
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Payam Kalhor | + 5102 word(s) | 5102 | 2021-02-23 07:12:18 | | | |
| 2 | Payam Kalhor | + 16 word(s) | 5118 | 2021-03-16 02:53:47 | | | | |
| 3 | Payam Kalhor | + 16 word(s) | 5118 | 2021-03-16 02:55:24 | | | | |
| 4 | Camila Xu | Meta information modification | 5118 | 2021-03-18 03:28:49 | | |
Video Upload Options
Deep eutectic solvents (DESs) are promising green solvents, due to their versatility and properties such as high biodegradability, inexpensiveness, ease of preparation and negligible vapor pressure. They have been employed as green catalysts in biomass transformations and its upgrading into valuable chemicals.
1. Introduction
In 2003, Abbott et al. proposed the concept of deep eutectic solvents (DESs) as a new generation of ionic liquid (IL) analogs, and probably the most novel class of solvents, composed of two or three components with a relatively large depression of melting points relative to those of the ideal liquid mixtures [1]. These components, performing the role of either hydrogen bond donors (HBDs) or hydrogen bond acceptors (HBAs) can be Lewis or Brønsted acids and bases involving a variety of neutral and ionic species [2]. The environmentally friendly properties of DESs such as low vapor pressure, recyclability and low toxicity have motivated research on the applications of DESs as alternative novel solvents to common organic ones [2][3]. DESs have found applications in several fields such as biofuel production [4][5], bio-oil production [6][7], catalysis [8][9][10][11][12], extraction [13][14][15] and separation processes [16][17].
They are easily prepared with usually no need for purification [3][18]. A remarkable property of DESs is the possibility to tailor the solvent in a task-specific way [2] and to a greater extent compared to ILs [19] that are already very good in this regard. This can be done by varying the molar ratio of the components, substituting a component with the one that brings the favorable property or by simply adding a specific amount of a cosolvent [20][21][22] such as water [23][24][25][26]. The DES–cosolvent preparation is especially important as most DESs have high viscosities [2] due to the extensive hydrogen bonding (H-bonding) networks [27][28] and van der Waals and electrostatic forces between species [29]. The high viscosity of the DESs can be mitigated via mixing with water [23][24][25][26]. However, care should be taken when diluting a DES with a cosolvent as the fundamental and desired properties of the DES may be affected by the cosolvent [25]. Most of the distinct properties of DESs are because of some specific underlying intermolecular interactions, with the most important one being the H-bonding. For instance, the significant decrease in the melting point of a typical DES is assumed to arise from the charge transfer between components, usually from the halide anion of an HBA to the HBD through H-bonds [22][26][30]. It has been found that as the H-bonds between HBD and HBA strengthen, the melting points depress more [1]. Depending on the DES constituents and their molar ratio, DESs may have reduced thermal stability [31][32][33].
The diminishing reserves of easily available fossil fuels and growing concerns about the global pollution as well as the upward demand for energy have significantly affected the research directions towards developing sustainable energy resources [34][35][36][37][38][39][40][41][42][43][44]. Therefore, inventive and appropriate uses of the naturally abundant supplies are important towards a sustainable future. As a very promising alternative to fossil fuels, biomass is increasingly drawing attention. Biomass is widely abundant, distributed worldwide and relatively inexpensive and has been used to produce various value-added chemicals [45][46][47][48][49][50][51]. Biomass transformation into valuable chemicals not only has revived the green chemistry principles but has also paved the way to alleviate the current high reliance on fossil fuels [48]. However, the optimization of chemical transformations towards a sustainable methodology and investigation of pivotal factors affecting process efficiency remain as challenges. Among all the factors affecting the conversion efficiency and the selectivity of the obtained products, the development and design of catalysts play a crucial role [52][53][54][55][56][57][58][59]. Catalysts have been regarded as important tools to accomplish a more sustainable chemical industry [56]. In this context, various catalytic systems have been proposed to produce upgraded chemicals and value-added products, with the purpose of drawing the full chemical potential of the biomass [11][53][55][60][61][62]. In recent years, DESs have been extensively used as active catalysts in valorization and upgrading of biomass in various types of reactions [10][63] where they integrate the advantages of both homogenous and heterogeneous catalysts [64][65]. The catalytic DESs can dissolve a wide range of reactants and consequently change a heterogeneous catalytic mechanism into a homogeneous one [66].
The catalytic DESs can be categorized as either Lewis acid-type or Brønsted acid-type DESs [10]. The Lewis acid-type DESs consist of a few DESs, usually chlorides of transition metals such as Zn [67], Fe [68] and Cr [69] combined with mainly choline chloride (ChCl) in different molar ratios. On the other hand, there are diverse Brønsted acid-type DESs mostly containing ChCl combined with organic acids such as oxalic acid [70], citric acid [71], acetic acid [72], malonic acid [8], formic acid [73], p-toluene sulfonic acid (p-TSA) [74] or alcohols such as ethylene glycol [75] or glycerol [76] or amides such as urea [77]. Figure 1 shows the general scheme for the conversion of biomass to value-added chemicals using catalytic biomass.

Figure 1. Conversion of biomass to value-added chemicals using catalytic deep eutectic solvents (DESs).
2. Catalytic Application of Deep Eutectic Solvents in Upgrading Biomass
Table 1 lists a collection of processes where DESs are employed as either Lewis acid-type or Brønsted acid-type catalysts to convert biomass to value-added chemicals.
Table 1. Catalytic DESs used in biomass upgrading.
| DES | Feedstock | Process | Conditions/Results | DES Recyclability | Ref. |
|---|---|---|---|---|---|
| Lewis acid-type DESs | |||||
| ChCl:2FeCl3 | Bagasse lignin | Fractionation of lignin | 74% selectivity for methyl p-hydroxycinnamate, after 8 h, at 160 °C. | 6 runs | [78] |
| ChCl:ZnCl2 ChCl:2ZnCl2 ChCl:3ZnCl2 |
Soybean oil | Transesterification of soybean oil to biodiesel | 55% transesterification yield. 16:1, Methanol: oil ratio. (ChCl:2ZnCl2) DES 10% at 70 °C for 72 h. | Unable to recycle | [67] |
| ChCl:2FeCl3 | Seaweed | Production of Fe3O4/Fe-doped graphene nanosheets (GNs) from seaweed | Formation of Fe3O4/Fe-GN with high surface area and electrical conductivity under 95% N2 and 5% H2, pyrolysis of DES + seaweed at 700–900 °C | Not reported | [68] |
| 10dimethylurea:3ZnCl2 | Cellulose | Synthesis of cellulose methyl carbamate (CMeC) | The degree of substitution was 0.17 after 3 h of reaction at 150 °C. | Not reported | [79] |
| FeCl3·6H2O-based DESs (different ratios) | Cellulose | Conversion of cellulose to gluconic acid | 2FeCl3·6H2O:ethylene glycol DES provided the highest yield (53%) of gluconic acid at 120 °C for 60 min. | 5 runs (FeCl3·6H2O:ethylene glycol) | [66] |
| 4dimethylurea: Zn(OAc)2 |
