG300 (Toagosei Co Ltd., Tokyo, Japan) [11]; and sealer (GuttaFlow™ Coltène-Whaledent) [12][13].
2. Synthesis of Silver Nanoparticles
Silver nanoparticles are synthesized using a precursor (often silver nitrate), a reducing agent that reduces silver ions from Ag+ to Ag0, and a stabilizing agent that ensures the stabilization of suspended nanoparticles and prevents nucleation and aggregation, since metallic nanoparticles have a high surface energy. Therefore, the synthesis of silver nanoparticles can be chemical, physical, or biological (Figure 1). In dentistry, the most common synthesis is the chemical route, as shown in Table 1.
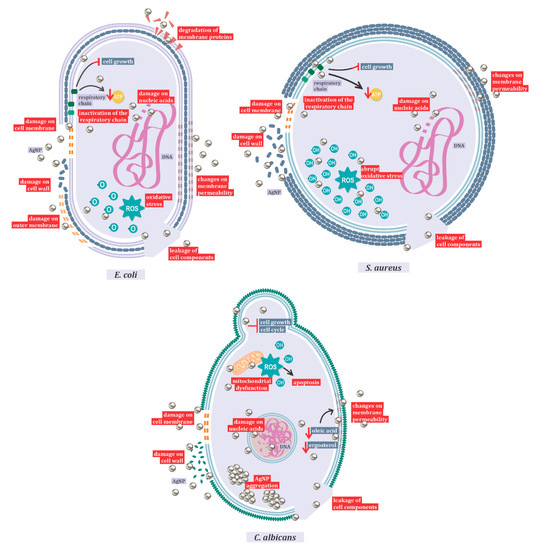
Figure 1. Mechanisms of action of AgNPs against Candida albicans, Escherichia coli, and Staphylococcus aureus.
Table 1. Synthesis methods of silver nanoparticles used in dentistry.
| Synthesis Method | References | Total | % |
|---|---|---|---|
| Commercial synthesis | [5][14][15][16][17][18][19][20][21][22][23][24] | 14.6 | |
| Chemical synthesis | [25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40] [41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57] [58][59][60] |
43.9 | |
| Physical synthesis | [61][62][63][64] | 4.9 | |
| Physicochemical synthesis | [65][66][67][68] | 4.9 | |
| Biosynthesis | [3][69][70][71][72][73][74][75][76][77] | 12.3 | |
| Uninformed | [78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93] | 19.5 |
The synthesis of AgNP is based on the chemical reduction of Ag+1 to Ag0. The differentiation between chemical processes is represented by the reduction agents and stabilizers used, such as sodium citrate, ascorbate, sodium borohydride (NaBH4), elemental hydrogen, polyol process, Tollens reagent, n,n-dimethylformamide (DMF), and poly (ethylene glycol)-block copolymers. Several protective agents (stabilizers) have been used, such as thiols, amines, acids, alcohols [93], and polymeric compounds such as chitosan [25][26][56] and polymethylmethacrylate [27][51][53][68][92]. These agents stabilize dispersive NPs during their synthesis and protect NPs that can be absorbed on, or bind onto, nanoparticle surfaces, avoiding their agglomeration and sedimentation [94].
Physical synthesis uses ultraviolet irradiation [61][65], thermal synthesis [62], and spray pyrolysis [63]. In addition, other researchers have reported unconventional synthesis by direct metal sputtering into anhydrous glycerol [95]. However, the essential approaches to physical synthesis include evaporation-condensation and laser ablation [96]. The benefit of physical approaches is the absence of solvent contamination in the preparation of thin films, the uniformity of nanoparticle distribution, high purity, and quick processing time. Small-scale production, high energy consumption, and thermal stability have been described as disadvantages [96][97].
However, the release of silver nanoparticles and harmful reducing agents such as sodium borohydride into the environment has become a concern. Thus, there is a search for low-cost synthesis processes and eco-friendly methods, which do not use toxic chemicals in synthesis protocols [98]. The biological synthesis emerges as a sustainable alternative and as an attempt to make the process less complicated compared to chemical and physical syntheses. It uses prokaryotic organisms, such as bacteria and eukaryotic organisms such as fungi and plants as potential reducing agents. In this method, the selection of solvents and nontoxic stabilizing agents are also taken into consideration. Our research showed that in dentistry, the most common organisms used to synthesize silver nanoparticles are plants (Table 2). Another advantage of this method is that it increases biocompatibility in living organisms, which is a desired feature for its use in human and veterinary health fields [99][100]. It is important to notice that the effectiveness of biosynthesized silver nanoparticles is related to the stabilization of the metal core with biological polymers [98][101].
