
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Theodoros Varzakas | + 4096 word(s) | 4096 | 2021-03-15 05:11:53 | | | |
| 2 | Vivi Li | Meta information modification | 4096 | 2021-03-16 02:35:38 | | | | |
| 3 | Vivi Li | Meta information modification | 4096 | 2021-03-24 11:14:12 | | |
Video Upload Options
Over the last decades, the incidence of diabetes has increased in developed countries and beyond the genetic impact, environmental factors, which can trigger the activation of the gut immune system, seem to affect the induction of the disease process. Since the composition of the gut microbiome might disturb the normal interaction with the immune system and contribute to altered immune responses, the restoration of normal microbiota composition constitutes a new target for the prevention and treatment of diabetes. Thus, the interaction of gut microbiome and diabetes, focusing on mechanisms connecting gut microbiota with the occurrence of the disorder, is discussed in the present review. Finally, the challenge of functional food diet on maintaining intestinal health and microbial flora diversity and functionality, as a potential tool for the onset inhibition and management of the disease, is highlighted by reporting key animal studies and clinical trials. Early onset of the disease in the oral cavity is an important factor for the incorporation of a functional food diet in daily routine.
1. Introduction-Diabetes as a Disease
The purpose of this non-systematic review is to discuss diabetes and see how the gut microbiome interacts with diabetes, describing the main mechanisms. The incorporation of fermented foods in order to maintain digestive health is very critical and we will demonstrate how this is achieved by description of animal and health models. This tool will aid in the prevention and management of this disease that so many people suffer nowadays.
According to the World Health Organization (WHO) and the International Diabetes Federation (IDF), the prevalence of diabetes has risen continuously over time from 108 million in 1980 [1] to approximately 463 million adults (20–79 years) living with diabetes in 2019; by 2045 this will rise to 700 million [2]. Furthermore, 4.2 million deaths were caused by diabetes and it costed at least 760 billion dollars (USD) in health expenditure in 2019. The global disease prevalence in adult population has risen from 4.7% to 8.5% during the years from 1980 to 2014 [1].
T2DM is described as a heterogeneous group of disorders. Most diabetic patients suffer from type 2 diabetes mellitus (T2DM) as a result of excess body weight and sedentary lifestyle. It is characterized by decline in insulin-producing β-cells, progressive peripheral insulin resistance and increased hepatic glucose production [3][4]. Without any doubt, diabetes etiology is strictly related to environmental and hereditary factors [5][6]. Women developing gestational diabetes mellitus (GDM) following pregnancy have high risk of developing Τ2DM [7][8]. In pregnant women, GDM is closely associated with phenotypes of metabolic disorders and more specifically obesity, insulin resistance and low-grade inflammation [7]. Chronic and low-grade inflammation is the hallmark of metabolic diseases, along with lipotoxicity-mediated production of cytokines, recruitment and phenotype changes of B and T cells, which promote macrophages infiltration into adipose tissue [9][10].
In T1DM, there is destruction of β-cells and little or no insulin is produced [11][12]. Viruses also seem to induce type 1 diabetes mellitus (T1DM) via molecular mimicry mechanism [13][14][15].
Symptoms may be almost identical in all types of disease including high blood glucose rates, polydipsia, polyuria, neuropathy, kidney failure, blindness, stroke, heart attack and limb amputation [16]. Oral manifestations are among the first to be seen in the human body and need to be early related to DM. Periodontal disease, periapical lesions, xerostomia and taste disturbance were more prevalent among diabetic patients as authors stated in a recent meta-analysis [17].
Heredity, ethnicity and feeding habits seem to increase diabetes burden. Asiatic populations for example, showed a lower prevalence of disease when compared with European populations [16]. Socio-economic status is another factor related to the disease prevalence. The increased rate of T2DM in urban Western societies has been linked to food selection, obesity, physical inactivity and lifestyle [18]. In this vein, urbanism in India favors the increasing rates of diabetes from 2.1% in 1970 at 11.6% in 1996 in adults [19]. Accordingly, a recent cross-sectional study showed variations in the diabetes picture between India’s different states [20] ranging from 4.3% to 11.8% [20]. Low socio-economic status groups living in disadvantaged urban areas showed a higher prevalence of diabetes [20]. Surprisingly, Germany had the highest prevalence rates in Europe in 2019 (15.3%) followed by Portugal (14.2%) and Malta (2.2%), while at the other end Ireland showed 4.4% [21]. However, most European countries showed a rate ranging from 6.3% to 10% [21]. Agglomeration index was positively associated to the diabetes prevalence while urban percentage was negatively associated. It seems that the model of urban development and not the urbanization as such determine disease prevalence [21].
DM as a ‘’flame within’’ causes different complications mainly through two mechanisms. Firstly, the polyol pathway converts glucose into sorbitol, by aldose reductase enzyme, that causes tissue damage and numerous diabetic complications. Secondly, the formation of advanced glycosylation end products (AGEs), due to binding of glucose to proteins, lipids and nucleic acids, results in the alteration of structures and functions, in addition to its deposition in specific organs that causes various complications. Atheroma deposits are formed in cells, which accumulate in the basal membrane and lumen causing decreased cellular defense capacity and impaired polymorphonuclear leukocyte response. This makes diabetic patients more susceptible to infections especially by anaerobic bacteria due to the reduction of oxygen diffusion through the capillary wall [17] (Figure 1).