Polyethylene terephthalate (PET) | Glycolysis of PET to yield bis(hydroxyalkyl) terephthalate (BHET) | With 5 g PET, 20 g ethylene glycol, 0.25 g DES at 190 °C for 20 min, the yield of BHET was 82%. | 6 runs | [80] |
| ChCl:4.43oxalic acid·2H2O: 0.1FeCl3·6H2O |
Bleached eucalyptus Kraft pulp (BEKP) |
Fabricate cellulose nanocrystals (CNCs) from BEKP | The yield of CNCs was higher than 90% under mild conditions, i.e., 80 °C and 6 h. | 3 runs | [81] |
| Brønsted acid-type DESs | |||||
| ChCl:oxalic acid | Corncob | Change corncob to furfuryl alcohol | 46% furfural alcohol yield at 120 °C for 30 min. | 3 runs | [82] |
| ChCl:p-TSA 2 | Alkali lignin | Degradation of alkali lignin (cleavage of β-O-4) | At 130 °C, the content of phenolic hydroxyl species increased. Alakali lignin underwent decarbonylation during treatment. | Not reported | [83] |
| ChCl:2urea ChCl:2ZnCl2 ChCl:2CrCl3· 6H2O ChCl:malonic acid ChCl:oxalic acid·2H2O 2ChCl:citric acid·H2O 2ChCl:citric acid |
Fructose | Conversion of fructose to hydroxymethylfurfural (HMF) | Most DESs were effective to convert fructose (91–100%). The Lewis acid-based DESs were not efficient to produce HMF. More than 90% of fructose conversion was obtained with ChCl:malonic acid/oxalic acid/citric acid·H2O at 80 °C for 1 h | 8 runs (2ChCl:citric acid·H2O) | [69] |
| ChCl:oxalic acid·2H2O 2ChCl:citric acid·H2O |
Inulin | Conversion of inulin to HMF | Using (ChCl: oxalic acid·2H2O) and (2ChCl: citric acid·H2O), at 80 °C for 2 h, the yields of HMF were 64 and 57%, respectively. | 6 runs (ChCl:oxalic acid·2H2O) | [70] |
| ATPPB 1:3p-TSA 2 | Low-grade crude palm oil (LGCPO) | Biodiesel production from LGCPO via esterification process | The esterification in 1 wt% DES, 10:1 methanol to LGCPO, at 60 °C in 30 min. <2% free fatty acid (FFA). | 4 runs | [84] |
| 2ChCl:citric acid·H2O | Xylan and xylose | Conversion of xylan and xylose to furfural, co-catalyzed by the DES and metal chlorides | In monophasic approach (DES + metal chloride), furfural yield from xylose and xylan were 59 and 54%, respectively. The yields increased to 73.1 and 67% in biphasic system (DES + metal chloride + methyl isobutyl ketone (MIBK)). | 5 runs | [71] |
| DEACl 3:3p-TSA 2 | Crude palm oil | Decreasing the level of FFAs for biodiesel production | The FFAs were reduced to <1%. The DES: palm oil was 0.75% (wt/wt). Methanol:oil ratio was 8:1 at 60 °C for 30 min. | 4 runs | [85] |
| ChCl:3p-TSA 2 | Acidic crude palm oil (ACPO) | Biodiesel production from ACPO (conversion of FFAs in ACPO to fatty acid methyl esters (FAME)) | The 1:10 molar ratio of Methanol:oil with 0.75 mass ratio of DES:ACPO reduced FFAs to <1% at 60 °C in 30 min. | 3 runs | [86] |
| ChCl:oxalic acid | Xylose and xylan | Furfural production from xylose and xylan using monophasic (DES) or biphasic (DES + MIBK) systems | Addition of metal chlorides to the DES led to improved furfural yields (14–44%). In the biphasic procedure, the yields from xylose and xylan were, respectively, 60 and 56% in AlCl3·6H2O presence. | 5 runs | [87] |
| ChCl:p-TSA 2 (1:0.5 to 1:2) | Fructose | Conversion of fructose to 5-HMF | 91% 5-HMF yield at 80 °C for 1 h in (ChCl: p-TSA) DES. | Not reported | [88] |
| ChCl:2urea ChCl:4p-TsOH 2 ChCl:3glycerol |
Pyrolysis oil (PO) | Esterification of acids in PO to fatty acid methyl esters (FAME) | Highest total acid number (TAN) reduction (86.62%) was achieved with (ChCl:4p-TsOH) DES with 1:50 molar ratio of oil: methanol in 40 min. | Not reported | [76] |
| ChCl:4p-TSA 2 | Pongamia pinnata seed oil | Biodiesel production from seed oil using either silica support DES (So-DES) and no support DES (Un-DES) | Using So-DES at 353 K for 240 min with catalyst loading of 5 (wt%/v), the biodiesel conversion was 89%. Using Un-DES at 343 K for 120 min with catalyst loading of 1 (wt%/v), the biodiesel conversion was 98%. |
4 runs (Un-DES) 7 runs (So-DES) |
[64] |
| ChCl:4KOH ChCl:4p-TsOH 2 ChCl:3glycerol ChCl:3FeCl3 |
De-oiled Jatropha curcas cake | Hydrothermal liquefaction of de-oiled Jatropha curcas cake to produce biocrude oil | DESs formed with HBDs preferentially favored the production of aromatic oil through condensation and hydrolysis of lipids. The highest biocrude yield was achieved by (ChCl: 4KOH) DES (44%). | Not reported | [6] |
| DEACl 3:0.5p-TSA 2 | Fructose | Dehydration of fructose to 5-HMF | 85% HMF yield at 80 °C with a 5% feed ratio at 1 h. | Not reported | [89] |
| ChCl:acetic acid ChCl:lactic acid ChCl:levulinic acid ChCl:glycerol |
Hardwood (poplar) and softwood (D. fir) | Extraction of lignin from woody biomass | The purity of the extracted lignin was 95%. The DESs could selectively cleavage the ether linkage in wood. | Not reported | [72] |
| ChCl:3p-TSA 2 ChCl:5p-TSA 2 ChCl:7p-TSA 2 |
Glycerol and whole Jatropha curcas seed | Co-liquefaction of glycerol and whole Jatropha curcas seed to produce biocrude oil | The biocrude oil yield was 9-wt% with 30-wt% glycerol, 6 wt% moisture, 22 wt% oxygen content and 1:3 molar ratio of the DES. | Not reported | [7] |
| ATPPB1:3p-TSA 2 | Oleic acid | Esterification of FFAs with glycerol | With 5 wt% DES and 6:1 molar ratio of glycerol: oleic acid and at 150 °C for 30 min, 95% of FFA is converted to yield 85% mono- and di-glyceride. | 5 runs | [90] |
| ChCl:oxalic acid·2H2O ChCl:malonic acid ChCl:citric acid·H2O |
Soybean oil | Epoxidation of soybean oil | The (ChCl:oxalic acid·2H2O) DES yielded high selectivity (94%) and conversion (89%) at 50 °C for 8 h | 5 runs (ChCl:oxalic acid·2H2O) | [8] |
| ChCl:2formic acid ChCl:4acetic acid ChCl:6glycolic acid ChCl:4levulinic acid |
Herbal residues of Akebia | Ethanol production from herbal residues of Akebia | The maximum levels of lignin, xylan and glucan removal with (ChCl: 6glycolic acid) DES at 120 °C. | Not reported | [73] |
| ChCl:oxalic acid | Fructose | Synthesis of biofuel ethoxymethyl furfural (EMF) from fructose | Under microwave irradiation, 92% of fructose was converted to yield 74% EMF in 3 h at 343 K. | 4 runs | [91] |
| ChCl:oxalic acid | Cellulose and native biomass | Selective conversion of cellulose and native biomass into the low molecular weight saccharides | The conversion yield and total selectivity of carbohydrate are as high as 85% and 98%, respectively. | Not reported | [92] |
| 3DEACl 3:2oxalic acid | Sunflower stalk | Conversion of cellulose to levulinic acid, 5-HMF, furfural and formic acid | The maximum carbon conversion was achieved as 38% at 170 °C in 5 min in microwave reactor. | Not reported | [93] |
| 3ChCl:oxalic acid | Furfural | Oxidation of furfural to maleic acid and fumaric acid | Used H2O2 as the oxidizer at 50 °C. 100% conversion of furfural and the yield of maleic acid and fumaric acid reached 96%. | Not reported | [12] |
| Taurine:3TfOH 4 | Isobutane and isobutene | Alkylation of isobutane and isobutene to high-octane alkylate gasoline | The DES in polyethylene glycol-200 (PEG-200) had a high catalytic activity and good recyclability with 83% conversion and 86% C8 selectivity. | 8 runs | [94] |
| ChCl:lactic acid (1:10 to 1:250) | Eucalyptus globulus chips | Delignification of Eucalyptus globulus chips (increase in cleavage rate of β-O-4) | The pupping experiments were performed at 120 °C for 8 h. The DES: biomass molar ratio was 20:1. | Not reported | [95] |
| ChCl:2malic acid ChCl:3malic acid ChCl:3glycolic acid |
Xylan from Beachwood | Furfural production from xylan | Higher furfural yield with (ChCl: 3malic acid) + 0.5 wt% water under microwave heating (25 min) at 150 °C. | 3 runs (ChCl:3malic acid) | [96] |
| TPAB 5:4ethylene glycol ChCl:2ethylene glycol |
Heavy residual Fuel oil (Mazut280) |
