Silver nanoparticles (AgNPs) have been successfully applied in several areas due to their significant antimicrobial activity against several microorganisms. In dentistry, AgNP can be applied in disinfection, prophylaxis, and prevention of infections in the oral cavity.
- endodontics
- nanotechnology
- oral microbiology
- periodontology
- prosthetics
1. Introduction
The use of silver in dentistry has been documented since 1840, mainly in the prevention and treatment of dental caries [1]. Initially, it was used as silver nitrate (AgNO3), and then in association with fluorine (AgF). In the 2000s, silver started to be also used in restorative materials such as silver amalgam. In the 20th century, the study of nanomaterials started a new field in health sciences, then named nanotechnology. The nanometric dimension of the particles used in this new field altered the usual properties of biomaterials, showing new characteristics, processability, and capabilities [2].
Among metallic nanoparticles, silver nanoparticles (AgNP) have stood out in scientific research for presenting antimicrobial properties and biological activity against bacteria, fungi, and enveloped viruses [3][4][3,4]. The mechanism of action of AgNPs is mainly associated with the release of cationic silver and its oxidative potential [5]. Particle size and shape can also influence the mechanism of action of AgNPs, as well as their synthesis.
Therefore, silver nanoparticles emerged as a promising compound to be used in dentistry, since the incorporation of antimicrobial substances in dental biomaterials has been a strategy adopted by some researchers [6][7][6,7]. Silver nanoparticles have already proved to be effective against several multi-drug-resistant microorganisms [8][9][8,9]. However, the commercial use of silver nanoparticles (NP) in dentistry is incipient, with only three products with AgNPs in their composition being commercially available: dental adhesive (NanoCare Gold DNT™) [5][10][5,10]; Novaron AG300 (Toagosei Co Ltd., Tokyo, Japan) [11]; and sealer (GuttaFlow™ Coltène-Whaledent) [12][13][12,13].
2. Synthesis of Silver Nanoparticles
Silver nanoparticles are synthesized using a precursor (often silver nitrate), a reducing agent that reduces silver ions from Ag+ to Ag0, and a stabilizing agent that ensures the stabilization of suspended nanoparticles and prevents nucleation and aggregation, since metallic nanoparticles have a high surface energy. Therefore, the synthesis of silver nanoparticles can be chemical, physical, or biological (Figure 1). In dentistry, the most common synthesis is the chemical route, as shown in Table 1.
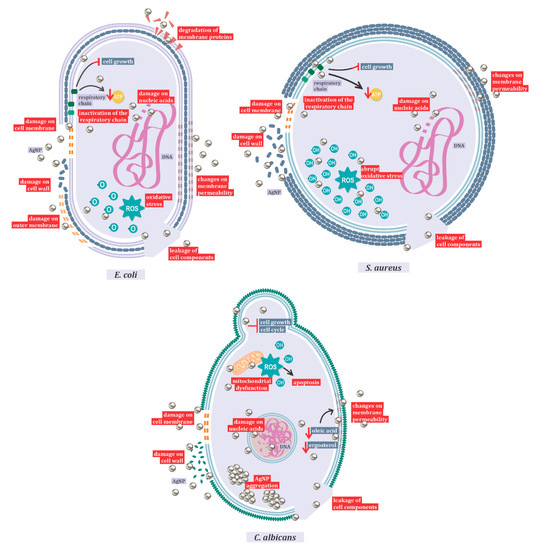
Figure 1. Mechanisms of action of AgNPs against Candida albicans, Escherichia coli, and Staphylococcus aureus.