Table 2. Species used for the biosynthesis of silver nanoparticles.
| Reference | Kingdom | Species |
|---|---|---|
| [69] | Algae | Spirulina platensis |
| [70] | Fungae | Fusarium oxysporum |
| [71] | Plantae | Heterotheca Inuloides |
| [72] | Plantae | Cassia roxburghii |
| [73] | Plantae | Geranium maculatum |
| [75] | Plantae | Allium cepa, Azadirachta indica, Solanum lycopersicum |
| [76] | Plantae | Salix alba |
| [77] | Plantae | Aloe vera |
| [3] | Plantae | Triticum aestivum |
| [74] | Viridae | M13 phage |
3. Types of AgNPs Used in Dentistry
The biological activity of AgNPs, like other products containing silver, occurs through the gradual release of silver as a consequence of redox reactions in the presence of water [102]. In addition, the antiproliferative action against bacteria, fungi, and viruses is related to the nanoparticle size and shape, in which sizes smaller than 10 nm have higher antimicrobial activity [103]. The diversity in sizes and shapes can be explained by the different nano-ionic origins of nanoparticles [14].
In dentistry, silver nanoparticles are used in association with composites, such as Chitalac-Ag [25], AgNP-methyl polymethylmethacrylate [53][73], amorphous calcium AgNP-phosphate [52], and fluorides (Nano Silver Fluoride) [26]. It can also be used alone in the form of silver nanoparticles or silver plasma [79][87].
4. Mechanisms of Action of AgNPs
Silver nanoparticles are frequently associated with their antimicrobial and antioxidant activities [3]. The action of silver nanoparticles is mainly related to their nanoscale, which alters the level of silver ion release and interferes with the surface energy [5]. Nanoparticles show good antimicrobial effects due to their large surface area, providing high contact with microorganisms when compared to other antimicrobial agents [104].
The action of AgNPs against several microorganisms, including bacteria, fungi, and viruses, has already been described, showing their therapeutic potential [4]. Even multi-resistant bacteria are susceptible to AgNP, which indicates that the mechanisms that confer the resistance of these strains to commercial antibiotics have no protective activity when exposed to nanoparticles [8].
One of the most important mechanisms of action of AgNP is represented by the induction of reactive oxygen species (ROS) production, and hydroxyl radicals are the main species responsible for the oxidative damage [105]. However, it also damages the membrane and cell walls, interferes in the respiratory chain, exhausts the levels of intracellular ATP, and shatters nucleic acids [3][5]. This mechanism of action varies with nanoparticle size and shape, and with the different target species. In this review, the mechanism of antibacterial action against Gram-positive and Gram-negative bacteria and the antifungal mechanism against Candida albicans (Figure 1) were highlighted. In Gram-negative bacteria, with Escherichia coli as a representative species, studies have shown action primarily on the outer membrane, resulting in the leakage of cell components.
After entering the cell, it has also been shown that AgNPs inactivate the respiratory chain dehydrogenases, inhibiting cell growth and respiration. In addition, these nanoparticles can act on phospholipids and membrane proteins, causing a breakdown in the plasma membrane and changes in its permeability [106]. The main responsible for the oxidation of lipids in E. coli is reactive oxygen [105]. Electron microscopy analyses indicated the fragmentation of E. coli after treatment with silver nanoparticles [106]. Gram-negative bacteria exhibited no resistance to the antimicrobial action of silver [2].
The difference between the action of silver nanoparticles on Gram-positive and Gram-negative bacteria is related to the structure of the peptidoglycan cell wall. When comparing inhibition between Escherichia coli and Staphylococcus aureus, the latter being considered as a model microorganism for Gram-positive bacteria studies, it was observed that Gram-negative bacteria are more easily inhibited than Gram-positive ones [107]. Gram-positive bacteria also show changes in membrane permeability and protein composition in the respiratory chain, and the formation of ROS [107]. Oxidative stress in Gram-positive bacteria is more abrupt than in Gram-negative ones. As in Gram-negative bacteria, high ROS concentrations lead to protein degradation by activation of the proteolytic pathway and lipid oxidation. However, in S. aureus, the hydroxyl radical is responsible for lipid oxidation. As in Gram-negative microorganisms, there are also changes in membrane potential, as well as DNA degradation in Gram-positive bacteria [105].
When the mechanism of action of silver nanoparticles in bacteria and fungi is compared, the aggregation of nanoparticles only occurs in eukaryotic cells, resulting in larger particles [98]. In Candida species, it has been shown that the toxic action of AgNP is related both to the ROS-mediated pathway, inducing dysfunctional mitochondrial apoptosis, and to the ROS-independent pathway, culminating in the same cell death outcome [108]. Similar to the antibacterial action, in Candida species, AgNP acts by interfering with the membrane potential, in its integrity and fluidity, in its growth, and in the cell cycle [108][109]. In addition, the synthesis method influences the action of silver nanoparticles, with biosynthesis showing better results [110].
References
- Peng, J.Y.; Botelho, M.G.; Matinlinna, J.P. Silver compounds used in dentistry for caries management: A review. J. Dent. 2012, 40, 531–541.
- Duran, N.; Marcato, P.D.; Conti, R.D.; Alves, O.L.; Costa, F.; Brocchi, M. Potential Use of Silver Nanoparticles on Pathogenic Bacteria, their Toxicity and Possible Mechanisms of Action. J. Braz. Chem. Soc. 2010, 21, 949–959.