Upgrading Mazut280 to light fuels | (TPAB:4ethylene glycol) DES was more efficient than (ChCl: 2ethylene glycol) DES with higher asphaltene reduction and desulfurization, resulting in high and stable light oil. | Not reported | [97] |
| betaine: oxalic acid·2H2O (1:1 to 1:28) |
α-pinene | Hydration of α-pinene to α-terpineol | The 1:2.7 molar ratio of the DES achieved the best catalytic and recyclability performance. | 5 runs | [98] |
| TBAB 6:2p-TSA 2 TBAC 7:2p-TSA 2 ChCl:2p-TSA 2 BTAB 8:2p-TSA 2 BTAC 9:2p-TSA 2 |
Yellow horn seed | Biodiesel production from the yellow horn seed via extraction of oil and conversion of fatty acid methyl esters | 11 wt% of (TBAB: 2p-TSA) DES exhibited the best catalytic activity with the maximum oil extraction (90%) and fatty acid conversion (97%) at 72 °C for 40 min. | 5 runs (TBAB:2p-TSA) | [74] |
| ChCl:urea (1:1 to 1:4) ChCl:oxalic acid ChCl:benzoic acid ChCl:p-TSA 2 ChCl:2MnCl2 ChCl:2CuCl2 ChCl:2acetamide |
Polycarbonate | Methanolysis of polycarbonate to obtain bisphenol A | The most effective catalyst was (ChCl:2urea) DES with ~100% polycarbonate conversion at 130 °C for 2.5 h. | 5 runs (ChCl:2urea) | [77] |
| urea:2propionic acid ChCl:10lactic acid ChCl:p-TSA 2 |
2-phenoxy-1- phenyl ethanol (PPE) (a lignin model compound) | Cleavage of the β-O-4 ether bond in the model biomass |
(ChCl: p-TSA) demonstrated the highest performance in the PPE cleavage. | Not reported | [99] |
| imidazole:1.5BSA 10 | Fructose | Dehydration of fructose to 5-HMF | The 5-HMF yield was 90.1% at 100 °C in 3 min. | 1 run | [100] |
| ATPPB1:3p-TSA 2 | Vegetable oil deodorizer distillate | Esterification of FFA in vegetable oil into glycerides | Glycerolysis reaction reached equilibrium at FFA conversion of 90%. The optimum condition was determined at 160 °C, 5 wt% of DES in 10 min. | Not reported | [101] |
| ChCl:2oxalic acid | Cotton fiber | Production of CNCs from cotton fiber | The used DES showed a high recyclability (>85%). | 5 runs | [102] |
| ChCl:2acetic acid ChCl:malonic acid ChCl:oxalic acid ChCl:citric acid ChCl:2formic acid 3ChCl:7p-TSA 2 |
Levulinic acid | Esterification of levulinic acid to produce ethyl levulinate | The most active catalyst: (3ChCl:7p-TSA) DES. ~100% yield with 5-wt% of the DES at 353 K for 1 h. | Not reported | [65] |
| ChCl:8formic acid | Furfural, xylose and corncob | Synthesis of cyclic biofuel intermediates | The direct conversion of furfural, xylose, and corncob to cyclic biofuel intermediates were as high as 92, 88, and 57%, respectively. | Not reported | [103] |
| ChCl:2ethylene glycol (CrCl3 had synergistic catalytic effect with ChCl) | Glucose | Dehydration of glucose to 5-HMF | At 150 °C for 3.64 min, the yield of 5-HMF reached 42%. | 4 runs | [75] |
| ChCl:2lactic acid | Isolated lignin from Eucalyptus tree and a series of β-O-4 lignin model compounds | The β-O-4 bonds in either realistic lignin or model compounds were cleaved by the DES |
Increase of the reaction temperature and time resulted in a decrease of insoluble lignin fraction and average molecular weights, as well as a sustained increase of hydroxyl groups. | 1 run | [104] |
| ChCl:10lactic acid | Eucalyptus | Depolymerization of the double enzymatic lignin (DEL) via a novel biorefinery process | DES pretreatment in 60–140 °C for 6 h: cleavage of C-O and C-C bonds in the lignin, dehydration, and acylation of hydroxyl groups of lignin, and recondensation of lignin. | Not reported | [105] |
| ChCl:oxalic acid ChCl:2glycerol |
Moso bamboo | Extraction of phenolic lignin from bamboo by subcritical ethanol catalyzed by DES | At 160 °C, the (ChCl: oxalic acid) DES obtained lignin with high UV-blocking and high phenolic hydroxyl content. | Not reported | [106] |
| ChCl:oxalic acid 2ChCl:oxalic acid:p-TSA 2 |
Softwood thermomechanical pulp (TMP) | Lignin-containing cellulose nanocrystals (LCNCs) from TMP |
LCNCs were isolated from DESs, showing a higher yield (66%) when using the ternary DES (3 h). | Not reported | [107] |
| ChCl:oxalic acid | Native biomass (poly)carbohydrates | The catalytic reactions of the native biomass to transform into value-added chemicals | Conversion yield as high as 68 wt% for glucose, 60 wt% for fructose, 73 wt% for xylose, 14 wt% for 5-HMF & 72 wt% for furfural. 0% for cellulose. | Not reported | [108] |
| ChCl:2formic acid ChCl:2acetic acid ChCl:2lactic acid |
Poplar wood shavings | Extraction of lignin from Poplar biomass and enhance cellulose reactivity | 6.3–7.9% lignin selectivity & delignification (73–77%) along with increase in the available area and porosity of cellulose were achieved. | Not reported | [109] |
| TOAB 11:2p-TsOH 2 | Cooked and waste vegetable oils | Transesterification of the cooked and waste vegetable oils into biodiesel | The catalytic DES enhanced the direct contact between MeOH and oil. The yield of FAME was 99% at 70.5 °C, DES dosage of 24.6 wt%and a molar ratio of 12.5. | 5 runs | [110] |
1 allyl tri-phenyl phosphonium bromide. 2 p-toluene sulfonic acid monohydrate. 3 N,N-di-ethyl ethanol ammonium chloride. 4 tri-fluoro methane sulfonic acid. 5 tetra-propyl ammonium bromide. 6 tetra-butyl ammonium bromide. 7 tetra-butyl ammonium chloride. 8 benzyl tri-methyl ammonium bromide. 9 benzyl tri-methyl ammonium chloride. 10 benzene sulfonic acid. 11 tetraoctyle ammonium bromide. All yields were rounded to the nearest integer.
2.1. Processes Catalyzed by Lewis Acid-Type DESs
Lewis acid-based transition-metal chlorides can catalytically activate the electron-rich substrates due to their electron deficiency [111]. The DESs containing metal chlorides are Lewis acidic. In catalytic upgrading of biomass, there are a few cases with catalytic DESs composed of metal chlorides. However, the most widely used metal chlorides are ZnCl2 [67], FeCl3 [68], CrCl3 [69], MnCl2 [77] and CuCl2 [77] to form DESs often with ChCl. This part discusses the application of DESs as Lewis acid catalysts in biomass conversion.
Long et al. [67] studied the transesterification of soybean oil to biodiesel using (ChCl:xZnCl2) DES as the catalyst, where methanol was used as a solvent. They found that the strength of the Lewis acidity of the DES increased upon increase of x. They suggested that Zn2Cl5− mainly catalyzed the transesterification, as Zn2Cl5− was the predominant species in the mixture. However, the yield of biodiesel was not satisfying because of the weak acidity of Zn2Cl5−. With the addition of 10% (ChCl:2ZnCl2) catalyst and a 16:1 molar ratio of methanol to soybean oil, the conversion rate reached 54.52% after 72 h at 70 °C. Finally, they proposed the transesterification mechanism as shown in Figure 2.
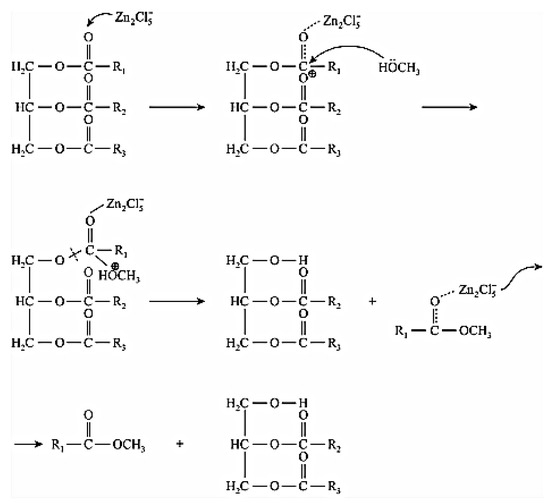
Figure 2. The proposed transesterification mechanism. Reprinted from reference [67] with permission.
Mondal et al. [68] produced Fe3O4/Fe-doped graphene nanosheets (GNs) by pyrolysis of granules of seaweed biomass and used (ChCl:FeCl3) DES as catalyst. The reaction was performed at 700–900 °C under an atmosphere of 95% N2 and 5% H2. The resulted Fe3O4/Fe-doped GN had favorable properties such as high surface area (220 m2·g−1) and high electrical conductivity (2384.6 mS·m−1). It was found that the nanosheets had highly stable oxygen reduction reaction (ORR) electrocatalytic activity after 30,000 cycles. This would make the produced graphene sheets a sustainable substitution for the existing catalysts composed of expensive metal-based ORRs. Figure 3 displays the proposed flowchart for production of Fe3O4/Fe-GN from Sargassum tenerrimum biomass.
