| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vasso Apostolopoulos | + 2776 word(s) | 2776 | 2021-03-05 09:58:12 | | | |
| 2 | Rita Xu | -1480 word(s) | 1296 | 2021-03-16 09:00:07 | | |
Video Upload Options
Functional and nutraceutical foods provide an alternative way to improve immune function to aid in the management of various diseases. With the development of research into nutraceuticals, dietary polyphenols are getting attention due to their immunomodulatory role. There is evidence that dietary polyphenols can influence dendritic cells, have an immunomodulatory effect on macrophages, increase proliferation of B cells, T cells, and suppress Type 1 T helper (Th1), Th2, Th17, and Th9 cells. Further, polyphenols have a beneficial role in the prevention and treatment of inflammatory bowel disease, allergy, asthma, and auto-immune diseases (type 1 diabetes, rheumatoid arthritis, and multiple sclerosis).
1. Introduction
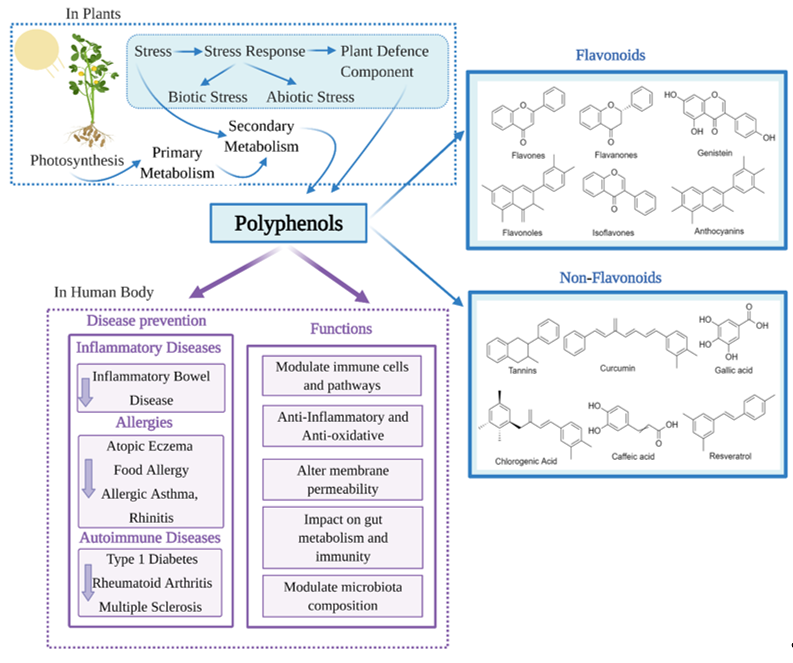
With advancing knowledge of the importance of adequate nutrition, and increased public health awareness about diet, there is growing attention on the health benefits of natural products including those that are rich in polyphenols. Polyphenols are the most extensive group of non-energetic secondary metabolites and are produced by plants in response to stress [1] (Figure 1). Polyphenols have been called ‘lifespan essentials’ due to their significant impact on health [2]. There are as many as 8000 different polyphenols which are divided into different classes based on their chemical structure. Despite the different classifications, all polyphenols have the key structural features of an aromatic ring and at least one hydroxyl group [3][4]. Dietary polyphenols are abundant in plant-based foods such as fruits, vegetables, dry legumes, cereals, olives, cocoa, tea, coffee and wine [5]. Some common dietary polyphenols include the lignins present in nuts and whole-grain cereals; pro-anthocyanidins in grapes, pine bark and cocoa; anthocyanins/anthocyanidins in brightly colored fruits and vegetables like berries; isoflavones in soybeans; catechins in green tea, grapes and wine; tannins in tea and nuts; quercetin in grapes and onion; resveratrol in wines and naringenin/hesperidin in citrus fruits [6].
Figure 1. Classification and health benefits of polyphenols.