- Gupta, S.; Jangir, O.P.; Sharma, M. The green synthesis, characterization and evaluation of antioxidant and antimicrobial efficacy of silver and gold nanospheres synthesized using wheat bran. Asian J. Pharm. Clin. Res. 2016, 9, 103–106.
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, e30.
- Porenczukl, A.; Grzeczkowicz, A.; Maciejewska, I.; Gołaś, M.; Piskorska, K.; Kolenda, A.; Gozdowski, D.; Kopeć-Swoboda, E.; Granicka, L.; Olczak-Kowalczyk, D. An initial evaluation of cytotoxicity, genotoxicity and antibacterial effectiveness of a disinfection liquid containing silver nanoparticles alone and combined with a glass-ionomer cement and dentin bonding systems. Adv. Clin. Exp. Med. 2019, 28, 75–83.
- Brennan, S.A.; Fhoghlú, C.N.; Devitt, B.M.; O’mahony, F.J.; Brabazon, D.; Walsh, A. Silver nanoparticles and their orthopaedic applications. Bone Jt. J. 2015, 97, 582–589.
- Zhang, Y.; Zheng, Y.; Li, Y.; Wang, L.; Bai, Y.; Zhao, Q.; Xiong, X.; Cheng, Y.; Tang, Z.; Deng, Y.; et al. Tantalum nitride-decorated titanium with enhanced resistance to microbiologically induced corrosion and mechanical property for dental application. PLoS ONE 2015, e0130774.
- Lara, H.H.; Ayala-Nuñez, N.V.; Turrent, L.D.C.I.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621.
- Panáček, A.; Kvítek, L. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71.
- Mackiewicz, A.; Olczak-Kowalczyk, D. Microscopic evaluation of surface topography and chemical composition of Nanocare Gold. J. Stomatol. 2014, 67, 826–840.
- Kiriyama, T.; Kuroki, K.; Sasaki, K.; Tomino, M.; Asakura, M.; Kominami, Y.; Takahashi, Y.; Kawai, T. Antibacterial properties of a self-cured acrylic resin composed of a polymer coated with a silver-containing organic composite antibacterial agent. Dent. Mater. J. 2013, 32, 679–687.
- De-Deus, G.; Brandão, M.C.; Fidel, R.A.S.; Fidel, S.R. The sealing ability of GuttaFlow™ in oval-shaped canals: An ex vivo study using a polymicrobial leakage model. Int. Endod. J. 2017, 40, 794–799.
- Patil, P.; Rathore, V.P.; Hotkar, C.; Savgave, S.S.; Raghavendra, K.; Ingale, P. A comparison of apical sealing ability between GuttaFlow and AH plus: An in vitro study. Int. Soc. Prev. Community Dent. 2016, 6, e377.
- Nozari, A.; Ajami, S.; Rafiei, A.; Niazi, E. Impact of Nano Hydroxyapatite, Nano Silver Fluoride and Sodium Fluoride Varnish on Primary Teeth Enamel Remineralization: An In Vitro Study. J. Clin. Diagnostic. Res. 2017, 11, zc97–zc100.
- Besinis, A.; Peralta, T.; Handy, R.D. The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of biossays. Nanotoxicology 2014, 8, 1–16.
- Chladek, G.; Mertas, A.; Barszczewska-Rybarek, I.; Nalewajek, T.; Żmudzki, J.; Król, W.; Łukaszczyk, J. Antifungal activity of denture soft lining material modified by silver nanoparticles—a pilot study. Int. J. Mol. Sci. 2011, 12, 4735–4744.
- Chandra, A.; Yadav, R.K.; Shakya, V.K.; Luqman, S.; Yadav, S. Antimicrobial efficacy of silver nanoparticles with and without different antimicrobial agents against Enterococcus faecalis and Candida albicans. Dent. Hypotheses 2017, 8, e94.
- Munikamaiah, R.L.; Jain, S.K.; Pal, K.S.; Gaikwad, A. Evaluation of Flexural Strength of Polymethyl Methacrylate modified with Silver Colloidal Nanoparticles subjected to Two Different Curing Cycles: An in vitro Study. J. Contemp. Dent. Pract. 2018, 19, 262–268.
- Chladek, G.; Mertas, A.; Krawczyk, C.; Stencel, R. The influence of silver nanoparticles introduced into RTV-silicone matrix on the activity against Streptococcus mutans. Arch. Mater. Sci. Eng. 2016, 78, 59–65.
- Inkielewicz-Stepniak, I.; Santos-Martinez, M.J.; Medina, C.; Radomski, M.W. Pharmacological and toxicological effects of co-exposure of human gingival fibroblasts to silver nanoparticles and sodium fluoride. Int. J. Nanomed. 2014, 9, e1677.
- Barszczewska-Rybarek, I.; Chladek, G. Studies on the curing efficiency and mechanical properties of Bis-GMA and TEGDMA nanocomposites containing silver nanoparticles. Int. J. Mol. Sci. 2018, 19, 3937.