Table 1. Synthesis methods of silver nanoparticles used in dentistry.
| Synthesis Method | References | Total | % |
|---|
| Commercial synthesis | [5][14][15][16][17][18][19][20][21][22][23][24][5,14,15,16,17,18,19,20,21,22,23,24] | 14.6 | |
| Chemical synthesis | [25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] [41][42][4151,42[43][44][45][46][47],43],44[48][49][50][[52,45][53,46][54,47],48[,4955][56][57,50],51,52,53,54,55,56,57] [58][59][60][58,59,60] |
43.9 | |
| Physical synthesis | [61][62][63][64][61,62,63,64] | 4.9 | |
| Physicochemical synthesis | [65][[68][6566][67],66,67,68] | 4.9 | |
| Biosynthesis | [3][69][70][71][72][73][74][75][76][77][3,69,70,71,72,73,74,75,76,77] | 12.3 | |
| Uninformed | [78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93] | 19.5 |
The synthesis of AgNP is based on the chemical reduction of Ag+1 to Ag0. The differentiation between chemical processes is represented by the reduction agents and stabilizers used, such as sodium citrate, ascorbate, sodium borohydride (NaBH4), elemental hydrogen, polyol process, Tollens reagent, n,n-dimethylformamide (DMF), and poly (ethylene glycol)-block copolymers. Several protective agents (stabilizers) have been used, such as thiols, amines, acids, alcohols [93], and polymeric compounds such as chitosan [25][26][56][25,26,56] and polymethylmethacrylate [27][51][53][68][92][27,51,53,68,92]. These agents stabilize dispersive NPs during their synthesis and protect NPs that can be absorbed on, or bind onto, nanoparticle surfaces, avoiding their agglomeration and sedimentation [94].
Physical synthesis uses ultraviolet irradiation [61][65][61,65], thermal synthesis [62], and spray pyrolysis [63]. In addition, other researchers have reported unconventional synthesis by direct metal sputtering into anhydrous glycerol [95]. However, the essential approaches to physical synthesis include evaporation-condensation and laser ablation [96]. The benefit of physical approaches is the absence of solvent contamination in the preparation of thin films, the uniformity of nanoparticle distribution, high purity, and quick processing time. Small-scale production, high energy consumption, and thermal stability have been described as disadvantages [96][97][96,97].
However, the release of silver nanoparticles and harmful reducing agents such as sodium borohydride into the environment has become a concern. Thus, there is a search for low-cost synthesis processes and eco-friendly methods, which do not use toxic chemicals in synthesis protocols [98]. The biological synthesis emerges as a sustainable alternative and as an attempt to make the process less complicated compared to chemical and physical syntheses. It uses prokaryotic organisms, such as bacteria and eukaryotic organisms such as fungi and plants as potential reducing agents. In this method, the selection of solvents and nontoxic stabilizing agents are also taken into consideration. Our research showed that in dentistry, the most common organisms used to synthesize silver nanoparticles are plants (Table 2). Another advantage of this method is that it increases biocompatibility in living organisms, which is a desired feature for its use in human and veterinary health fields [99][100][99,100]. It is important to notice that the effectiveness of biosynthesized silver nanoparticles is related to the stabilization of the metal core with biological polymers [98][101][98,101].
Table 2. Species used for the biosynthesis of silver nanoparticles.
| Reference | Kingdom | Species |
|---|
| [69] | Algae | Spirulina platensis |
| [70] | Fungae | Fusarium oxysporum |
| [71] | Plantae | Heterotheca Inuloides |
| [72] | Plantae | Cassia roxburghii |
| [73] | Plantae | Geranium maculatum |
| [75] | Plantae | Allium cepa, Azadirachta indica, Solanum lycopersicum |
| [76] | Plantae | Salix alba |
| [77] | Plantae | Aloe vera |
| [3] | Plantae | Triticum aestivum |
| [74] | Viridae | M13 phage |
3. Types of AgNPs Used in Dentistry
The biological activity of AgNPs, like other products containing silver, occurs through the gradual release of silver as a consequence of redox reactions in the presence of water [102]. In addition, the antiproliferative action against bacteria, fungi, and viruses is related to the nanoparticle size and shape, in which sizes smaller than 10 nm have higher antimicrobial activity [103]. The diversity in sizes and shapes can be explained by the different nano-ionic origins of nanoparticles [14].