Research into the beneficial health effects of polyphenols has increased considerably over the last two decades [7]. Polyphenols have shown anti-inflammatory, antimicrobial, antioxidant, anticarcinogenic, antiadipogenic, antidiabetic and neuroprotective effects [8][9][10][11][12]. Polyphenols may also counteract cytotoxicity and apoptosis due to their immunomodulatory properties [13] and regulate innate and adaptive immunity. Polyphenols have also been shown to reduce oxidative stress and inflammation [14], modulate immune cells, regulate gut microbiota composition and immunity (Figure 1). Through this regulation of the immune system, polyphenols could beneficially impact a number of chronic diseases [15].
2. Immune Modulation of Polyphenols to Immune Cells
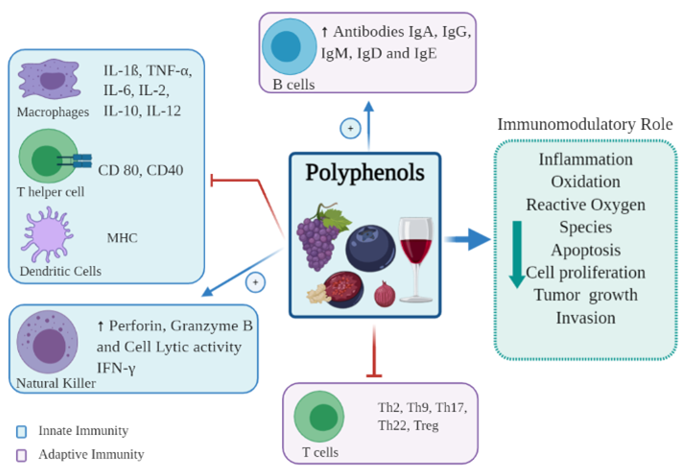
The immune system as a whole consists of innate and adaptive immunity, each with different roles and functions [16]. The innate immune system is the first line of defense, and protects against foreign antigens through the skin, pulmonary system, and gut epithelial cells, forming a barrier between the organism and its environment [17]. The innate system is broadly divided into cellular and non-cellular systems. The cellular system consists of several cell subsets, including dendritic cells (DCs), monocytes, macrophages, granulocytes and natural killer (NK) cells. The non-cellular system is very diverse, ranging from simple mucus barriers to complex protein pathways, such as the complement cascade, however all function to prevent pathogen entry, and facilitate pathogen destruction by phagocytosis [18]. The adaptive immune system comprises T and B cells. B cells secrete antibodies, whilst T cells are involved in the production of cytokines, direct cytotoxic destruction of infected or malignant tissue, and activation of other immune cells [16]. Polyphenols modulate immune responses in both the innate and adaptive systems, having both stimulatory and inhibitory effects in different areas [19] (Figure 2).
Figure 2. Immunomodulatory effects of polyphenols on immune cells.
2.1. Effects of Polyphenols on Dendritic Cells
DCs are the most potent antigen-presenting cells which act to prime the adaptive immune system to recognize foreign antigens, and so are vital in the initiation and regulation of the adaptive immune response [20]. It has been shown that polyphenols can influence the differentiation of DCs [21]. In fact, resveratrol has been identified as affecting human DC differentiation from monocytes, with a strong potential for regulatory action [22]. Likewise, epigallocatechin gallate (EGCG) induces apoptosis and affects the phenotype of developing DCs. Molecules that are essential for antigen presentation by DCs such as CD83, CD80, CD11c, and major histocompatibility complex (MHC) class II, are downregulated by EGCG, suggesting an immunosuppressive action [23]. Other polyphenols, including EGCG, curcumin, quercetin, apigenin, silibinin, and blackberry polyphenols cause inhibition of murine bone marrow-derived DC maturation and expression of MHC molecules, reducing antigen uptake and decreasing secretion of the pro-inflammatory cytokines interleukin-1 (IL-1), IL-2, IL-6, IL-12 [24][25][26][27]. A study in an animal model showed that administration of fisetin (50 mg/kg) decreased DC migration and DC allo-stimulatory capacity [28]. Similarly, in vitro resveratrol has an inhibitory effect on DC maturation [29].