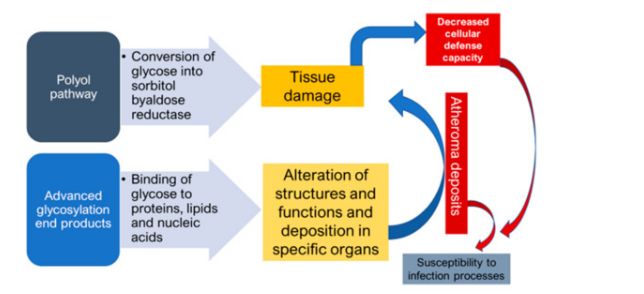
Figure 1. Mechanisms of formation of diabetic complications.
2. Functional Foods and Diabetes
2.1. The Challenge of Functional Foods
Functional foods are foods that surpass classic nutrition and exert beneficial effects connected to their consumption [22] with optimization of markers related to the disease.
Undoubtedly, economic, political and social trends, together with technological advantages, lead people to migrate to towns [23]. As humans become more urban, the society meets the negative impact of urbanization due to the changing lifestyle. Living-conditions, restricted green space, scant sanitation, fat-food eating habits affect human health. Efforts have been made to prevent human disease development. Education and increased information on health issues make people shift their habits to more sustainable healthy solutions. In this vein, during the last decades, functional foods have gained particular attention, due to their relationship to nutrition and health [24].
Without any doubt, healthy nutrition preserves the intestinal ecosystem and beneficially affects metabolic regulation [25]. From another aspect, this interest in functional foods has played a significant role in the adoption of healthy habits, due to the increasing consumer health concerns [24][26].
Fermented foods were known since ancient times. Fermentation seems to be firstly used in the fertile crescent area of the Middle East in 6000 B.C. [26]. The term ‘acid milk’ was also mentioned in the Bible [24]. For centuries, the fermentation process was pragmatically used for food preservation and production in every culture. People foremost understood that fermentation enhance food shelf-life and improve organoleptic characteristics of foods [27]. As known, fermented foods contain edible microorganisms whose enzymes hydrolyze food polysaccharides, proteins and lipids to non-toxic products. As a result, a food transformation is taking place and ingredients beneficial for human health, such as SCFAs, are produced [28]. During the last years, higher throughput biotechnologies serve to promote the fermented food industrial production in a large scale and genome sequencing provided a global picture on the biodiversity of microorganisms in food fermentation processes [27]. New technologies tailor food with important characteristics through overexpression or disruption of respective metabolic genes [27]. Moreover, microbial interplay in the fermented food matrix affects food quality and safety, organoleptic properties and finally food digestibility and beneficial modulation of the host immune system [29].
A plethora of fermented products was developed, ranging from drinks to foods in every culture; kimchi in Asia, cassava in West Africa, kombucha tea in China, kefir yoghurt in Caucasian and Balkan countries, sauerkraut, pickles, apple vinegar in most Western countries.
Nevertheless it is notable that most research focuses on the development of fermented dairy products. Metchnikoff was enrolled in a precursory research in the field of dairy fermentation in 1908. In his thesis under the title “The Prolongation of Life” evaluated the properties of lactic bacteria, specifically Lactobacillus delbrueckii subsp. bulgaricus and the longevity of Bulgarian farmers attributed to the consumption of fermented dairy products. Probiotics seem to prevent and reduce symptoms of multiple diseases, such as infections, autoimmune and allergic diseases and many others [30][31]. Even they are used as an adjunct therapy they maintain the balance of the intestinal microbiota [32].
Nevertheless, the country supporting vigorously the use of labeling in functional food products is Japan [33]. Since 1991, under the Foods for Specialized Health Use (FOSHU) label the Ministry of Health, Labor and Welfare in Japan issued a functional food regulation and launched their use in the market [33]. More than 200 functional products were branded under FOSHU legislation. In this vein, USA issued a regulation called “Foods with Function Claims” based on the Dietary Supplement Health and Education Act system (DSHEA) in 2015 [34]. This system seems to be more flexible in terms of health claims and in use of clinical protocols and thus launched functional foods market all over the world [34]. Registration of clinical studies must be under a University Hospital Medical Information Network (UMIN) protocol in both systems. While the FOSHU requires evidence that shows the reason for the minimum dosage from a dose-dependent study, as well as striking evidence based on the ways of action of the active compounds and the analytical methodology used, the American regulation system embeds on clinical studies proving a significant effect of functional foods when compared with a placebo intake group [34].
Functional food ingredients, such as probiotics, prebiotics, synbiotics and fermented as well, could amend the good condition of oral cavity and the activity of the gastrointestinal tract in a beneficial way. It is actually more than an axiom that their healthful effects are attributed to probiotic lactic acid bacteria (LAB) and Bifidobacterium [25].
The major effect of their intake is associated with the improvement of the host intestinal immune system through the ‘barrier effect’ and alleviation of the gut inflammatory response [31] through the production of immunoglobulin A (IgA) and balance between pro-inflammatory and anti-inflammatory cytokines [35]. It seems that diabetes is a disease related to the modern nutritional habits and lifestyle. Consumer’s interest in functional foods has increased due to their connection to health issues, such as the balance of gut microbiota and stimulation of the immune system [36].