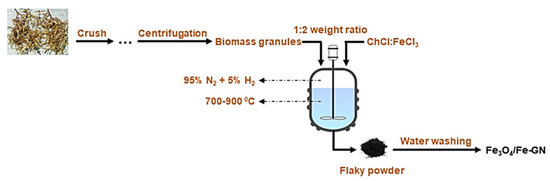
Figure 3. The flowchart for production of Fe3O4/Fe-GN from Sargassum tenerrimum biomass.
The Lewis acid-type DESs have also been employed as catalysts for chemical modifications of cellulose to value-added chemicals. For example, Sirvio et al. [79] found that a reactive DES composed of dimethyl-urea and ZnCl2 (10:3) could be used to synthesize cellulose methyl carbamate (CMeC) at elevated temperature (150 °C). The CMeC had a degree of substitution (DS) of 0.17 after 3 h at 150 °C. The original cellulose fibers had a very poor alkaline solubility, whereas the product with a DS of 0.17 exhibited a good alkaline solubility. In this process, dimethyl-urea degraded to methylamine and methylisocyanate to react with the hydroxyl groups of cellulose. The heat-driven reaction was significantly catalyzed by ZnCl2. Figure 4 illustrates the proposed mechanism for CMeC synthesis.
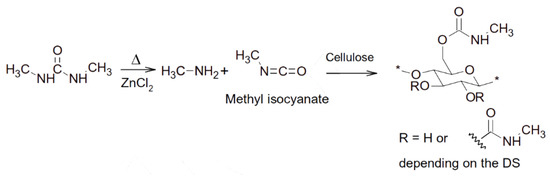
Figure 4. The proposed reaction mechanism for CMeC synthesis. Reprinted from reference [79] with permission.
Liu et al. [66] prepared a series of FeCl3·6H2O-based catalytic DESs with ethylene glycol, glycerol, malonic acid, L-alanine, L-serine, xylitol, pentaerythritol, and glycine as HBDs for transformation of cellulose to gluconic acid where the ferric chloride behaved as the catalyst. Most of the DESs had low viscosities, low melting points, and high conductivities. The high conductivities of the prepared DESs compared to other DESs and ILs are important for their electrochemical applications. Among the DESs, the (FeCl3·6H2O:ethylene glycol) DES showed the best performance, with 100% cellulose conversion and 53% gluconic acid yield in 1 h at 120 °C. The interesting point was that gluconic acid could self-precipitate, and the process of product separation could be skipped. Therefore, the employed method had the advantage of integrating solvent and catalyst as well as reaction and separation. Figure 5 illustrates the experimental procedure to obtain gluconic acid from glucose or cellulose.

Figure 5. The experimental procedure to obtain gluconic acid from glucose or cellulose.
Recently, Yang et al. [81] fabricated cellulose nanocrystals (CNCs) from bleached eucalyptus kraft pulp (BEKP) using a FeCl3-catalyzed DES with a composition of (ChCl:oxalic acid·2H2O:FeCl3·6H2O) in a 1:4.43:0.1 molar ratio. This composition had the best swelling ability and strongest hydrolysis activity. Based on the cellulose content in BEKP, more than 90% CNC yield was obtained by a one-step DES treatment where the reaction took 6 h at 80 °C. Under these conditions, glucose and xylose were also obtained. They found that the resultant CNCs had higher thermal stability than the traditional H2SO4-hydrolyzed ones.
2.2. Processes Catalyzed by Brønsted Acid-Type DESs
The Brønsted acidic DESs involve chemicals with an acidic character that can protonate other compounds for further reactions. The Brønsted acidity of DESs is mainly provided by their organic acid constituents. Therefore, a direct proportionality between the acidity of the HBD and that of the Brønsted acid-type DES is expected [112]. The common organic acids used to prepare Brønsted acid-type DESs include p-TSA [84], oxalic acid [69], malonic acid [69], citric acid [70], formic acid [73], glycolic acid [73], lactic acid [72], acetic acid [73], levulinic acid [72] and propionic acid [99], which are ordered from the strongest (p-TSA) to the weakest (propionic acid) [113][114][115][116][117][118][119]. The DESs involving stronger Brønsted acids can fractionate the biomass more efficiently and consequently have higher catalytic capability [112]. DESs containing amides [69][76][77] and alcohols [76][97] can also perform as Brønsted acidic catalysts, which are inherently less acidic than those composed of organic acids. For example, Kumar et al. [120] found that the organic acid-based DESs could remove lignin and hemicellulose from biomass more efficiently than those DESs based on alcohols or amides. Moreover, the DESs with higher acidic characters were found to be more effective in lignin removal and glucan recovery [121]. However, the effect of the HBAs on the acidity of the liquids should not be ignored. For example, it has been found that the ammonium salts have major roles on the H-bond basicity of DESs [122]. Moreover, it was suggested that as the alkyl side chain of HBD or HBA in a specific DES increases, the solvent is less able to donate protons [122]. In another study on (ChCl:ethylene glycol) DES, it was found that as the ratio of ChCl to ethylene glycol increases, the acidity of the liquid decreases which is due to the relatively more basic character of chloride compared to ethylene glycol [123]. Furthermore, it has been indicated that the higher number of H-bond-forming groups (such as hydroxyl or amide groups) in a DES enhances the fractionation of the lignocellulosic biomass [124][125]. By the catalytic function of Brønsted acidic DESs, the biomass can be upgraded to biodiesel [64][74][84][85][86][94], biofuel [73][91][103], biocrude [6][7], hydroxymethylfurfural (HMF) [69][70][75][88][89][93][100], furfural [71][87][93][96][108] and other value-added chemicals [8][12][65][76][77][92][93][98][102][108] such as organic acids [12][93] and glucose and xylose [92][108]. The Brønsted acidic DESs can also be used in esterification of free fatty acids (FFAs) to glycerides [90][101] and delignification of biomass [72][95][99][104][105][106][107][109].
3. Recyclability of Catalytic DESs
In the chemical industry, recycling technology is an important stage towards sustainability [85]. Recyclability usually involves separation, purification and reusing a solvent or catalyst in successive steps, namely recovering step, refining step and recycling (reusing) step [47]. The whole process greatly affects the solvent or catalyst’s utilization in industrial processes from economic and environmental aspects. In general, the more recyclable a solvent or catalyst, the better it is. In most cases, the chemical efficiency is directly correlated with the recyclability. The recyclability of the applied catalytic DESs have been evaluated in several studies. For example, Song et al. [77] performed a reusability experiment of ChCl:2urea DES in methanolysis of polycarbonate under the optimum reaction conditions. They found that the DES could be reused five times with no significant reduction in polycarbonate conversion, indicating the good reusability of the DES. In the process of conversion of cellulose to gluconic acid, Liu et al. [66] reported no loss of the catalytic activity of 2FeCl3·6H2O:ethylene glycol DES, which was due to the self-precipitation of gluconic acid from the reaction system. Liu et al. [80] used the catalytic DES (1,3-dimethylurea:ZnCl2) in 4:1 molar ratio to convert polyethylene terephthalate (PET) to bis(hydroxyalkyl) terephthalate (BHET). As is shown in Figure 6, the catalyst worked efficiently after being recycled five times.
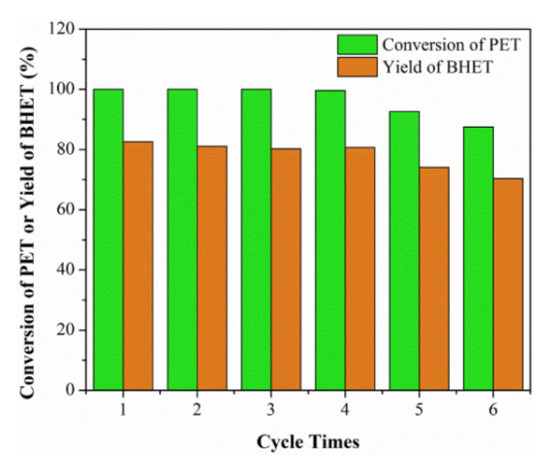
Figure 6. Recycling efficiency of the employed catalyst. Reprinted from reference [80] with permission.