- Mohammed, H.F.; Riad, M.I. The effect of silver nanoparticles incorporation in the self-etch adhesive system on its antibacterial activity and degree of conversion: An in-vitro study. F1000Research 2019, 8, e244.
- Jonaidi-Jafari, N.; Izadi, M.; Javidi, P. The effects of silver nanoparticles on antimicrobial activity of ProRoot mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM). J. Clin. Exp. Dent. 2016, 8, e22.
- Matsubara, V.H.; Igai, F.; Tamaki, R.; Tortamano Neto, P.; Nakamae, A.E.M.; Mori, M. Use of silver nanoparticles reduces internal contamination of external hexagon implants by Candida albicans. Braz. Dent. J. 2015, 26, 458–462.
- Cataldi, A.; Gallorini, M.; Di Giulio, M.; Guarnieri, S.; Mariggiò, M.A.; Traine, T.; Di Pietro, R.; Cellini, L.; Marsich, E.; Sancilio, S. Adhesion of human gingival fibroblasts/Streptococcus mitis co-culture on the nanocomposite system Chitlac-nAg. J. Mater. Sci. Mater. Med. 2016, 27, e88.
- Freire, P.L.L.; Albuquerque, A.J.R.; Sampaio, F.C.; Galembeck, A.; Flores, M.A.; Stamford, T.; Rosenblatt, A. AgNPs: The New Allies against S. Mutans Biofilm Radhakrishnan—A Pilot Clinical Trial and Microbiological Assay. Braz. Dent. J. 2017, 28, 417–422.
- Chen, S.; Yang, J.; Jia, Y.G.; Lu, B.; Ren, L. A study of 3D-printable reinforced composite resin: PMMA modified with Silver nanoparticles Loaded Cellulose Nanocrystal. Materials 2018, 11, 2444.
- Fujieda, T.; Uno, M.; Ishigami, H.; Kurachi, M.; Wakamatsu, N.; Doi, Y. Addition of platinum and silver nanoparticles to toughen dental porcelain. Dent. Mater. J. 2012, 31, 711–716.
- Fan, W.; Wu, D.; Ma, T.; Fan, B. Ag-loaded mesoporous bioactive glasses against Enterococcus faecalis biofilm in root canal of human teeth. Dent. Mater. J. 2015, 34, 54–60.
- Schwass, D.R.; Lyons, K.M.; Love, R.; Tompkins, G.R.; Meledandri, C.J. Antimicrobial activity of a colloidal AgNP suspension demonstrated in vitro against monoculture biofilms: Toward a novel tooth disinfectant for treating dental caries. Adv. Dent. Res. 2018, 29, 117–123.
- Espinosa-Cristóbal, L.F.; Holguín-Meráz, C.; Zaragoza-Contreras, E.A.; Martínez-Martínez, R.E.; Donohue-Cornejo, A.; Loyola-Rodríguez, J.P.; Cuevas-González, J.C.; Reyes-López, S.Y. Antimicrobial and Substantivity Properties of Silver Nanoparticles against Oral Microbiomes Clinically Isolated from Young and Young-Adult Patients. J. Nanomater. 2019, 2019, ID3205971.
- Gligorijević, N.; Kostić, M.; Tačić, A.; Nikolić, L.; Nikolić, V. Antimicrobial properties of acrylic resins for dentures impregnated with silver nanoparticles. Acta Stomatol. Naissi 2017, 33, 1696–1702.
- Martínez-Robles, Á.M.; Loyola-Rodríguez, J.P.; Zavala-Alonso, N.V.; Martínez-Martínez, R.E.; Ruiz, F.; Lara-Castro, R.H.; Donohué-Cornejo, A.; Reyes-López, S.Y.; Espinosa-Cristóbal, L.F. Antimicrobial properties of biofunctionalized silver nanoparticles on clinical isolates of Streptococcus mutans and its serotypes. Nanomaterials 2016, 6, 136.
- González-Luna, I.V.P.; Martínez-Castañón, G.A.; Zavala-Alonso, N.V.; Patiño-Marin, N.; Niño-Martínez, N.; Móran-Martínez, J.; Ramírez-González, J.H. Bactericide effect of silver nanoparticles as a final irrigation agent in endodontics on Enterococcus faecalis: An ex vivo study. J. Nanomater. 2016, 2016, 7597295.
- Sancilio, S.; di Giacomo, V.; Di Giulio, M.; Gallorini, M.; Marsich, E.; Travan, A.; Tarusha, L.; Cellini, L.; Cataldi, A. Biological responses of human gingival fibroblasts (HGFs) in an innovative co-culture model with Streptococcus mitis to thermosets coated with a silver polysaccharide antimicrobial system. PLoS ONE 2014, 9, e96520.
- Niska, K.; Knap, N.; Kędzia, A.; Jaskiewicz, M.; Kamysz, W.; Inkielewicz-Stepniak, I. Capping agent-dependent toxicity and antimicrobial activity of silver nanoparticles: An in vitro study. Concerns about potential application in dental practice. Int. J. Med. Sci. 2016, 13, e772.