As stated before, PRRs are of accrue importance for the deployment of the innate immune response [37]. Considering the above, therapeutic modulation of the gut microbiome via functional foods with probiotic properties may slow down the development of diabetes disease and its complications through a beneficial balance of the microbiota [38]. Knowledge gathered from animal models and human studies have shown that foods enriched with probiotics may impede postprandial hyperglycemia and adipose tissue and lipid metabolism occurring during diabetes inflammatory processes [38]. Furthermore, they seem to regulate dyslipidemia and insulin resistance status and reduce oxidative stress and inflammation [38]. In addition, they modify and regulate in a beneficial way the development of long-term diabetes complications, such as cardiovascular disease, neuropathy, nephropathy and retinopathy and oral manifestations [17][39]
Many studies have examined the influence of specific eating patterns to the gut microbiome [40][41][42]. Animal studies stated that several Lactobacillus and Bifidobacterium species could prevent or even impede the severity of T2DM [43]. Additionally, studies in humans aimed to clarify metabolic shifts, oxidative stress and inflammation linked to diabetes [43].
Meta-analysis studies of multiple control trials stated that probiotics improved the fasting plasma glucose (FPG) and the glycosylated hemoglobin (HbA1c) in T2DM [44]. Similarly, probiotics given in people developing T2DM improve glycemic control [45].
Without any doubt, unraveling and exploring the involved microbial patterns and getting a better knowledge of the microbiota profile should clarify their role in health and disease and should lead to the development of more effective or even alternative therapeutic strategies and nutritional habits.
2.2. Animal Studies
A great amount of work has been dedicated in unveiling the health benefits of functional food ingredients, such as prebiotic fibers and probiotics on mechanisms regulating immunity system via modulation of the intestinal microbiota in diabetic animal models. There is an increasing number of studies concerning how functional foods can improve or be supplemented as an auxiliary treatment in metabolic disorders, such as obesity, atherosclerosis or diabetes. Functional foods seem to have additional physiological benefits and contribute to the reduction of the risk of chronic diseases beyond their basic nutritional functions [46][47].
The use of animal models in the study of diabetes, especially in the last decade, is a common practice [48]. The most widespread animals that are equipped in a variety of experimental protocols belong to the family of rodents, mainly mice and rats because they have a very similar genetic background to that of humans, as well as a short lifespan. Furthermore, they are highly productive, less expensive and easily treated by scientists and researchers than other animal models [49].
Xue et al. [50] employed T2DM rats and investigated the potential health effects of propolis. Interestingly, propolis led to lower fasting blood glucose (FBG), reduced insulin resistance and improved intestinal mucosal injury in ileum tissue. Microbiota of diabetic rats were normalized with predominant being the Lactobacillus genera that consists mainly of probiotic bacteria, whereas Enterococcus, Clostridium, Turicibacter and Arthrobacter were lower compared to the control group [50].
Another interesting study underlined the effects of pistachio nuts supplementation on amelioration of inflammation by lower inflammatory foci, IL-1β, CCL-2 gene expression and inflammatory markers (TNF-α and IL-1β) in Wistar rats under high fat diet (HFD). These obese pistachio-supplemented rats exhibited lower Firmicutes/Bacteroidetes ratio and increased health-related bacteria, such as Parabacteroides, Dorea, Allobaculum, Turicibacter, Lactobacillus and Anaeroplasma, while inflammation-associated genera like Oscillospira, Desulfovibrio, Coprobacillus and Biophila were decreased [51]. The health benefit of pistachios on the microbiome of T1DM rats has also been studied by our group [52]. In healthy animals receiving pistachios lactobacilli and bifidobacteria were found in increased numbers, as well as increased populations of the Firmicutes phylum were reported, but decreased amounts of Bacteroidetes phylum were recorded.
Dietary supplementation on rats in HFD with barley or malt revealed decreased ratio of colonic Firmicutes/Bacteroidetes and increased numbers of Actinobacteria and Verrucomicrobia after barley supplementation. Furthermore, Akkermansia, Ruminococcus, Blautia, Biophila, Turicibacter and Roseburia genera were elevated after barley malt intake and shed some light in the manner of optimizing the health benefits of whole-brain barley products [53]. Cornstarch diet has been also associated with benefits in alleviating the adversity of diabetes mellitus. Studies that were performed in STZ-induced diabetic rats showed increased diversity of gut microbiota. It was observed a decrease of Actinobacteria and Bacteriodetes with increased abundance of Firmicutes [54][55]. In another similar study, the RA of these 6 OTUs, Christensenellaceae R-7 group, Prevotella 9, an unknown species of the Prevotellaceae family, Prevotellaceae UCG-001 and Ruminococcaceae UCG-005, and Ruminococcus 1, were all increased after starch feeding. Starch feeding also led to a reduction in RA of an uncultured species of the Erysipelotrichaceae family, Escherichia-Shigella, Klebsiella, an unknown species of the Peptostreptococcaceae family and Turicibacter [56].
Sane et al. [57] tested the effects of lone human milk administration in nod mice and underlined the prevention of diabetes onset and progression with elevated fecal Bifidobacterium and Akkermansia abundances and lower cecal B. fragilis and E. coli. Cecal and colonic B. vulgatus were enhanced by human milk intake [57].
Shikano et al. [58] used Green loofah L. cylindrica homogenate (LH) and fermented LH (FL) with L. lactis subsp. lactis Uruma-SU1 and L. plantarum Uruma-SU4, isolated from algal beach casts in a dietary supplementation experiment on a specific pathogen free (SPF) mice Kwl: ddY mice in high-fat diet. After FL consumption, TC, LDL-C and the ratio LDL-C/HDL-C were lower, whereas cecal L. johnsonii and C. disporicum were increased [58].