In dentistry, silver nanoparticles are used in association with composites, such as Chitalac-Ag [25], AgNP-methyl polymethylmethacrylate [53][73][53,73], amorphous calcium AgNP-phosphate [52], and fluorides (Nano Silver Fluoride) [26]. It can also be used alone in the form of silver nanoparticles or silver plasma [79][87][79,87].
4. Mechanisms of Action of AgNPs
Silver nanoparticles are frequently associated with their antimicrobial and antioxidant activities [3]. The action of silver nanoparticles is mainly related to their nanoscale, which alters the level of silver ion release and interferes with the surface energy [5]. Nanoparticles show good antimicrobial effects due to their large surface area, providing high contact with microorganisms when compared to other antimicrobial agents [104].
The action of AgNPs against several microorganisms, including bacteria, fungi, and viruses, has already been described, showing their therapeutic potential [4]. Even multi-resistant bacteria are susceptible to AgNP, which indicates that the mechanisms that confer the resistance of these strains to commercial antibiotics have no protective activity when exposed to nanoparticles [8].
One of the most important mechanisms of action of AgNP is represented by the induction of reactive oxygen species (ROS) production, and hydroxyl radicals are the main species responsible for the oxidative damage [105]. However, it also damages the membrane and cell walls, interferes in the respiratory chain, exhausts the levels of intracellular ATP, and shatters nucleic acids [3][5][3,5]. This mechanism of action varies with nanoparticle size and shape, and with the different target species. In this review, the mechanism of antibacterial action against Gram-positive and Gram-negative bacteria and the antifungal mechanism against Candida albicans (Figure 1) were highlighted. In Gram-negative bacteria, with Escherichia coli as a representative species, studies have shown action primarily on the outer membrane, resulting in the leakage of cell components.
After entering the cell, it has also been shown that AgNPs inactivate the respiratory chain dehydrogenases, inhibiting cell growth and respiration. In addition, these nanoparticles can act on phospholipids and membrane proteins, causing a breakdown in the plasma membrane and changes in its permeability [106]. The main responsible for the oxidation of lipids in E. coli is reactive oxygen [105]. Electron microscopy analyses indicated the fragmentation of E. coli after treatment with silver nanoparticles [106]. Gram-negative bacteria exhibited no resistance to the antimicrobial action of silver [2].
The difference between the action of silver nanoparticles on Gram-positive and Gram-negative bacteria is related to the structure of the peptidoglycan cell wall. When comparing inhibition between Escherichia coli and Staphylococcus aureus, the latter being considered as a model microorganism for Gram-positive bacteria studies, it was observed that Gram-negative bacteria are more easily inhibited than Gram-positive ones [107]. Gram-positive bacteria also show changes in membrane permeability and protein composition in the respiratory chain, and the formation of ROS [107]. Oxidative stress in Gram-positive bacteria is more abrupt than in Gram-negative ones. As in Gram-negative bacteria, high ROS concentrations lead to protein degradation by activation of the proteolytic pathway and lipid oxidation. However, in S. aureus, the hydroxyl radical is responsible for lipid oxidation. As in Gram-negative microorganisms, there are also changes in membrane potential, as well as DNA degradation in Gram-positive bacteria [105].
When the mechanism of action of silver nanoparticles in bacteria and fungi is compared, the aggregation of nanoparticles only occurs in eukaryotic cells, resulting in larger particles [98]. In Candida species, it has been shown that the toxic action of AgNP is related both to the ROS-mediated pathway, inducing dysfunctional mitochondrial apoptosis, and to the ROS-independent pathway, culminating in the same cell death outcome [108]. Similar to the antibacterial action, in Candida species, AgNP acts by interfering with the membrane potential, in its integrity and fluidity, in its growth, and in the cell cycle [108][109][108,109]. In addition, the synthesis method influences the action of silver nanoparticles, with biosynthesis showing better results [110].