2.2. Effects of Polyphenols on Monocytes and Macrophages
Macrophages are phagocytes that ingest pathogens and dead cells, which differentiate from the transitory monocyte. Like DCs, macrophages can also function as antigen-presenting cells (albeit with less potent activity) being able to activate naïve T cells into effector T cells in the presence of an antigen [19]. Macrophages play an important role in inflammation, host defense, and tissue repair [30][31]. Importantly, macrophages also play a pathogenic role in various chronic diseases including asthma, inflammatory bowel disease, atherosclerosis and rheumatoid arthritis [31][32][33]. Macrophages are classically considered in two categories, known as polarization: the classical inflammatory M1 and immunosuppressive/anabolic M2 phenotypes. Initiation of M1 differentiation is by interferon-γ (IFN-γ) stimulation and the activation of toll-like receptors (TLRs) by bacterial lipopolysaccharides (LPS); while M2 polarization is triggered by IL-4 [34]. It has been shown that polyphenolic cocoa extract suppressed M1 mediated inflammation and drove M2 polarization of activated macrophages [35]. Polyphenol-rich green tea has anti-tumor effects secondary to the activation of macrophages and NK cells [36]. Inonotus sanghuang, a plant known for its medicinal value, rich in rutin, quercetin, quercitrin, isorhamnetin and chlorogenic acid, has been shown to reduce inflammation by modulating the interaction between macrophages and adipocytes. It was suggested that in this way it may improve insulin resistance and metabolic syndrome [37]. Moreover, Overman et al. reported that grape powder extract decreased LPS-stimulated inflammation in macrophages and reduce insulin resistance [38].
Monocytes and macrophages play a fundamental role in the progression of atherosclerosis [35]. Increased oxidative stress causes low-density lipoprotein oxidation (oxLDL), with the resulting lipoproteins engulfed by macrophages resulting in the formation of foam cells. This process triggers an inflammatory response in the neighboring endothelial cells which secrete pro-inflammatory cytokines and chemokines [39][40][41]. When monocytes migrate towards the intima, they transform into macrophages on stimulation by macrophage colony-stimulating factor, increasing the expression of scavenger receptors outside the cell [39][40]. Polyphenols are known to regulate this interplay between immune and vascular endothelial function. Evidence has shown that polyphenols reduce atherosclerotic progression by increasing high-density lipoprotein (HDL) levels and decrease LDL accumulation in macrophages, reducing foam cell formation [3][42].
2.3. Effects of Polyphenols on Natural Killer Cells
NK cells are a subset of lymphocytes, but are part of the innate immune response, with the function of eliminating infected or malignant cells [19]. NK cells have a strong cytolytic function and a considerable role in immune regulation [43]. NK cells are activated by CD4+ T cell secretion of IL-2 and IFN-γ [44]. Once activated NK cells secrete perforin and granzyme B, which induce apoptosis and necrosis in target cells. Polyphenols have immunomodulatory effects on NK cells, increasing their number and activity. Green tea catechin metabolites increase NK cell cytotoxicity [45] and quercetin enhances NK cell lytic activity [46] in animal models. In a clinical trial, healthy participant prescribed a diet low in polyphenols and supplemented with juices rich in polyphenols increased lymphocyte proliferative responsiveness, IL-2 secretion and lytic activity by NK cells [47]. Berries rich in flavonoids and pro-anthocyanidins have a cancer-preventive effect but are also involved in the modulation of NK cells [48]. A study in marathon runners noted that daily consumption of 250 g of blueberries for six weeks resulted in doubled NK cell counts [49]. Evidence showed that purple sweet potato leaves that are rich in flavonoids enhanced the lytic activity of NK cells in 16 healthy participants [50].
References
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020, 2020, 9081686.
- Chandrasekara, A.; Shahidi, F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 2010, 58, 6706–6714.
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65.
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 2015, 31, 28–37.
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422.
- Martin, K.R.; Appel, C.L. Polyphenols as dietary supplements: A double-edged sword. Nutr. Diet. Suppl. 2009, 2, 1–12.
- Quiñones, M.; Miguel, M.; Aleixandre, A. Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 2013, 68, 125–131.
- Cassidy, A.; O’Reilly, É.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347.