The recyclability of (ChCl:oxalic acid·2H2O) DES, used as a catalyst in the conversion of inulin to 5-HMF, was investigated. The results indicated that activity of the recycled DES was very high even after utilization six times. The same DES was investigated for its reusability as a catalyst in the epoxidation of soybean oil. It was found that ChCl:oxalic acid·2H2O DES had good catalytic activity, with 90% selectivity after five recycle runs under optimum conditions. Hayyan et al. studied the recyclability of (ATPPB:3p-TSA) DES employed as a catalyst in biodiesel production from LGCPO. They found that the DES had a high conversion yield in the first three recycle runs, while the efficiency reduced in the fourth recycle run. They suggested that the main advantage of using the DES was its reusability andthat using p-TSA alone as a catalyst would not facilitate acid recycling, because of the loss of the acid in the product. The same research group performed the recyclability experiment of the employed catalytic DES, ChCl:3p-TSA, in the process of FFA conversion to FAME. The results revealed that first and second runs of recycling had high efficiencies, while for the third run, a slight reduction in the conversion yield was observed. Therefore, a lengthened reaction time was suggested to have the same yield as the first two runs. However, the successful reusability of the DES was dependent on the efficiency of the catalyst separation by centrifugation. Alhassan et al. examined the recycling of ChCl:4p-TSA catalytic DESs (So-DES and Un-DES). A high-speed centrifuge was used to regenerate the catalysts. Then, the biodiesel production was performed, employing the recycled DESs under optimum conditions. For Un-DES, marginal reduction in the catalytic activity was observed for the first four runs. For So-DES, after eight successive experiments, catalytic activity reduced after, which the catalyst became deactivated. Deactivation was proposed to be due to the decrease in the density of the acidic sites.
The catalytic (ATPPB:3p-TSA) DES was used to reduce the oleic acid in low-grade oil via esterification process. The results showed that for five repeated recycle runs, the catalytic efficiency of the DES did not change significantly, implying that the DES catalyst regained its high catalytic activity even after several usages. Compared to the study by Hayyan et al. where the same DES catalyst was employed, the DES catalyst in this study worked more efficiently (five recycle runs compared to three recycle runs). However, it should be noted that in the present study, more catalyst was used (5.0 wt%) than 1.0 wt% used by Hayyan et al. Yu et al. used (taurine:3TfOH) DES dissolved in PEG-200 to alkylate isobutane and isobutene for production of gasoline. After the reaction was completed, the alkylate oil was simply separated from taurine:3TfOH + PEG-200 catalytic system via decantation. They found that after eight times of using the catalyst, the isobutane and isobutene conversion rates and octane selectivity did not drop significantly. Additionally, compared to pure TfOH, taurine:3TfOH or TfOH/PEG-200, the taurine:3TfOH + PEG-200 catalytic system worked remarkably better in terms of reusability and octane selectivity.
In order to investigate the reusability of (TBAB:2p-TSA) catalyst in a process of biodiesel production from yellow horn seed biomass, Shen et al. examined the FAMEs conversion yield and oil extraction yield in several runs. They found that the oil extraction yield was nearly unchanged and the conversion yield of FAME reached 80% after four recycle runs. They did not recommend more than four runs of recycling because glycerol, as the by-product of the process, hindered the transesterification reaction. Zhang et al. tested the reusability of the (ChCl:2ethylene glycol) + CrCl3 catalytic system employed for 5-HMF production. After the decomposition of glucose, ethyl acetate was employed as an extraction solvent to separate 5-HMF, levulinic acid and formic acid from the reaction mixture. Because of the very low solubility of (ChCl:2ethylene glycol) and CrCl3 in ethyl acetate, 5-HMF was readily separated from the mixture. The remaining liquid was heated to remove the residual solvent from the catalytic system. The catalytic system could be used up to four times, with 38% yield of 5-HMF. Therefore, the catalytic system could be successfully recycled for decomposition of glucose to 5-HMF for four times.
4. Limitations of DESs
DESs are superior over other common organic solvents, due to their features mentioned above, for sustainability. However, despite the many remarkable properties of DESs, acting either as catalysts and/or reaction media in biomass transformations, there are a number of limitations restricting their use. For instance, the biomass conversions are usually carried out at high temperatures. Some DESs may decompose or undergo side reactions under those conditions. For example, there are studies reporting the decomposition of the DES constituents and formation of unwanted chemicals at high temperatures. Moreover, it has been reported that some types of DESs composed of ChCl and carboxylic acids underwent esterification reactions as a consequence of interactions between hydroxyl groups of the salt and the acids. For similar reasons, the suitability of DESs with high melting points, such as type I DESs, which are composed of an organic salt and a non-hydrated metal salt, for industrial applications is highly limited.
The other concern about the applicability of DESs in biomass conversions is their recyclability. As given in Table 1, the recyclability of DESs of both types of Brønsted acids and Lewis acids is hardly greater than 6 times, after which their efficiencies drop significantly. Difficulties in purification of DESs after several recycling processes has created serious constraints towards scaling up. There are therefore only a few studies reporting scaling-up processes containing DESs. The usually high viscosities of DESs along with high melting points of some of them would result in reduced mass transfer during the pretreatment process, hampering their applications and recyclability. However, as a well-practiced approach, water addition would circumvent the high viscosities of DESs. This practice may have also a positive influence on the efficiency of the process.
Finally, it has been argued that the impurities and minerals present in feedstock can change the physicochemical properties of the DESs negatively and impact their catalytic properties.
References
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082.
- Zhang, Q.; Vigier, K.D.O.; Franc, S.R.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146.
- Xu, G.C.; Ding, J.C.; Han, R.Z.; Dong, J.J.; Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2016, 2–3, 364–369.
- Procentese, A.; Raganati, F.; Olivieri, G.; Elena, M.; Rehmann, L.; Marzocchella, A. Low-energy biomass pretreatment with deep eutectic solvents for bio-butanol production. Bioresour. Technol. 2017, 243, 464–473.
- Alhassan, Y.; Kumar, N.; Bugaje, I.M. Hydrothermal liquefaction of de-oiled Jatropha curcas cake using deep eutectic solvents (DESs) as catalysts and co-solvents. Bioresour. Technol. 2016, 199, 375–381.
- Alhassan, Y.; Pali, H.S.; Kumar, N.; Bugaje, I.M. Co-liquefaction of whole Jatropha curcas seed and glycerol using deep eutectic solvents as catalysts. Energy 2017, 138, 48–59.
- Wang, J.; Liu, Y.; Zhou, Z.; Fu, Y.; Chang, J. Epoxidation of soybean oil catalyzed by deep eutectic solvents based on the choline chloride-carboxylic acid bifunctional catalytic system. Ind. Eng. Chem. Res. 2017, 56, 8224–8234.
- Jerome, F.; Vigier, K.D.O. Catalytic conversion of carbohydrates to furanic derivatives in the presence of choline chloride. Catalysts 2017, 7, 218.
- Unlu, A.E.; Arikaya, A.; Takac, S. Use of deep eutectic solvents as catalyst: A mini-review. Green Process. Synth. 2019, 8, 355–372.
- Körner, S.; Albert, J.; Held, C. Catalytic low-temperature dehydration of fructose to 5-hydroxymethylfurfural using acidic deep eutectic solvents and polyoxometalate catalysts. Front. Chem. 2019, 7, 661.
- Ni, Y.; Bi, Z.; Su, H.; Yan, L. Deep eutectic solvent (DES) as both solvent and catalyst for oxidation of furfural to maleic acid and fumaric acid. Green Chem. 2019, 21, 1075–1079.
- Bai, C.; Wei, Q.; Ren, X. Selective extraction of collagen peptides with high purity from cod skins by deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 7220–7227.
- Chen, Z.; Bai, X.; Lusi, A.; Wan, C. High-solid lignocellulose processing enabled by natural deep eutectic solvent for lignin extraction and industrially relevant production of renewable chemicals. ACS Sustain. Chem. Eng. 2018, 6, 12205–12216.
- Cao, Q.; Li, J.; Xia, Y.; Li, W.; Luo, S.; Ma, C.; Liu, S. Green extraction of six phenolic compounds from rattan (calamoideae faberii) with deep eutectic solvent by homogenate-assisted vacuum-cavitation method. Molecules 2019, 24, 113.
- Garc, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep eutectic solvents: Physicochemical properties and gas separation applications. Energy Fuels 2015, 29, 2616–2644.
- Tan, T.; Zhang, M.L.; Wan, Y.Q.; Qiu, H.D. Utilization of deep eutectic solvents as novel mobile phase additives for improving the separation of bioactive quaternary alkaloids. Talanta 2016, 149, 85–90.
- Florindo, C.; Lima, F.; Ribeiro, B.D.; Marrucho, I.M. Deep eutectic solvents: Overcoming 21st century challenges. Curr. Opin. Green Sustain. Chem. 2019, 18, 31–36.
- Ghandi, K. A review of ionic liquids, their limits and applications. Green Sustain. Chem. 2014, 4, 44–53.
- Sas, O.G.; Fidalgo, R.; Domínguez, I.; Macedo, E.A.; González, B. Physical properties of the pure deep eutectic solvent, [ChCl]:[Lev] (1:2) DES, and its binary mixtures with alcohols. J. Chem. Eng. Data 2016, 61, 4191–4202.
- Harifi-Mood, A.R.; Buchner, R. Density, viscosity, and conductivity of choline chloride + ethylene glycol as a deep eutectic solvent and its binary mixtures with dimethyl sulfoxide. J. Mol. Liq. 2017, 225, 689–695.