STZ-induced diabetic Wistar rats in high-fat diet (T2DM) were supplemented with fermented milk that was produced by inoculation of skim milk with probiotic cultures, L. rhamnosus NCDC 17 and L. rhamnosus GG, at 1% (v/v) [59]. Both probiotic treatments increased the population of total bacteria. L. rhamnosus NCDC 17 supplementation group had higher populations of E. rectale- C. coccoides, Bacteroides, lactobacilli and bifidobacteria. LGG and L. rhamnosus NCDC 17 decreased FGB and increased insulin levels and had a positive effect on glycosylated hemoglobin [59]. Free fatty acids levels and lipid profile were improved after HFD + L. rhamnosus NCDC 17 administration and triglycerides were reduced, whilst both probiotics increased HDL-C levels. Finally, L. rhamnosus NCDC 17 supplementation decreased expression levels of TNF-α and IL-6 genes and increased mRNA of the adiponectin gene [59].
A non-dairy fermented product with a combination of specific LABs and non-bitter beer yeast was administered to Zucker diabetic fatty (ZDF) rats, a model for T2DM associated with obesity [60]. Decreased glucose absorption was observed in treated group, along with decreased blood glucose. Microbial diversity was enriched after administration and Firmicutes, Saturella, Proteus, Alistipes, Anaerococcus were increased in the supplemented group, while Streptococcacae, Anaerococcus and Streptococcus, Barnesiella and Blautia were enriched in the control group. Hu et al. [61] showed that mixed fermentation by L. fermentum and Saccharomyces cerevisiae enhanced DNJ extraction efficiency from mulberry leaves. When implemented to STZ-induced diabetic mice, the extract seems to have relieved gut dysbiosis by promoting the growth of Lactobacillus, Lachnospiraceae NK4A136 group, Oscillibacter, Alistipes and Bifidobacterium [61]. At the same time, the growth of Ruminococcaceae UCG-014, Weissella, Ruminococcus, Prevotellaceae Ga6A1 group, Anaerostipes, Klebsiella, Prevotellaceae UCG-001 and Bacteroidales S24-7 group were significantly suppressed [61].
The efficacy of probiotics in diabetes relies on their ability to lower FBG and insulin levels in preclinical setting, as well as human trials [62]. VSL#3 is a probiotic product available in the market that contains strains of Bifidobacteriaceae (B. longum, B. infantis and B. breve), Lactobacillaceae (L. acidophilus, L. paracasei, L. delbrueckii subsp. Bulgaricus and L. plantarum) and S. thermophilus. When it was administrated to the non-obese diabetic (nod) mice model, where T1DM occurs as a result of insulitis, ameliorated diabetes progression took place, which was accompanied by reduced degree of insulitis in histological examination [63]. Inflammation markers were decreased as there was an inhibition of IL-1β expression and also enhancement of indoleamine-2, 3-dioxygenase (IDO) and IL-33 was documented [63]. An interesting outcome included the reduced differentiation of T-helper cells in autoimmune sites of the pancreatic lymph nodes (PLN), the site where the autoimmune response is regulated in T1DM [63]. The potential health benefits of probiotic administration were maximized with the increased Lactobacillacae, clostridia and Rikenellaceae after VSL#3 treatment and decreased abundance of Bacteroidetes strain S24-7 [63].
Other animal models, such as STZ-induced diabetic Wistar rats in HFD (T2DM), were utilized in exploring the supplementation with L. plantarum (probiotic), inulin (prebiotic) or in combination (symbiotic) [64]. Probiotic and synbiotic treatment led to an increase of Firmicutes phylum and of Lactobacillales family, while Clostridiales, Enterococcaceae and Bacteroidales were decreased [64]. Prebiotic treatment increased Streptococcaceae classification [64]. All treated groups were dominated with Lactobacillus genera and were characterized by enhanced Lactobacillus/Firmicutes ratio, whilst only the synbiotic supplementation increased specifically the probiotic L. plantarum population [64]. An improvement of oxidative stress status in hippocampus and prefrontal cortex, as well as a neuropsychological improvement and reversion of cognitive impairment were observed after symbiotic administration, underlying the variety of health benefits that probiotics can offer [64].
L. rhamnosus BSL and L. rhamnosus R23, when administered in STZ-induced diabetic Sprague-Dawley rats, led to elevated LAB levels after 30 days of probiotic supplementation and improved glucose tolerance and glucose control, as FBG was significantly reduced. After probiotic treatment, there was a decrease in TC and in atherogenic index [65].
Tian et al. [66], and Li et al. [67] used L. paracasei subsp. paracasei G15 and/or L. casei Q14 isolated from dairy food in STZ-induced diabetic Wistar rats in high-fat diet (T2DM) and concluded that glucose tolerance was restored and TC and triacylglycerol level was suppressed after 6 weeks of probiotic administration [66][67]. Hyperinsulinemia was ameliorated with insulin and glucagon levels being lower after probiotic ingestion and concentration of antidiabetic hormones GLP-1 and PYY was augmented after probiotic supplementation [66][67]. Furthermore, plasma LPS was reduced and a healthier intestinal microenvironment was achieved by improving intestinal barrier structure. The epithelial and mucosal structure was normalized and consisted of more integral mucosa [66][67]. Interleukins were also affected; specifically, IL-1β, IL-8 and IL-6 levels were diminished. The relative abundances of Lactobacillus, Bifidobacterium, Clostridium leptum, Bacteroides and Prevotella were increased after probiotic treatment [66][67].