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: A randomized clinical trial. Clin. Nutr. 2013, 32, 200–206.
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402.
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751.
- Ahtesh, F.B.; Stojanovska, L.; Feehan, J.; de Courten, M.P.; Flavel, M.; Kitchen, B.; Apostolopoulos, V. Polyphenol Rich Sugar Cane Extract Inhibits Bacterial Growth. Prilozi 2020, 41, 49–57.
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306.
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797.
- Wolowczuk, I.; Verwaerde, C.; Viltart, O.; Delanoye, A.; Delacre, M.; Pot, B.; Grangette, C. Feeding our immune system: Impact on metabolism. Clin. Dev. Immunol. 2008, 2008, 639803.
- Medzhitov, R.; Janeway, C. Innate immunity. N. Engl. J. Med. 2000, 343, 338–344.
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859.
- Clark, R.; Kupper, T. Old meets new: The interaction between innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 629–637.
- Hachimura, S.; Totsuka, M.; Hosono, A. Immunomodulation by food: Impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 2018, 82, 584–599.
- Buckwalter, M.R.; Albert, M.L. Orchestration of the immune response by dendritic cells. Curr. Biol. 2009, 19, R355–R361.
- del Cornò, M.; Scazzocchio, B.; Masella, R.; Gessani, S. Regulation of dendritic cell function by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2016, 56, 737–747.
- Švajger, U.; Obermajer, N.; Jeras, M. Dendritic cells treated with resveratrol during differentiation from monocytes gain substantial tolerogenic properties upon activation. Immunology 2010, 129, 525–535.
- Yoneyama, S.; Kawai, K.; Tsuno, N.H.; Okaji, Y.; Asakage, M.; Tsuchiya, T.; Yamada, J.; Sunami, E.; Osada, T.; Kitayama, J. Epigallocatechin gallate affects human dendritic cell differentiation and maturation. J. Allergy Clin. Immunol. 2008, 121, 209–214.
- Lee, J.S.; Kim, S.G.; Kim, H.K.; Lee, T.H.; Jeong, Y.I.; Lee, C.M.; Yoon, M.S.; Na, Y.J.; Suh, D.S.; Park, N.C. Silibinin polarizes Th1/Th2 immune responses through the inhibition of immunostimulatory function of dendritic cells. J. Cell. Physiol. 2007, 210, 385–397.
- Huang, R.-Y.; Yu, Y.-L.; Cheng, W.-C.; OuYang, C.-N.; Fu, E.; Chu, C.-L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010, 184, 6815–6821.
- Yoon, M.-S.; Lee, J.S.; Choi, B.-M.; Jeong, Y.-I.; Lee, C.-M.; Park, J.-H.; Moon, Y.; Sung, S.-C.; Lee, S.K.; Chang, Y.H. Apigenin inhibits immunostimulatory function of dendritic cells: Implication of immunotherapeutic adjuvant. Mol. Pharmacol. 2006, 70, 1033–1044.
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99.
- Liu, S.-H.; Lin, C.-H.; Hung, S.-K.; Chou, J.-H.; Chi, C.-W.; Fu, S.-L. Fisetin inhibits lipopolysaccharide-induced macrophage activation and dendritic cell maturation. J. Agric. Food Chem. 2010, 58, 10831–10839.
- Buttari, B.; Profumo, E.; Facchiano, F.; Ozturk, E.I.; Segoni, L.; Saso, L.; Riganò, R. Resveratrol prevents dendritic cell maturation in response to advanced glycation end products. Oxidative Med. Cell. Longev. 2013, 2013, 574029.
- Bottazzi, B.; Doni, A.; Garlanda, C.; Mantovani, A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu. Rev. Immunol. 2009, 28, 157–183.
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737.
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204.
- Kamada, N.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kobayashi, T.; Sato, T.; Sakuraba, A.; Kitazume, M.T.; Sugita, A.; Koganei, K. Unique CD14+ intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-γ axis. J. Clin. Investig. 2008, 118, 2269–2280.
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795.