- Kalhor, P.; Xu, J.; Ashraf, H.; Cao, B.; Yu, Z.W. Structural properties and hydrogen-bonding interactions in binary mixtures containing a deep-eutectic solvent and acetonitrile. J. Phys. Chem. B 2020, 124, 1229–1239.
- Meng, X.; Ballerat-busserolles, K.; Andanson, J. Impact of water on the melting temperature of urea+choline chloride deep eutectic solvent. New J. Chem. 2016, 40, 4492–4499.
- Zhekenov, T.; Toksanbayev, N.; Kazakbayeva, Z.; Shah, D. Formation of type III Deep Eutectic Solvents and effect of water on their intermolecular interactions. Fluid Phase Equilibria 2017, 441, 43–48.
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The effect of water upon deep eutectic solvent nanostructure: An unusual transition from ionic mixture to aqueous solution. Angew.Chem. Int. Ed. 2017, 56, 9782–9785.
- Kalhor, P.; Zheng, Y.Z.; Ashraf, H.; Cao, B.; Yu, Z.W. Influence of hydration on the structure and interactions of Ethaline deep-eutectic solvent: A spectroscopic and computational study. ChemPhysChem 2020, 21, 995–1005.
- Kalhor, P.; Li, Q.Z.; Zheng, Y.Z.; Yu, Z.W. Is the Fourier transform infrared free-OH band of t-butanol only from free OHs? case studies on the binary systems of the alcohol with CCl4 and CHCl3. J. Phys. Chem. A 2020, 124, 6177–6185.
- Kalhor, P.; Yu, Z.W. Structural and hydrogen-bonding properties of neat t-BuNH2 and its binary mixtures with CCl4, CHCl3 and DMSO. J. Mol. Struct. 2020, 1215, 128257.
- Kalhor, P.; Ghandi, K. Deep eutectic solvents for pretreatment, extraction, and catalysis of biomass and food waste. Molecules 2019, 24, 4012.
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147.
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marueho, I.M. Insights into the synthesis and properties of deep eutectic solvents based on cholinum chloride and carboxylic acid. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425.
- Simeonov, S.P.; Afonso, C.A.M. Basicity and stability of urea deep eutectic solvents. RSC Adv. 2016, 6, 5485–5490.
- Kroon, M.C.; Binnemans, K. Degradation of deep-eutectic solvents based on choline chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2019, 7, 11521–11528.
- Ishida, N.; Miura, T.; Murakami, M. Hydrothermal liquefaction of model food waste biomolecules and ternary mixtures under isothermal and fast conditions. Chem. Commun. 2007, 6, 4381–4383.
- Tan, Y.; Chen, Y.; Mahimwalla, Z.; Johnson, M.B.; Sharma, T.; Brüning, R.; Ghandi, K. Novel synthesis of rutile titanium dioxide—Polypyrrole nano composites and their application in hydrogen generation. Synth. Met. 2014, 189, 77–85.
- Kannan, S.; Gariepy, Y.; Raghavan, G.S.V. Conventional hydrothermal carbonization of shrimp waste. Energy Fuels 2018, 32, 3532–3542.
- Chen, W.T.; Zhang, Y.; Zhang, J.; Schideman, L.; Yu, G.; Zhang, P.; Minarick, M. Co-liquefaction of swine manure and mixed-culture algal biomass from a wastewater treatment system to produce bio-crude oil. Appl. Energy 2014, 128, 209–216.
- Ghandi, S.K.; Gordon, M.S. Ultra-fast electron capture by electrostericallystabilized gold nanoparticles. Nanoscale 2015, 7, 11545–11551.
- Wu, C.; Wang, Q.; Xiang, J.; Yu, M.; Chang, Q.; Gao, M.; Sonomoto, K. Enhanced productions and recoveries of ethanol and methane from food waste by a three-stage process. Energy Fuels 2015, 29, 6494–6500.
- Yu, X.; Yin, J.; Wang, K.; Shen, D.; Long, Y.; Chen, T. Enhancing food waste hydrolysis and the production rate of volatile fatty acids by prefermentation and hydrothermal pretreatments. Energy Fuels 2016, 30, 4002–4008.
- Déniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Bio-oil production from food processing residues: Improving the bio-oil yield and quality by aqueous phase recycle in hydrothermal liquefaction of blackcurrant (Ribes nigrum L.) pomace. Energy Fuels 2016, 30, 4895–4904.
- Dagle, V.L.; Smith, C.; Flake, M.; Albrecht, K.O.; Gray, M.J.; Ramasamy, K.K.; Dagle, R.A. Integrated process for the catalytic conversion of biomass-derived syngas into transportation fuels. Green Chem. 2016, 18, 1880–1891.
- Zhang, B.; Lin, Q.; Zhang, Q.; Wu, K.; Pu, W.; Yang, M.; Wu, Y. Catalytic hydrothermal liquefaction of Euglena sp. microalgae over zeolite catalysts for the production of bio-oil. RSC Adv. 2017, 7, 8944–8951.
- Posmanik, R.; Cantero, D.A.; Malkani, A.; Sills, D.L.; Tester, J.W. Biomass conversion to bio-oil using sub-critical water: Study of model compounds for food processing waste. J. Supercrit. Fluids 2017, 119, 26–35.
- Burns, F.P.; Themens, P.; Ghandi, K. Assessment of phosphonium ionic liquid- dimethylformamide mixtures for dissolution of cellulose. Compos. Interfaces 2013, 21, 59–73.
- Tan, Y.; Johnson, M.B.; Ghandi, K. Synthesis of Polypyrrole/TiO2 Nanoparticles in Water by Chemical Oxidative Polymerization. In Biomaterial Aplications; Thomas, S., Nandhakumar, K., Weimen, Y., Babu, B.M., Eds.; Apple Academic Press: Palm Bay, FL, USA; Burlington, ON, Canada, 2014.
- Troter, D.Z.; Todorović, Z.B.; Dokić-Stojanović, D.R.; Stamenković, O.S.; Veljković, V.B. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 61, 473–500.
- Zhang, Z.; Song, J.; Han, B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem. Rev. 2017, 117, 6834–6880.
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380.
- Amiri, H.; Karimi, K. Pretreatment and hydrolysis of lignocellulosic wastes for butanol production: Challenges and perspectives. Bioresour. Technol. 2018, 270, 702–721.
- Lang, J.; Lu, J.; Lan, P.; Wang, N.; Yang, H.; Zhang, H. Preparation of 5-HMF in a DES/ethyl n-butyrate two-phase system. Catalysts 2020, 10, 636.
- Pham, T.N.; Sooknoi, T.; Crossley, S.P.; Resasco, D.E. Ketonization of carboxylic acids: Mechanisms, catalysts, and implications for biomass conversion. ACS Catal. 2013, 3, 2456–2473.
- Gilkey, M.J.; Xu, B. Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal. 2016, 6, 1420–1436.
- Aitchison, H.; Wingad, R.L.; Wass, D.F. Homogeneous ethanol to butanol catalysis—Guerbet renewed. ACS Catal. 2016, 6, 7125–7132.
- Li, H.; Riisager, A.; Saravanamurugan, S.; Pandey, A.; Sangwan, R.S.; Yang, S.; Luque, R. Carbon-increasing catalytic strategies for upgrading biomass into energy-intensive fuels and chemicals. ACS Catal. 2018, 8, 148–187.
- Li, A.Y.; Moores, A. Carbonyl reduction and biomass: A case study of sustainable catalysis. ACS Sustain. Chem. Eng. 2019, 7, 10182–10197.
- Li, S.; Yu, D.; Cheng, S.; Cross, S.J. Recyclabl metal (Ni, Fe) cluster designed catalyst for cellulose pyrolysis to upgrade bio-oil. Catalysts 2020, 10, 1160.
- Traboni, S.; Bedini, E.; Vessella, G.; Iadonisi, A. Solvent-free approaches in carbohydrate synthetic chemistry: Role of catalysis in reactivity and selectivity. Catalysts 2020, 10, 1142.
- Sun, L.; Wang, Z.; Chen, L.; Yang, S.; Xie, X.; Gao, M.; Zhao, B.; Si, H.; Li, J.; Hua, D. Catalytic fast pyrolysis of biomass into aromatic hydrocarbons over Mo-modified ZSM-5 catalysts. Catalysts 2020, 10, 1051.
- Al-sabawi, M.; Chen, J.; Ng, S. Fluid catalytic cracking of biomass-derived oils and their blends with petroleum feedstocks: A review. Energy Fuels 2012, 26, 5355–5372.