Similar findings were observed in STZ-induced diabetic C57BL/6J mice in high-fat diet (T2DΜ) supplemented with L. casei CCFM419 [68]. Microbiota modulation was conducted with increased Allobaculum and Bacteroides genera and Bacteroidetes phylum abundances and decreased Firmicutes [68]. Post-probiotic ingestion, FBG and HBA1c and leptin levels were lower, 2-h postprandial blood glucose was reduced, and insulin sensitivity was improved by decreased fasting insulin concentration, as well as improvement of HOMA-IR value was noticed [68]. Inflammation was ameliorated with decreased levels of TNF-α, IL-6 and IL-10 [68]. Lipid control was conducted with reduced LDL-C and increased HDL-C levels [68]. Finally, there was a recovery of impaired islet cells and the expression of mRNAs of PI3K and GS, concerning the insulin resistance, was increased and GSK-3β mRNA expression was decreased [68].
L. casei Zhang, when administrated in STZ-induced diabetic Sprague-Dawley rats in high-fat sucrose diet (HFS) (T2DΜ), led to more abundant cecal Bifidobacterium and Lactobacillus genera and lower levels of C. coccoides–E. rectale group and C. scindens members [69]. Probiotic supplementation led to reduced endotoxin LPS production that had been induced by STZ and the onset and development of hyperglycemia in both fasting and postprandial 2 h blood glucose levels and OGTT levels was reversed [69]. What is more, pro-inflammatory cytokines (IFN-c and TNF-α) was inhibited after probiotic administration [69].
L. rhamnosus CCFM0528 provoked increased Bacteroidetes and decreased Firmicutes in phyla level and elevated Bifidobacterium, Lactobacillus, Allobaculum and Bacteroides genera in STZ-induced diabetic C57BL/6J mice in high-fat diet (T2DM) accompanied with amelioration of insulin resistance, glucose tolerance, FBG and postprandial 2-h blood glucose; TNF-α and IL-6 production were decreased and GLP-1 was increased [70].
All of the above-mentioned studies presented in detail in Table 1, provided similar results and reach to the same conclusion. Prebiotics, as well as probiotics and foods enriched with such ingredients can promote gut health via enhancement of the presence of beneficial bacteria genera and accumulation of advantages for the host immune system and metabolic regulation.
2.3. Human Studies
A range of food supplements are consumed by various groups of people with the aim of improving health. Several clinical studies have been conducted so far, especially with probiotics per se. There are also a small number of studies in which food products enriched with specific beneficial microbes are examined. These foods include mainly fermented milk products.
Allen et al. [71] evaluated the safety of a bacterial dietary supplement for the prevention of atopy in infants in a randomized, double-blinded, placebo-controlled trial. Two strains of lactobacilli (L. salivarius CUL61 and L. paracasei CUL08) and bifidobacteria (B. animalis subsp. lactis CUL34 and B. bifidum CUL20) with a total of 1 x 1010 colony-forming units were administered daily to women during the last month of pregnancy and to infants aged 0–6 months, with beneficial results [71].
Kassaian et al. [72] assessed the effects of probiotics and synbiotics on metabolic syndrome in individuals with prediabetes. One hundred and twenty adults with prediabetes were enrolled in a double-blind, placebo-controlled randomized parallel-group clinical trial [72]. Participants were randomized to a multi-species probiotic or inulin-based synbiotic or placebo. The potential benefits of using probiotic and synbiotic for metabolic syndrome management in prediabetes have been supported by the results, which provided an important strategy to combat metabolic syndrome-associated diseases [72].
Khalili et al. [73] divided forty patients with T2DM (n=20 for each group) into intervention (probiotic) and placebo groups. The intervention group received a daily capsule containing 108 cfu of L. casei for eight weeks [73]. The patients in placebo group took capsules containing maltodextrin for the same time duration. In comparison with the placebo, L. casei supplementation significantly increased SIRT1 and decreased fetuin-A levels at the end of the trial in a way that improved glycemic response in subjects with T2DM [73]. Affecting the SIRT1 and fetuin-A levels introduced a new known mechanism of probiotic action in diabetes management [73].
Medina-Vera et al. [74] studied the effects of a functional food-based dietary intervention on fecal microbiota and biochemical parameters in patients with T2DΜ. In a placebo-controlled, randomized, double-blind study 81 patients with T2DM were divided into two 3-month treatment groups: one following a reduced-energy diet with a dietary portfolio (DP) comprising high-fiber, polyphenol-rich and vegetable-protein functional foods, the other taking a placebo (P) [74]. Patients with T2DΜ exhibited intestinal dysbiosis characterized by an increase in Prevotella copri [74]. Dietary intervention with functional foods significantly modified fecal microbiota compared with P group by increasing alpha diversity and modifying the abundance of specific bacteria, independently of antidiabetic drugs [74]. There was a decrease in P. copri and an increase in Faecalibacterium prausnitzii and Akkermansia muciniphila, two bacterial species known to have anti-inflammatory effects [74]. The DP group also exhibited significant reductions in areas under the curve for glucose, TC and LDL-C [74].
Sabico et al. [75] studied the effects of 6-months multi-strain probiotics supplementation in T2DM in a randomized, double-blind, placebo-controlled trial and concluded in the beneficial role of probiotics in inflammation, promising adjuvant anti-diabetes therapy [75].
Gut bacterial translocation to the blood may play an important role in the development of insulin resistance in T2DM. Sato et al. [76] investigated whether probiotics could reduce bacterial translocation and cause changes in the gut microbiota, in two groups of Japanese; the probiotic group that consumed Lactobacillus casei strain Shirota-fermented milk, and the control group administered no probiotics [76]. Probiotic administration reduced bacterial translocation and altered the gut microbiota in Japanese patients with T2DM [76].