- Nam, K.Y. Characterization and antimicrobial efficacy of Portland cement impregnated with silver nanoparticles. J. Adv. Prosthodont. 2017, 9, 217–223.
- Xiao, S.; Liang, K.; Weir, M.D.; Cheng, L.; Liu, H.; Zhou, X.; Ding, Y.; Xu, H.H. Combining bioactive multifunctional dental composite with PAMAM for root dentin remineralization. Materials 2017, 10, 89.
- Tirupathi, S.; Nirmala, S.V.S.G.; Rajasekhar, S.; Nuvvula, S. Comparative cariostatic efficacy of a novel Nano-silver fluoride varnish with 38% silver diamine fluoride varnish a double-blind randomized clinical trial. J. Clin. Exp. Dent. 2019, 11, e105.
- Siqueira, P.C.; Magalhães, A.P.R.; Pires, F.C.P.; Silveira-Lacerda, E.P.; Carrião, M.S.; Bakuzis, A.F.; Souza-Costa, C.A.; Lopes, L.G.; Estrela, C. Cytotoxicity of glass ionomer cements containing silver nanoparticles. J. Clin. Exp. Dent. 2015, 7, e622.
- Espinosa-Cristóbal, L.F.; López-Ruiz, N.; Cabada-Tarín, D.; Reyes-López, S.Y.; Zaragoza-Contreras, A.; Constandse-Cortéz, D.; Donohué-Cornejo, A.; Tovar-Carrilo, K.; Cuevas-González, J.C.; Kobayashi, T. Antiadherence and antimicrobial properties of silver nanoparticles against Streptococcus mutans on brackets and wires used for orthodontic treatments. J. Nanomater. 2018, 2018, 9248527.
- Ghorbanzadeh, R.; Pourakbari, B.; Bahador, A. Effects of baseplates of orthodontic appliances with in situ generated silver nanoparticles on cariogenic bacteria: A randomized, double-blind cross-over clinical. J. Contemp. Dent. Pract. 2015, 16, 291–298.
- Torres-Mendez, F.; Martinez-Castanon, G.A.; Torres-Gallegos, I.; Zavala-Alonso, N.V.; Patino-Marin, N.; Nino-Martinez, N.; Ruiz, F. Effects of silver nanoparticles on the bonding of three adhesive systems to fluorotic enamel. Dent. Mater. J. 2017, 36, 266–274.
- Wang, Z.; Sun, Y.; Wang, D.; Liu, H.; Boughton, R.I. In situ fabrication of silver nanoparticle-filled hydrogen titanate nanotube layer on metallic titanium surface for bacteriostatic and biocompatible implantation. Int. J. Nanomed. 2013, 8, 2903–2916.
- Nam, K.Y. In vitro antimicrobial effect of the tissue conditioner containing silver nanoparticles. J. Adv. Prosthodont. 2011, 3, 20–24.
- Pokrowiecki, R.; Zaręba, T.; Szaraniec, B.; Pałka, K.; Mielczarek, A.; Menaszek, E.; Tyski, S. In vitro studies of nanosilver-doped titanium implants for oral and maxillofacial surgery. Int. J. Nanomed. 2017, 12, 4285–4297.
- Wu, R.; Zhao, Q.; Lu, S.; Fu, Y.; Yu, D.; Zhao, W. Inhibitory effect of reduced graphene oxide-silver nanocomposite on progression of artificial enamel caries. J. Appl. Oral Sci. 2019, 27, e20180042.
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombrádi, Z.; Hajdu, P.; Hegedús, V.; Rácz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721.
- Venugopal, A.; Muthuchamy, N.; Tejani, H.; Gopalan, A.I.; Lee, K.P.; Lee, H.J.; Kyung, H.M. Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties. Korean J. Orthod. 2017, 47, 3–10.
- Fernandes, G.L.; Delbem, A.C.B.; Do Amaral, J.G.; Gorup, L.F.; Fernandes, R.A.; de Souza Neto, F.N.; Souza, J.A.S.; Monteiro, D.R.; Hunt, A.M.A.; Camargo, E.R.; et al. Nanosynthesis of Silver-Calcium Glycerophosphate: Promising Association against Oral Pathogens. Antibiotics 2018, 7, 52.
- Zhang, N.; Melo, M.A.S.; Antonucci, J.M.; Lin, N.J.; Lin-Gibson, S.; Bai, Y.; Xu, H.H.K. Novel dental cement to combat biofilms and reduce acids for orthodontic applications to avoid enamel demineralization. Materials 2016, 9, 413.
- Cheng, L.; Zhang, K.; Zhou, C.C.; Weir, M.D.; Zhou, X.D.; Xu, H.H.K. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int. J. Oral Sci. 2016, 8, 172–181.