- Kostyniuk, A.; Grilc, M.; Likozar, B. Catalytic cracking of biomass-derived hydrocarbon tars or model compounds to form biobased benzene, toluene, and xylene isomer mixtures. Ind. Eng. Chem. Res. 2019, 58, 7690–7705.
- Chen, X.; Liu, Y.; Wang, J. Lignocellulosic biomass upgrading into valuable nitrogen- containing compounds by heterogeneous catalysts. Ind. Eng. Chem. Res. 2020, 59, 17008–17025.
- Liu, P.; Hao, J.W.; Mo, L.P.; Zhang, Z.H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in. RSC Adv. 2015, 5, 48675–48704.
- Alhassan, Y.; Kumar, N. Single step biodiesel production from pongamia pinnata (Karanja) seed oil using deep eutectic solvent (DESs) catalysts. Waste Biomass Valorization 2016, 7, 1055–1065.
- Sert, M. Catalytic effect of acidic deep eutectic solvents for the conversion of levulinic acid to ethyl levulinate. Renew. Energy 2020, 153, 1155–1162.
- Liu, F.; Xue, Z.; Zhao, X.; Mou, H.; He, J.; Mu, T. Catalytic deep eutectic solvents for highly efficient conversion of cellulose to gluconic acid with gluconic acid self-precipitation separation. Chem. Commun. 2018, 54, 6140–6143.
- Long, T.; Deng, Y.; Gan, S.; Chen, J. Application of choline chloride·xZnCl2 ionic liquids for preparation of biodiesel. Chin. J. Chem. Eng. 2010, 18, 322–327.
- Mondal, D.; Sharma, M.; Wang, C.; Lin, Y.; Huang, H.; Saha, A.; Nataraj, S.K.; Prasad, K. Deep eutectic solvent promoted one step sustainable conversion of fresh seaweed biomass to functionalized graphene as a potential electrocatalyst. Green Chem. 2016, 18, 2819–2826.
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 2008, 10, 1280–1283.
- Hu, S.; Zhang, Z.; Zhou, Y.; Song, J.; Fan, H.; Han, B. Direct conversion of inulin to 5-hydroxymethylfurfural in biorenewable ionic liquids. Green Chem. 2009, 11, 873–877.
- Zhang, L.; Yu, H. Conversion of xylan and xylose into furfural in biorenewable deep eutectic solvent with trivalent metal chloride added. BioResources 2013, 8, 6014–6025.
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141.
- Yu, Q.; Zhang, A.; Wang, W.; Chen, L.; Bai, R.; Zhuang, X.; Wang, Q.; Wang, Z.; Yuan, Z. Deep eutectic solvents from hemicellulose-derived acids for the cellulosic ethanol refining of Akebia’ herbal residues. Bioresour. Technol. 2018, 247, 705–710.
- Shen, C.; Wang, X.; Zhu, Y.; Jiao, J.; Bao, S.; Kou, P.; Pan, H.; Li, Y.; Fu, Y. A green one-pot method for simultaneous extraction and transesterification of seed oil catalyzed by a p -toluenesulfonic acid based deep eutectic solvent. Ind. Crops Prod. 2020, 152, 112517.
- Zhang, H.; Yu, Z.; Gu, T.; Xiang, L.; Shang, M.; Shen, C.; Su, Y. Continuous synthesis of 5-hydroxymethylfurfural using deep eutectic solvents and its kinetic study in microreactors. Chem. Eng. J. 2020, 391, 123580.
- Alhassan, Y.; Kumar, N.; Bugaje, I.M. Catalytic upgrading of waste tire pyrolysis oil via supercritical esterification with deep eutectic solvents (green solvents and catalysts). J. Energy Inst. 2016, 89, 683–693.
- Song, X.; Hu, W.; Huang, W.; Wang, H.; Yan, S.; Yu, S. Methanolysis of polycarbonate into valuable product bisphenol A using choline chloride-based deep eutectic solvents as highly active catalysts. Chem. Eng. J. 2020, 388, 124324.
- Li, Z.; Long, J.; Zeng, Q.; Wu, Y.; Cao, M.; Liu, S.; Li, X. Production of methyl p-hydroxycinnamate by selective tailoring of herbaceous lignin using metal-based deep eutectic solvents (DES) as catalyst. Ind. Eng. Chem. Res. 2020, 59, 17328–17337.
- Sirvio, J.A.; Heiskanen, J.P. Synthesis of alkaline-soluble cellulose methyl carbamate using reactive deep eutectic solvent. ChemSusChem 2017, 10, 455–460.
- Liu, B.; Fu, W.; Lu, X.; Zhou, Q.; Zhang, S. Lewis acid-base synergistic catalysis for polyethylene terephthalate degradation by 1,3-dimethylurea/Zn(OAc)2 deep eutectic solvent. Sustain. Chem. Eng. 2019, 7, 3292–3300.
- Yang, X.; Xie, H.; Du, H.; Zhang, X.; Zou, Z.; Zou, Y.; Liu, W.; Lan, H.; Zhang, X.; Si, C. Facile extraction of thermally stable and dispersible cellulose nanocrystals with high yield via a green and recyclable FeCl3-catalyzed deep eutectic solvent system. ACS Sustain. Chem. Eng. 2019, 7, 7200–7208.
- Bu, C.; Yan, Y.; Zou, L.; Ouyang, S.; Zheng, Z. Comprehensive utilization of corncob for furfuryl alcohol production by chemo-enzymatic sequential catalysis in a biphasic system. Bioresour. Technol. 2021, 319, 124156.
- Li, L.; Wu, Z.; Xi, X.; Liu, B.; Cao, Y.; Xu, H.; Hu, Y. A bifunctional brønsted acidic deep eutectic solvent to dissolve and catalyze the depolymerization of alkali lignin. J. Renew. Mater. 2021, 9, 219–235.
- Hayyan, A.; Ali Hashim, M.; Mjalli, F.S.; Hayyan, M.; AlNashef, I.M. A novel phosphonium-based deep eutectic catalyst for biodiesel production from industrial low grade crude palm oil. Chem. Eng. Sci. 2013, 92, 81–88.
- Hayyan, A.; Hashim, M.A.; Hayyan, M.; Mjalli, F.S.; Alnashef, I.M. A novel ammonium based eutectic solvent for the treatment of free fatty acid and synthesis of biodiesel fuel. Ind. Crops Prod. 2013, 46, 392–398.
- Hayyan, A.; Ali, M.; Hayyan, M.; Mjalli, F.S.; Alnashef, I.M. A new processing route for cleaner production of biodiesel fuel using a choline chloride based deep eutectic solvent. J. Clean. Prod. 2014, 65, 246–251.
- Zhang, L.; Yu, H.; Yu, H.; Chen, Z.; Yang, L. Conversion of xylose and xylan into furfural in biorenewable choline chloride-oxalic acid deep eutectic solvent with the addition of metal chloride. Chin. Chem. Lett. 2014, 25, 1132–1136.
- Assanosi, A.A.; Farah, M.M.; Wood, J.; Al-duri, B. A facile acidic choline chloride–p-TSA DES catalysed dehydration of fructose to 5-hydroxymethylfurfural. RSC Adv. 2014, 4, 39359–39364.
- Assanosi, A.; Farah, M.M.; Wood, J.; Al-duri, B. Fructose dehydration to 5HMF in a green self-catalysed DES composed of N,N-diethylethanolammonium chloride and p-toluenesulfonic acid monohydrate (p-TSA). C. R. Chim. 2016, 19, 450–456.
- Williamson, S.T.; Shahbaz, K.; Mjalli, F.S.; Alnashef, I.M.; Farid, M.M. Application of deep eutectic solvents as catalysts for the esterification of oleic acid with glycerol. Renew. Energy 2017, 114, 480–488.
- Gawade, A.B.; Yadav, G.D. Microwave assisted synthesis of 5-ethoxymethylfurfural in one pot from D-fructose by using deep eutectic solvent as catalyst under mild condition. Biomass Bioenergy 2018, 117, 38–43.
- Bodachivskyi, I.; Kuzhiumparambil, U.; Williams, D.B.G. High yielding acid-catalysed hydrolysis of cellulosic polysaccharides and native biomass into low molecular weight sugars in mixed ionic liquid systems. ChemistryOpen 2019, 8, 1316–1324.
- Arslanoğlu, A.; Sert, M. Direct conversion of biomass to platform chemicals, catalyzed using a deep eutectic solvent of N,N diethyl ethanol ammonium chloride-oxalic acid in a microwave reactor. Fuel 2019, 258, 116142.
- Yu, F.L.; Gu, Y.L.; Gao, X.; Liu, Q.C.; Xie, C.X.; Yu, S.T. Alkylation of isobutane and isobutene catalyzed by trifluoromethanesulfonic acid-taurine deep eutectic solvents in polyethylene glycol. Chem. Commun. 2019, 55, 4833–4836.