- Dugo, L.; Belluomo, M.G.; Fanali, C.; Russo, M.; Cacciola, F.; Maccarrone, M.; Sardanelli, A.M. Effect of cocoa polyphenolic extract on macrophage polarization from proinflammatory M1 to anti-inflammatory M2 state. Oxidative Med. Cell. Longev. 2017, 2017, 6293740.
- Park, H.-R.; Hwang, D.; Suh, H.-J.; Yu, K.-W.; Kim, T.Y.; Shin, K.-S. Antitumor and antimetastatic activities of rhamnogalacturonan-II-type polysaccharide isolated from mature leaves of green tea via activation of macrophages and natural killer cells. Int. J. Biol. Macromol. 2017, 99, 179–186.
- Zhang, M.; Xie, Y.; Su, X.; Liu, K.; Zhang, Y.; Pang, W.; Wang, J. Inonotus sanghuang polyphenols attenuate inflammatory response via modulating the crosstalk between macrophages and adipocytes. Front. Immunol. 2019, 10, 286.
- Overman, A.; Bumrungpert, A.; Kennedy, A.; Martinez, K.; Chuang, C.C.; West, T.; Dawson, B.; Jia, W.; McIntosh, M. Polyphenol-rich grape powder extract (GPE) attenuates inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. Int. J. Obes. 2010, 34, 800–808.
- McLaren, J.E.; Michael, D.R.; Ashlin, T.G.; Ramji, D.P. Cytokines, macrophage lipid metabolism and foam cells: Implications for cardiovascular disease therapy. Prog. Lipid Res. 2011, 50, 331–347.
- Moss, J.W.E.; Ramji, D.P. Nutraceutical therapies for atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 513.
- Ramji, D.P.; Davies, T.S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015, 26, 673–685.
- Sevov, M.; Elfineh, L.; Cavelier, L.B. Resveratrol regulates the expression of LXR-α in human macrophages. Biochem. Biophys. Res. Commun. 2006, 348, 1047–1054.
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100.
- Hu, J.-Y.; Zhang, J.; Cui, J.-L.; Liang, X.-Y.; Lu, R.; Du, G.-F.; Xu, X.-Y.; Zhou, G. Increasing CCL5/CCR5 on CD4+ T cells in peripheral blood of oral lichen planus. Cytokine 2013, 62, 141–145.
- Kim, Y.H.; Won, Y.-S.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green tea catechin metabolites exert immunoregulatory effects on CD4+ T cell and natural killer cell activities. J. Agric. Food Chem. 2016, 64, 3591–3597.
- Exon, J.H.; Magnuson, B.A.; South, E.H.; Hendrix, K. Dietary quercetin, immune functions and colonic carcinogenesis in rats. Immunopharmacol. Immunotoxicol. 1998, 20, 173–190.
- Bub, A.; Watzl, B.; Blockhaus, M.; Briviba, K.; Liegibel, U.; Müller, H.; Pool-Zobel, B.L.; Rechkemmer, G. Fruit juice consumption modulates antioxidative status, immune status and DNA damage. J. Nutr. Biochem. 2003, 14, 90–98.
- McAnulty, L.S.; Nieman, D.C.; Dumke, C.L.; Shooter, L.A.; Henson, D.A.; Utter, A.C.; Milne, G.; McAnulty, S.R. Effect of blueberry ingestion on natural killer cell counts, oxidative stress, and inflammation prior to and after 2.5 h of running. Appl. Physiol. Nutr. Metab. 2011, 36, 976–984.
- McAnulty, L.S.; Collier, S.R.; Landram, M.J.; Whittaker, D.S.; Isaacs, S.E.; Klemka, J.M.; Cheek, S.L.; Arms, J.C.; McAnulty, S.R. Six weeks daily ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014, 34, 577–584.
- Chen, C.-M.; Li, S.-C.; Lin, Y.-L.; Hsu, C.-Y.; Shieh, M.-J.; Liu, J.-F. Consumption of purple sweet potato leaves modulates human immune response: T-lymphocyte functions, lytic activity of natural killer cell and antibody production. World J. Gastroenterol. WJG 2005, 11, 5777.