- De Matteis, V.; Cascione, M.; Toma, C.C.; Albanese, G.; De Giorgi, M.L.; Corsalini, M.; Rinaldi, R. Silver Nanoparticles Addition in Poly (Methyl Methacrylate) Dental Matrix: Topographic and Antimycotic Studies. Int. J. Mol. Sci. 2019, 20, 4691.
- Jasso-Ruiz, I.; Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Lara-Carrillo, E.; Toral-Rizo, V.H.; López-Castañares, R.; Morales-Luckie, R.A. Synthesis and Characterization of Silver Nanoparticles on Orthodontic Brackets: A New Alternative in the Prevention of White Spots. Coatings 2019, 9, 480.
- Fatemeh, K.; Mohammad, J.; Samaneh, K. The effect of silver nanoparticles on composite shear bond strength to dentin with different adhesion protocols. J. Appl. Oral Sci. 2017, 25, 367–373.
- Santos, V.E., Jr.; Vasconcelos Filho, A.V.; Targino, A.G.R.; Flores, M.A.P.; Galembeck, A.; Caldas, A.F., Jr.; Rosenblatt, A. A new silver-bullet to treat caries in children Nano Silver Fluoride: A randomised clinical trial. J. Dent. 2014, 42, 945–951.
- Barot, T.; Rawtani, D.; Kulkarni, P. Physicochemical and biological assessment of silver nanoparticles immobilized Halloysite nanotubes-based resin composite for dental applications. Heliyon 2020, 6, e03601.
- Zannella, C.; Shinde, S.; Vitiello, M.; Falanga, A.; Galdiero, E.; Fahmi, A.; Santella, B.; Nucci, L.; Gasparro, R.; Galdiero, M.; et al. Antibacterial Activity of Indolicidin-Coated Silver Nanoparticles in Oral Disease. Appl. Sci. 2020, 10, 1837.
- Marques, L.; Martinez, G.; Guidelli, É.; Tamashiro, J.; Segato, R.; Payão, S.L.; Baffa, O.; Kinoshita, A. Performance on Bone Regeneration of a Silver Nanoparticle Delivery System Based on Natural Rubber Membrane NRL-AgNP. Coatings 2020, 10, 323.
- Guo, C.; Cui, W.; Wang, X.; Lu, X.; Zhang, L.; Li, X.; Li, W.; Zhang, W.; Chen, J. Poly-l-lysine/Sodium Alginate Coating Loading Nanosilver for Improving the Antibacterial Effect and Inducing Mineralization of Dental Implants. ACS Omega 2020, 20, 10562–10571.
- Choi, S.H.; Jang, Y.S.; Jhang, J.H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019847067.
- Yoshida, Y.; Churei, H.; Takeuchi, Y.; Wada, T.; Uo, M.; Izumi, Y.; Ueno, T. Novel antibacterial mouthguard material manufactured using silver-nanoparticle embedded ethylene-vinyl acetate copolymer masterbatch. Dent. Mater. J. 2018, 37, 437–444.
- Keskar, M.; Sabatini, C.; Cheng, C.; Swihart, M.T. Synthesis and characterization of silver nanoparticle-loaded amorphous calcium phosphate microspheres for dental applications. Nanoscale Adv. 2019, 1, 627–635.
- Hanif, A.; Ghani, F. Mechanical properties of an experimental resin based composite containing silver nanoparticles and bioactive glass. PaK J. Med. Sci. 2020, 36, 776–791.
- Paiva, L.; Fidalgo, T.K.S.; da Costa, L.P.; Maia, L.C.; Balan, L.; Anselme, K.; Ploux, L.; Thiré, R.M.S.M. Antibacterial properties and compressive strength of new one-step preparation silver nanoparticles in glass ionomer cements (NanoAg-GIC). J. Dent. 2018, 69, 102–109.
- Fu, C.; Zhang, X.; Savino, K.; Gabrys, P.; Gao, Y.; Chaimayo, W.; Miller, B.L.; Yates, M.Z. Antimicrobial silver-hydroxyapatite composite coatings through two-stage electrochemical synthesis. Surf. Coat. Technol. 2016, 30, 13–19.
- Yang, Y.; Ren, S.; Zhang, X.; Yu, Y.; Liu, C.; Yang, J.; Miao, L. Safety and efficacy of PLGA (Ag-Fe3O4)-coated dental implants in inhibiting bacteria adherence and osteogenic inducement under a magnetic field. Int. J. Nanomed. 2018, 13, 3751.
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The influence of graphene in improvement of physico-mechanical properties in PMMA Denture Base Resins. Materials 2019, 12, 2335.
- Rashad, S.; El-Chaghaby, G.; Elchaghaby, M.A. Antibacterial activity of silver nanoparticles biosynthesized using Spirulina platensis microalgae extract against oral pathogens. Egypt. J. Aquat. Biol. 2019, 23, 261–266.
- Sato, T.P.; Conjo, C.I.; Rossoni, R.D.; Junqueira, J.C.; De Melo, R.M.; Durán, N.; Borges, A.L.S. Antimicrobial and mechanical acrylic resin properties with silver particles obtained from Fusarium oxysporum. Braz. Dent. Sci. 2018, 21, 96–103.