In a randomized, double-blind, placebo-controlled study, participants were assigned into two groups: a probiotic group, consuming fermented milk containing L. acidophilus La-5 and B. animalis subsp lactis BB-12 (109 colony-forming units/d, each) and a control group, consuming conventional fermented milk [77]. Probiotic consumption improved the glycemic control in T2DM subjects. However, the intake of fermented milk seems to be involved with other metabolic changes, such as decrease in inflammatory cytokines (TNF-a and resistin) and increase in acetic acid [77]. Furthermore, dietary intervention by consumption of yogurt with live probiotics and other dairy products seems to improve the antioxidant status and FPG levels in T2DM patients [78][79].
References
- World Health Organization. Diabetes. Available online: (accessed on 19 November 2020).
- International Diabetes Federation. Facts and Figures. Available online: (accessed on 26 December 2020).
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223.
- Guo, C.; Chen, W.D.; Wang, Y.D. TGR5, Not only a metabolic regulator. Front. Physiol. 2016, 7, 646.
- WebMD. Types of Diabetes Mellitus. Available online: (accessed on 19 November 2020).
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553.
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Ismail, N.A.M.; Razalli, N.H.; Gnanou, J.V.; Ali, R.A.R. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188.
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779.
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42.
- Sell, H.; Habich, C.; Eckel, J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 2012, 8, 709–716.
- Yoon, J.W.; Jun, H.S. Autoimmune destruction of pancreatic beta cells. Am. J. Ther. 2005, 12, 580–591.
- Karlsson, F.A.; Berne, C.; Björk, E.; Kullin, M.; Li, Z.; Ma, J.Y.; Schölin, A.; Zhao, L. Beta-cell activity and destruction in type 1 diabetes. Ups. J. Med. Sci. 2000, 105, 85–95.
- Filippi, C.M.; von Herrath, M.G. Viral trigger for type 1 diabetes: Pros and cons. Diabetes 2008, 57, 2863–2871.
- Beik, P.; Ciesielska, M.; Kucza, M.; Kurczewska, A.; Kuźmińska, J.; Maćkowiak, B.; Niechciał, E. Prevention of Type 1 Diabetes: Past Experiences and Future Opportunities. J. Clin. Med. 2020, 9, 2805.
- Stefanaki, C.; Peppa, M.; Mastorakos, G.; Chrousos, G.P. Examining the gut bacteriome, virome, and mycobiome in glucose metabolism disorders: Are we on the right track? Metab. Clin. Exp. 2017, 73, 52–66.
- World Health Organization. Global Report on Diabetes. Available online: (accessed on 19 November 2020).
- Mauri-Obradors, E.; Estrugo-Devesa, A.; Jané-Salas, E.; Viñas, M.; López-López, J. Oral manifestations of Diabetes Mellitus. A systematic review. Med. Oral. Patol. Oral. Cir. Bucal. 2017, 22, e586–e594.
- Connolly, V.; Unwin, N.; Sherriff, P.; Bilous, R.; Kelly, W. Diabetes prevalence and socioeconomic status: A population based study showing increased prevalence of type 2 diabetes mellitus in deprived areas. J. Epidemiol. Community Health 2000, 54, 173–177.
- Rayappa, Ph.; Raju, K.; Kapur, A.; Björk, S.; Sylvest, C.; Kumar, D. The Impact of socio-economic factors on diabetes care. Int. J. Diab. Dev. Ctries. 1999, 19, 7–15.
- Anjana, R.M.; Deepa, M.; Pradeepa, R.; Mahanta, J.; Narain, K.; Das, H.K.; Adhikari, P.; Rao, P.V.; Saboo, B.; Kumar, A.; et al. Prevalence of diabetes and prediabetes in 15 states of India: Results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017, 5, 585–596.
- Statista. Prevalence of Diabetes in Europe in 2019. Available online: (accessed on 19 November 2020).
- Monjiote, D.P.; Leo, E.E.M.; Campos, M.R.S. Functional and Biological Potential of Bioactive Compounds in Foods for the Dietary Treatment of Type 2 Diabetes Mellitus. Functional Food—Improve Health through Adequate Food; Hueda, M.C., Ed.; IntechOpen: London, UK, 2017.
- Mancebo, F. Urbanism. In Encyclopedia of Community: From the Village to the Virtual World; Sage: New York, NY, USA, 2003; pp. 1428–1433.
- Stavropoulou, E.; Tsigalou, C.; Bezirtzoglou, E. Functions of the Human Intestinal Microbiota in Relation to Functional Foods. Erciyes Med. J. 2018, 40, 188–193.
- Bezirtzoglou, E.; Stavropoulou, E. Immunology and probiotic impact of the newborn and young children intestinal microflora. Anaerobe 2011, 17, 369–374.
- Food & Nutrition Magazine. The History and Health Benefits of Fermented Food. Available online: (accessed on 29 November 2020).
- Smid, E.J.; Hugenholtz, J. Functional Genomics for Food Fermentation Processes. Annu. Rev. Food Sci. Technol. 2010, 1, 497–519.
- Montet, D.; Ray, R. Fermented Foods: Part I: Biochemistry & Biotechnology. Fermented Foods; Routledge: Abingdon, UK, 2015.