- Smink, D.; Juan, A.; Schuur, B.; Kersten, S.R.A. Understanding the role of choline chloride in deep eutectic solvents used for biomass delignification. Ind. Eng. Chem. Res. 2019, 58, 16348–16357.
- Morais, E.S.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Enhanced conversion of xylan into furfural using acidic deep eutectic solvents with dual solvent and catalyst behavior. ChemSusChem 2020, 13, 784–790.
- Razavian, M.; Fatemi, S. Intensified transformation of low-value residual fuel oil to light fuels with TPABr:EG as deep eutectic solvent with dual functionality at moderate temperatures. Energy Fuels 2020, 34, 5497–5510.
- Zhang, D.; Chen, X.; Yuan, B.; Yu, F.; Xie, C.; Yu, S. A novel green catalytic strategy for hydration of α-pinene by a natural deep eutectic solvent. Biomass Convers. Biorefin. 2020.
- Lopes, A.M.D.; Gomes, J.R.B.; Coutinho, J.A.P.; Silvestre, A.J.D. Novel insights into biomass delignification with acidic deep eutectic solvents: A mechanistic study of β-O-4 ether bond cleavage and the role of the halide counterion in the catalytic performance. Green Chem. 2020, 22, 2474–2487.
- Ruan, C.; Mo, F.; Qin, H.; Cheng, H.; Chen, L.; Qi, Z. Bifunctional imidazole-benzenesulfonic acid deep eutectic solvent for fructose dehydration to 5-hydroxymethylfurfural. Catal. Lett. 2020.
- Zhang, T.; Shahbaz, K.; Farid, M.M. Glycerolysis of free fatty acid in vegetable oil deodorizer distillate catalyzed by phosphonium-based deep eutectic solvent. Renew. Energy 2020, 160, 363–373.
- Wang, H.; Li, J.; Zeng, X.; Tang, X.; Sun, Y.; Lei, T.; Lin, L. Extraction of cellulose nanocrystals using a recyclable deep eutectic solvent. Cellulose 2020, 27, 1301–1314.
- Liu, Y.; Wang, Y.; Cao, Y.; Chen, X.; Yu, Q.; Wang, Z.; Yuan, Z. One-pot synthesis of cyclic biofuel intermediates from biomass in choline chloride/formic acid-based deep eutectic solvents. ACS Sustain. Chem. Eng. 2020, 8, 6949–6955.
- Wang, S.; Li, H.; Xiao, L.; Song, G. Unraveling the structural transformation of wood lignin during deep eutectic solvent treatment. Front. Energy Res. 2020, 8, 48.
- Shen, X.; Chen, T.; Wang, H.; Mei, Q.; Yue, F.; Sun, S.; Wen, J.-L.; Yuan, T.-Q.; Sun, R.-C. Structural and morphological transformations of lignin macromolecules during bio-based deep eutectic solvent (DES) pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 2130–2137.
- Guo, D.; Guo, Y.; Sha, L.; Lyu, G.; Li, J.; Zhang, X.; Liu, B. Subcritical ethanol catalyzed with deep eutectic solvent extract phenolic lignin for preparation of an ultraviolet-blocking composite film. Energy Fuels 2020, 34, 8395–8402.
- Jiang, J.; Carrillo-Enriquez, N.C.; Oguzlu, H.; Han, X.; Bi, R.; Song, M.; Saddler, J.N.; Sun, R.; Jiang, F. High production yield and more thermally stable lignin-containing cellulose nanocrystals isolated using a ternary acidic deep eutectic solvent. ACS Sustain. Chem. Eng. 2020, 8, 7182–7191.
- Bodachivskyi, I.; Kuzhiumparambil, U.; Williams, D.B.G. Catalytic valorization of native biomass in a deep eutectic solvent: A systematic approach toward high-yielding reactions of polysaccharides. ACS Sustain. Chem. Eng. 2020, 8, 678–685.
- Tian, D.; Guo, Y.; Hu, J.; Yang, G.; Zhang, J.; Luo, L.; Xiao, Y.; Deng, S.; Deng, O.; Zhou, W.; et al. Acidic deep eutectic solvents pretreatment for selective lignocellulosic biomass fractionation with enhanced cellulose reactivity. Int. J. Biol. Macromol. 2020, 142, 288–297.
- Liu, Y.; Yan, H.; Liu, J.; Dong, W.; Cao, Z.; Hu, X.; Zhou, Z. Acidic deep eutectic solvents with long carbon chains as catalysts and reaction media for biodiesel production. Renew. Energy 2020, 162, 1842–1853.
- Maka, H.; Spychaj, T.; Adamus, J. Lewis acid type deep eutectic solvents as catalysts for epoxy resin crosslinking. RSC Adv. 2015, 5, 82813–82821.
- Xu, H.; Kong, Y.; Peng, J.; Song, X.; Liu, Y.; Su, Z.; Li, B.; Gao, C.; Tian, W. Comprehensive analysis of important parameters of choline chloride-based deep eutectic solvent pretreatment of lignocellulosic biomass. Bioresour. Technol. 2021, 319, 124209.
- Samaranayake, C.P.; Sastry, S.K. In-situ pH measurement of selected liquid foods under high pressure. Innov. Food Sci. Emerg. Technol. 2013, 17, 22–26.
- Taysun, M.B.; Sert, E.; Atalay, F.S. Physical properties of benzyl tri-methyl ammonium chloride based deep eutectic solvents and employment as catalyst. J. Mol. Liq. 2016, 223, 845–852.
- Sun, J.; Bostick, B.C.; Mailloux, B.J.; Ross, J.M.; Chillrud, S.N. Effect of oxalic acid treatment on sediment arsenic concentrations and lability under reducing conditions. J. Hazard. Mater. 2016, 311, 125–133.
- Miyaji, H.; Fujimoto, J.; Mabuchi, R.; Okumura, M.; Goto, S.; Honda, Y. A novel anthracene-based fluorescent colorimetric sensor for the simple naked-eye diagnosis of methylmalonic aciduria. Tetrahedron Lett. 2017, 58, 3623–3627.
- Eda, S.; Borra, A.; Parthasarathy, R.; Bankupalli, S.; Bhargava, S.; Thella, P.K. Recovery of levulinic acid by reactive extraction using tri-n-octylamine in methyl isobutyl ketone: Equilibrium and thermodynamic studies and optimization using Taguchi multivariate approach. Sep. Purif. Technol. 2018, 197, 314–324.
- Cecchin, D.; Bringhenti, I.L.; Bernardi, J.B.; Leal, L.O.; Souza, M.A.; Bedran-Russo, A.K.; Farina, A.P. Alpha-hydroxy glycolic acid for root dentin etching: Morphological analysis and push out bond strength. Int. J. Adhes. Adhes. 2019, 90, 138–143.
- Zhang, M.; Wei, Z.; Chen, W.; Xu, M.; Cai, J.; Chen, Y. Bell shape vs volcano shape pH dependent kinetics of the electrochemical oxidation of formic acid and formate, intrinsic kinetics or local pH shift? Electrochim. Acta 2020, 363, 137160.
- Kumar, N.; Gautam, R.; Stallings, J.D.; Coty, G.G.; Lynam, J.G. Secondary agriculture residues pretreatment using deep eutectic solvents. Waste Biomass Valorization 2020.
- Wang, Z.; Hong, S.; Wen, J.; Ma, C.Y.; Tang, L.; Jiang, H.; Chen, J.; Li, S.; Shen, X.; Yuan, T. Lewis acid-facilitated deep eutectic solvent (DES) pretreatment for producing high-purity and antioxidative lignin. ACS Sustain. Chem. Eng. 2020, 8, 1050–1057.
- Teles, A.R.R.; Capela, E.V.; Carmo, R.S.; Coutinho, J.A.P.; Silvestre, A.J.D.; Freire, M.G. Solvatochromic parameters of deep eutectic solvents formed by ammonium-based salts and carboxylic acids. Fluid Phase Equilibria 2017, 448, 15–21.
- Abbott, A.P.; Alabdullah, S.S.M.; Al-murshedi, A.Y.M.; Ryder, K.S. Brønsted acidity in deep eutectic solvents and ionic liquids. Faraday Discuss. 2018, 206, 365–377.
- Hou, X.; Li, A.; Lin, K.; Wang, Y.; Kuang, Z.; Cao, S. Insight into the structure-function relationships of deep eutectic solvents during rice straw pretreatment. Bioresour. Technol. 2018, 249, 261–267.
- Tan, Y.T.; Ngoh, G.C.; Seak, A.; Chua, M. Effect of functional groups in acid constituent of deep eutectic solvent for extraction of reactive lignin. Bioresour. Technol. 2019, 281, 359–366.