- Hernández-Gómora, A.E.; Lara-Carrillo, E.; Robles-Navarro, J.B.; Scougall-Vilchis, R.J.; Hernández-López, S.; Medina-Solís, C.E.; Morales-Luckie, R.A. Biosynthesis of silver nanoparticles on orthodontic elastomeric modules: Evaluation of mechanical and antibacterial properties. Molecules 2017, 22, 1407.
- Charannya, S.; Duraivel, D.; Padminee, K.; Poorni, S.; Nishanthine, C.; Srinivasan, M.R. Comparative evaluation of antimicrobial efficacy of silver nanoparticles and 2% chlorhexidine gluconate when used alone and in combination assessed using agar diffusion method: An in vitro study. Contemp. Clin. Dent. 2018, 9, S204–S209.
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012, 7, 4777–4786.
- Yang, T.; Li, N.; Wang, X.; Zhai, J.; Hu, B.; Chen, M.; Wang, J. Dual functional AgNPs-M13 phage composite serves as antibacterial film and sensing probe for monitoring the corrosion of chromium-containing dental alloys. Chin. Chem. Lett. 2020, 31, 145–149.
- Chand, K.; Abro, M.I.; Aftab, U.; Shah, A.H.; Lakhan, M.N.; Cao, D.; Mehdi, G.; Mohamed, A.M.A. Green synthesis characterization and antimicrobial activity against Staphylococcus aureus of silver nanoparticles using extracts of neem, onion and tomato. RSC Adv. 2019, 9, 17002–17015.
- Majeed, S.; Khanday, M. Green synthesis of silver nanoparticles using bark extract of Salix alba and its antimicrobial effect against bacteria isolated from dental plaque. Orient. J. Chem 2016, 32, e1611.
- Lazuardi, M.B.; Widiyanti, P.; Supardi, A. Physical evaluation of PCL-AgNPs biocomposites as guided tissue regeneration membrane. J. Teknol. 2020, 82, 155–161.
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotocixology 2017, 11, 327–338.
- Qiao, S.; Cao, H.; Zhao, X.; Lo, H.; Zhuang, L.; Gu, Y.; Shi, J.; Liu, X.; Lai, H. Ag-plasma modification enhances bone apposition around titanium dental implants: An animal study in Labrador dogs. Int. J. Nanomed. 2015, 10, 653–664.
- Saafan, A.; Zaazou, M.H.; Sallam, M.K.; Mosallam, O.; El Danaf, H.A. Assessment of photodynamic therapy and nanoparticles effects on caries models. Open Access Maced. J. Med. Sci. 2018, 6, 1289–1295.
- Munguía-Moreno, S.; Martínez-Castañón, G.A.; Patiño-Marín, N.; Cabral-Romero, C.; Zavala-Alonso, N.V. Biocompatibility and surface characteristics of resin-modified glass ionomer cements with ammonium quaternary compounds or silver nanoparticles: An in vitro study. J. Nanomater. 2018, 2018, 6401747.
- Poggio, C.; Trovati, F.; Ceci, M.; Chiesa, M.; Colombo, M.; Pietrocola, G. Biological and antibacterial properties of a new silver fiber post: In vitro evaluation. J. Clin. Exp. Dent. 2017, 9, e387.
- Omidkhoda, M.; Hasanzadeh, N.; Soleimani, F.; Shafaee, H. Antimicrobial and physical properties of alginate impression material incorporated with silver nanoparticles. Dent. Res. J. (Isfahan) 2019, 16, 372–376.
- Mahross, H.Z.; Baroudi, K. Effect of silver nanoparticles incorporation on viscoelastic properties of acrylic resin denture base material. Eur. J. Dent. 2015, 9, 207–212.
- Fujieda, T.; Uno, M.; Ishigami, H.; Kurachi, M.; Kamemizu, H.; Wakamatsu, N.; Doi, Y. Effects of dental porcelain containing silver nanoparticles on static fatigue. Dent. Mater. J. 2013, 32, 405–408.
- Kielbassa, A.M.; Leimer, M.R.; Hartmann, J.; Harm, S.; Pasztorek, M.; Ulrich, I.B. Ex vivo investigation on internal tunnel approach/internal resin infiltration and external nanosilver-modified resin infiltration of proximal caries exceeding into dentin. PLoS ONE 2020, 15, e0228249.
- Zhu, Y.; Cao, H.; Qiao, S.; Wang, M.; Gu, Y.; Luo, H.; Meng, F.; Liu, X.; Lai, H. Hierarchical micro/nanostructured titanium with balanced actions to bacterial and mammalian cells for dental implants. Int. J. Nanomed. 2015, 10, 6659–6674.
- Cabal, B.; Cafini, F.; Esteban-Tejeda, L.; Alou, L.; Bartolomé, J.F.; Sevillano, D.; López-Piris, R.; Torrecillas, R.; Moya, J.S. Inhibitory effect on in vitro Streptococcus oralis biofilm of a soda-lime glass containing silver nanoparticles coating on titanium alloy. PLoS ONE 2012, 7, e42393.