- Adesulu-Dahunsi, A.T.; Dahunsi, S.O.; Olayanju, A. Synergistic microbial interactions between lactic acid bacteria and yeasts during production of Nigerian indigenous fermented foods and beverages. Food Control 2020, 110, 106963.
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192.
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics as a weapon in the fight against COVID-19. Front. Nutr. 2020, 7, 614986.
- Elbron, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531.
- Iwatani, S.; Yamamoto, N. Functional food products in Japan: A review. Food Sci. Hum. Well. 2019, 8, 96–101.
- Nutraceutical and Functional Food Regulations in the United States and around the World; Elsevier: Amsterdam, The Netherlands, 2019.
- Fukushima, Y.; Kawata, Y.; Hara, H.; Terada, A.; Mitsuoka, T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int. J. Food Microbiol. 1998, 42, 39–44.
- Küster-Boluda, I.; Vidal-Capilla, I. Consumer attitudes in the election of functional foods. Spanish J. Mark. ESIC 2017, 21, 65–79.
- Steinhagen, F.; Schmidt, S.V.; Schewe, J.C.; Peukert, K.; Klinman, D.M.; Bode, C. Immunotherapy in sepsis—Brake or accelerate? Pharmacol. Ther. 2020, 208, 107476.
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281.
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310.
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean Diet as a Tool to Combat Inflammation and Chronic Diseases. An Overview. Biomedicines 2020, 8, 201.
- GMFH—Best of Nutrition Diet. Available online: (accessed on 29 November 2020).
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47.
- Tonucci, L.B.; Dos Santos, K.M.O.; Ferreira, C.L.D.L.F.; Ribeiro, S.M.R.; De Oliveira, L.L.; Martino, H.S.D. Gut microbiota and probiotics: Focus on diabetes mellitus. Crit. Rev. Food Sci. Nutr. 2017, 57, 2296–2309.
- Yao, K.; Zeng, L.; He, Q.; Wang, W.; Lei, J.; Zou, X. Effect of Probiotics on Glucose and Lipid Metabolism in Type 2 Diabetes Mellitus: A Meta-Analysis of 12 Randomized Controlled Trials. Med. Sci. Monit. 2017, 23, 3044–3053.
- Honda, K.; Moto, M.; Uchida, N.; He, F.; Hashizume, N. Anti-diabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J. Clin. Biochem. Nutr. 2012, 51, 96–101.
- Silva, J.C.P.; Jones, J.G. Improving Metabolic Control Through Functional Foods. Curr. Med. Chem. 2020, 26, 3424–3438.
- Kerry, R.G.; Das, G.; Golla, U.; Rodriguez-Torres, M.D.; Shin, H.; Patra, J.K. Engineered probiotic and prebiotic nutraceutical supplementations in combating non-communicable disorders: A review. Curr. Pharm. Biotechnol. 2020, 21, 33050862.
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894.
- Al-Awar, A.; Kupai, K.; Veszelka, M.; Szucs, G.; Attieh, Z.; Murlasits, Z.; Varga, C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016, 2016, 9051426.
- Xue, M.; Liua, Y.; Xu, H.; Zhou, Z.; Ma, Y.; Sun, T.; Liu, M.; Zhang, H.; Liang, H. Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomed. Pharmacother. 2019, 118, 109393.
- Terzo, S.; Mulè, F.; Caldara, G.F.; Baldassano, S.; Puleio, R.; Vitale, M.; Cassata, G.; Ferrantelli, V.; Amato, A. Pistachio Consumption Alleviates Inflammation and Improves Gut Microbiota Composition in Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2020, 21, 365.
- Yanni, AE.; Mitropoulou, G.; Prapa, I.; Agrogiannis, G.; Kostomitsopoulos, N.; Bezirtzoglou, E.; Kourkoutas, Y.; Karathanos, V.T. Functional modulation of gut microbiota in diabetic rats following dietary intervention with pistachio nuts (Pistacia vera L.). Metab. Open 2020, 7, 100040.
- Zhong, Y.; Nyman, M.; Fak, F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol. Nutr. Food Res. 2015, 59, 2066–2076.
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet-induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2018, 62, 78–88.
- Zhang, H.H.; Liu, J.; Lv, Y.J.; Jiang, Y.I.; Pan, J.X.; Zhu, Y.J.; Huang, M.G.; Zhang, S.K. Changes in Intestinal Microbiota of Type 2 Diabetes in Mice in Response to Dietary Supplementation With Instant Tea or Matcha. Can. J. Diabetes 2020, 44, 44–52.
- Surono, I.S.; Wardana, A.A.; Waspodo, P.; Saksono, B.; Verhoeven, J.; Venema, K. Effect of functional food ingredients on gut microbiota in a rodent diabetes model. Nutr. Metab. 2020, 17, 77.
- Sane, F.; Scuotto, A.; Pierrat, V.; Kacet, N.; Hober, D.; Romond, M.B. Diabetes progression and alterations in gut bacterial translocation: Prevention by diet supplementation with human milk in NOD mice. J. Nutr. Biochem. 2018, 62, 108–122.
- Shikano, A.; Kuda, T.; Shibayama, J.; Toyama, A.; Ishida, Y.; Takahashi, H.; Kimura, B. Effects of lactobacillus plantarum uruma-SU4 fermented green loofah on plasma lipid levels and gut microbiome of high-fat diet fed mice. Food Res. Int. 2019, 121, 817–824.