- Sasabe, E.; Tomomura, A.; Kitamura, N.; Yamamoto, T. Metal nanoparticles-induced activation of NLRP3 inflammasome in human oral keratinocytes is a possible mechanism of oral lichenoid lesions. Toxicol. In Vitro 2020, 62, e104663.
- Metin-Gürsoy, G.; Taner, L.; Akca, G. Nanosilver coated orthodontic brackets: In vivo antibacterial properties and ion release. Eur. J. Orthod. 2017, 39, 9–16.
- Mendes, M.S.; Resende, L.D.; Pinto, C.A.; Raldi, D.P.; Cardoso, F.G.; Habitante, S.M. Radiopacity of Mineral Trioxide Aggregate with and without Inclusion of Silver Nanoparticles. J. Contemp. Dent. Pract. 2017, 18, 448–451.
- Chen, R.; Han, Z.; Huang, Z.; Karki, J.; Wang, C.; Zhu, B.; Zhang, X. Antibacterial activity, cytotoxicity and mechanical behavior of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent. Mater. J. 2017, 36, 693–699.
- Krishnaveni, T.; Ramasubbu, A. Synthesis and characterization of biomimetic hydroxy apatite-silver impregnated soy protein isolate nanocomposites for dental implantations. Asian J. Chem. 2017, 29, 2634–2638.
- Oliveira, M.; Ugarte, D.; Zanchet, D.; Zarbin, A.J. Influence of synthetic parameters on the size, structure, and stability of dodecanethiol-stabilized silver nanoparticles. J. Colloid Interface Sci. 2005, 292, 429–435.
- Siegel, J.; Ondřej, K.; Ulbrich, P.; Kolská, Z.; Slepička, P.; Švorčík, V. Progressive approach for metal nanoparticle synthesis. Mater. Lett. 2012, 89, 47–50.
- Hashim, A. The Delivery of Nanoparticles; IntechOpen: London, UK, 2012; 554p, Available online: (accessed on 5 November 2020).
- Grumezescu, A. Antimicrobial Nanoarchitectonics: From Synthesis to Applications; Elsevier: Amsterdam, The Netherlands, 2017; 564p.
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406.
- Mohanty, S.; Mishra, S.; Jena, P.; Jacob, B.; Sarkar, B.; Sonawane, A. An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch-stabilized silver nanoparticles. Nanomedicine 2012, 8, 916–924.
- Gopinath, V.; Priyadarshini, S.; Loke, M.F.; Arunkumar, J.; Marsili, E.; MubarakAli, D.; Velusamy, P.; Vadivelu, J. Biogenic synthesis, characterization of antibacterial silver nanoparticles and its cell cytotoxicity. Arab. J. Chem. 2017, 10, 1107–1117.
- Mittal, J.; Batra, A.; Singh, A.; Sharma, M.M. Phytofabrication of nanoparticles through plant as nanofactories. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, e043002.
- Lee, Y.J.; Kim, J.; Oh, J.; Bae, S.; Lee, S.; Hong, I.S.; Kim, S.H. Ion-release kinetics and ecotoxicity effects of silver nanoparticles. Environ. Toxicol. Chem. 2012, 31, 155–159.
- Żarowska, B.; Koźlecki, T.; Piegza, M.; Jaros-Koźlecka, K.; Robak, M. New Look on Antifungal Activity of Silver Nanoparticles (AgNPs). Pol. J. Microbiol. 2019, 68, 515–525.
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of microbials. Biotechnol. Adv. 2009, 27, 76–83.
- Quinteros, M.A.; Viviana, C.A.; Onnainty, R.; Mary, V.S.; Theumer, M.G.; Granero, G.E.; Paraje, M.G.; Páez, P.L. Biosynthesized silver nanoparticles: Decoding their mechanism of action in Staphylococcus aureus and Escherichia coli. Int. J. Biochem. Cell B 2018, 104, 87–93.
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; You-Sheng, O.Y.; Chen, Y.B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122.
- Gomaa, E.Z. Silver nanoparticles as an antimicrobial agent: A case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43.
- Radhakrishnan, V.S.; Mudiam, M.K.R.; Kumar, M.; Dwivedi, S.P.; Singh, S.P.; Prasad, T. Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int. J. Nanomed. 2018, 13, 2647–2663.
- Kim, K.J.; Sung, W.S.; Suh, B.K.; Moon, S.K.; Choi, J.S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nanoparticles on Candida albicans. Biometals 2009, 22, 235–242.
- Ballottin, D.; Fulaz, S.; Cabrini, F.; Tsukamoto, J.; Duran, N.; Alves, O.L.; Tasic, L. Antimicrobial textiles: Biogenic silver nanoparticles against Candida and Xanthomonas. Mater. Sci. Eng. C 2017, 75, 582–589.