- Singh, S.; Sharma, R.K.; Malhotra, S.; Pothuraju, R.; Shandilya, U.K. Lactobacillus rhamnosus NCDC17 ameliorates type-2 diabetes by improving gut function, oxidative stress and inflammation in high-fat-diet fed and streptozotocin treated rats. Benef. Microbes 2017, 8, 243–255.
- Cabello-Olmo, Μ.; Oneca, Μ.; Torre, P.; Sainz, N.; Moreno-Aliaga, M.J.; Guruceaga, E.; Díaz, J.V.; Encio, I.J.; Barajas, M.; Araña, M. A Fermented Food Product Containing Lactic Acid Bacteria Protects ZDF Rats from the Development of Type 2 Diabetes. Nutrients 2019, 11, 2530.
- Hu, T.G.; Wen, P.; Shen, W.Z.; Liu, F.; Li, Q.; Li, E.N.; Liao, S.T.; Wu, H.; Zou, Y.X. Effect of 1-Deoxynojirimycin Isolated from Mulberry Leaves on Glucose Metabolism and Gut Microbiota in a Streptozotocin-Induced Diabetic Mouse Model. J. Nat. Prod. 2019, 82, 2189–2200.
- Shah, N.J.; Swami, O.C. Role of probiotics in diabetes: A review of their rationale and efficacy. EMJ Diabet. 2017, 5, 104–110.
- Dolpady, J.; Sorini, C.; Di Pietro, C.; Cosorich, I.; Ferrarese, R.; Saita, D.; Clementi, M.; Canducci, F.; Falcone, M. Oral Probiotic VSL#3 Prevents Autoimmune Diabetes by Modulating Microbiota and Promoting Indoleamine 2,3-Dioxygenase-Enriched Tolerogenic Intestinal Environment. J. Diabetes Res. 2016, 2016, 7569431.
- Morshedi, M.; Saghafi-Asl, M.; Hosseinifard, E.S. The potential therapeutic effects of the gut microbiome manipulation by symbiotic containing-Lactobacillus plantarum on neuropsychological performance of diabetic rats. Transl. Med. 2020. 18, 1–14.
- Farida, E.; Nuraida, L.; Giriwono, P.E.; Jenie, B.L.E. Lactobacillus rhamnosus Reduces Blood Glucose Level through Downregulation of Gluconeogenesis Gene Expression in Streptozotocin-Induced Diabetic Rats. International Journal of Food Science 2020, 2020, 6108575.
- Tian, P.; Li, B.; He, C.; Song, W.; Hou, A.; Tian, S.; Meng, X.; Lia, K.; Shan, Y. Antidiabetic (type 2) effects of Lactobacillus G15 and Q14 in rats through regulation of intestinal permeability and microbiota. Food Funct. 2016, 7, 3789–3797.
- Li, K.K.; Tian, P.J.; Wang, S.D.; Lei, P.; Qu, L.; Huang, J.P.; Shan, Y.J.; Li, B.L. Targeting gut microbiota: Lactobacillus alleviated type 2 diabetes via inhibiting LPS secretion and activating GPR43 pathway. J. Funct. Foods 2017, 38, 561–570.
- Li, X.; Wang, E.; Yin, B.; Fang, D.; Chen, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Effects of Lactobacillus casei CCFM419 on insulin resistance and gut microbiota in type 2 diabetic mice. Benef 2017, 8, 421–432.
- Zhang, Y.; Guo, X.; Guo, J.; He, Q.; Li, H.; Song, Y.; Zang, H. Lactobacillus casei reduces susceptibility to type 2 diabetes via microbiota-mediated body chloride ion influx. Sci. Rep. 2014, 4, 5654.
- Wang, G.; Li, X.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus casei CCFM419 attenuated type 2 diabetes via a gut microbiota dependent mechanism. Food Funct. 2017, 8, 3155–3164.
- Allen, S.J.; Jordan, S.; Storey, M.; Thornton, C.A.; Gravenor, M.; Garaiova, I.; Plummer, S.F.; Wang, D.; Morgan, G. Dietary supplementation with lactobacilli and bifidobacteria is well tolerated and not associated with adverse events during late pregnancy and early infancy. J. Nutr. 2010, 140, 483–488.
- Kassaian, N.; Feizi, A.; Aminorroaya, A.; Amini, M. Probiotic and synbiotic supplementation could improve metabolic syndrome in prediabetic adults: A randomized controlled trial. Diabetes Metab. Syndr. 2019, 13, 2991–2996.
- Khalili, L.; Alipour, B.; Jafar-Abadi, M.A.; Faraji, I.; Hassanalilou, T.; Mesgari Abbasi, M.; Vaghef-Mehrabany, E.; Sani, A.M. The Effects of Lactobacillus casei on Glycemic Response, Serum Sirtuin1 and Fetuin-A Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Iran Biomed. J. 2019, 23, 68–77.
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-López, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019, 45, 122–131.
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ansari, A.M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1561–1569.
- Sato, J.; Kanazawa, A.; Azuma, K.; Ikeda, F.; Goto, H.; Komiya, K.; Kanno, R.; Tamura, Y.; Asahara, T.; Takahashi, T.; et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Sci. Rep. 2017, 7, 12115.
- Tonucci, L.B.; Dos Santos, O.K.M.; de Oliveira, L.L.; Ribeiro, R.S.M.; Martino, D.H.S. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92.
- Yanni, A.E.; Kartsioti, K.; Karathanos, V.T. The role of yogurt consumption in the management of type 2 diabetes. Food Funct. 2020, 11, 10306–10316.
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543.





