
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | SAYAF MUSTAFA | + 2040 word(s) | 2040 | 2021-03-09 08:47:49 | | | |
| 2 | Rita Xu | Meta information modification | 2040 | 2021-03-10 04:17:16 | | |
Video Upload Options
Fe is extracted from Fe ore and converted into alloys. This metallurgical process is important. The raw materials of the iron-bearing mineral are introduced in the blast furnace (BF), wherein aside from Fe and C, other elements are also subjected to roasting in the furnace.
1. Introduction
A mineral is a naturally occurring chemical compound that has specific atomic structure, chemical composition, and physical properties [1]. Minerals can be found in different areas and phases. Most minerals (e.g., Fe) have wide industrial application in many aspects of the social economy, such as in steel making. Abundant iron-containing minerals are present in Fe ore, which mainly occurs in the form of rocks, magnetite (Fe3O4), hematite (Fe2O3), siderites (FeCO3), and limonite (2Fe2O3·3H2O) [2]. However, the ironmaking process causes environmental problems. The growing need for a dramatic reduction of greenhouse gas emissions leads to the development of innovative technologies to reduce energy consumption and emissions in this process [3].
1.1. Ironmaking Process
Fe is extracted from Fe ore and converted into alloys. This metallurgical process is important. The raw materials of the iron-bearing mineral are introduced in the blast furnace (BF), wherein aside from Fe and C, other elements are also subjected to roasting in the furnace [4]. Therefore, steel is produced by pig iron or hot metal and steel scrap. These methods can be performed using two types of furnaces, namely, basic oxygen (or basic oxygen steelmaking) and electric arc furnaces (BOF and EAF, respectively) [2][5]. In BOFs, approximately 75% of Fe comes from the hot metal produced during the BF process. In fact, BOF plays a key role in steel production [6] and produces almost 66% of the total crude steel. At present, over 93% of the total Fe production from ores is performed using BF. BF uses several Fe ores, such as magnetite (Fe3O4) and hematite (Fe2O3), as iron-bearing raw materials. These materials can use coke, pulverized coal, and lime or limestone as reducing agent, heat source, and fluxing agents, respectively. BF ironmaking mainly aims to produce a hot metal with consistent quality [5].
Pb–Zn refractory iron ore presents a complicated relationship because of Pb–Zn–Fe inlay, fine particles, and variable contents of lead and zinc impurities. Such variations influence the difference of migration and enrichment, which are important problems in Fe ore beneficiation and processing. Reduction roasting technology has become an important method for refractory Fe ore dressing because of its remarkable sorting index and the high utilization of Fe resources [7][8]. Fe can also be extracted from cyanide gold tailings through magnetization roasting process or by using sulfuric acid slag [7][9]. Fe and Zn are also recoverable through selective reduction and calcination from the Zn tailings in Ni Fe ores [10][11]. Fe and Ni can be recovered using microwave carbothermal reduction [8], in which a refractory Fe ore that contains Pb and Zn as impurities is subjected to roasting and reduction processes because of high temperature. Various types of Zn and Pb minerals influence complex intercalation relationships and diverse morphological changes.
However, the phase transition and migration of Pb and Zn impurities have not been explored. With the breakthrough of flash magnetization, relevant analysis can improve the efficiency of the magnetization roasting process and enhance the resources.
1.2. Impurities in the Ironmaking Process
The reactivity of coke in an operating BF is not only affected by an inherent mineral matter but also by recirculating alkalis [12]. BF sludge (BFS) is a dangerous waste product of pig ironmaking. In addition to Fe, BFS contains Zn, CaO, S, Pb, Cd, Cr, As, and alkali metals (e.g., K, Na, etc.) [13]. Cu, Ni, Cr, Cd, Pb, and Zn are among the most common heavy metal contaminants of industrial pollution [14][15]. The impacts of these chemicals in terms of impurities can be arranged according to the environmental risk indicator for heavy metals, where Cd > Zn > Cu > As > Pb [16]. Moreover, the deleterious elements that enter the smelting furnace include K, Na, Zn, and Pb [17].
2. Effect of Pb and Zn Impurities on the Ironmaking Process
The Fe and steel industry can directly determine the industrialization foundation of a country. In 2018, China’s crude steel output reached 928 million tons; the Fe ore dependence outside the country exceeded 80%. Given the complex metallogenic reasons and sources, Pb and Zn impurities are more or less contained in the raw materials for ironmaking. Both chemicals are harmful to BF ironmaking. The allowable amount of Pb and Zn in the raw materials should be less than 0.15 kg/t. The effect of these impurities on the furnace is evident, especially in refractory areas [18]. Traditional refractory materials for BF hearth lining comprise carbon bricks and ceramic cup. Nevertheless, these materials cannot meet the requirements for a long service life design of blast furnaces [19].
Pb and Zn are considered harmful impurities in BF ironmaking. Pb impurities can easily reduce, oxidize, expand, and form Pb-containing precipitation. Zn-containing dust seriously affects BF production. At present, problems related to the use and recycling of mineral wastes are common in integrated steel mills. The continued emphasis of environmental regulations on landfills and their storage has rendered the effective management of these wastes a high priority [20]. The direct recycling of Fe wastes in BF is disserved by their corresponding chemistry (0.1% Zn in the charge), as well as the presence of Pb [13]. To achieve effective continuous recycling of Fe units, the sources should be identified, and the structure should be determined to develop a device and suitable technology for the processing of Zn and Pb [21].
2.1. Effect of Pb on Ironmaking
The harmfulness of Pb to BF is mainly due to the destructive effect of liquid Pb that penetrates the furnace’s body and the oxidizing expansion. The economic and technical problems in conventional metal treatments are caused by complex compounds that are produced by a mixture of Pb sulfide and Zn ores [22]. The impact of Pb on BF can be summarized as follows. First, Pb infiltrates into the refractories of the furnace’s body. The oxidation and expansion produce internal stress, which damages the refractories, and even cracks the furnace shell. Pb infiltration in the carbon bricks of the furnace’s bottom causes the floatation of the refractory bricks and even leakage in the entire bottom part. Second, when the liquid Pb is denser than molten Fe, the former exhibits low fluidity and becomes insoluble in the latter. Excessive accumulation of liquid Pb in the hearth and hearth bottom causes the abnormal work of the furnaces, such as iron mouth and main ditch. Maintaining and plugging the skimmer is difficult, thereby resulting in iron-running accident. Third, Pb oxide and other components in the furnace form low melting point or eutectic compounds, adhere to the sinter and pellet, reduce the soft melting temperature of the sinter and pellet, adhere to the permeability of the BF column to the coke effects, and adhere to the furnace wall to form nodules, which affect the normal production of BF. In addition, the loading of Pb increases the coke rate [23]. Lastly, Pb vapor discharges outside the furnace, thereby causing potential safety hazards, environmental pollution, and Pb poisoning [15].
2.2. Effect of Zn on Ironmaking
The Zn content in the furnace, which enters through the ore and the furnace sludge, is high. The Zn input in the BF must be limited; hence, the excess Zn must be removed [4]. The effect of Zn on the BF can be elaborated as follows. First, the simultaneous evaporation, condensation, oxidation, and Zn circulation can lead to accumulation in the furnace. Second, the deposition of extremely fine particles on other molecules with large surface reduces the life of the BF and weakens the quality of the iron pig product [21][24]. Third, the increased Zn absorption of the sinter and pellet with temperature affects the permeability of the combustion furnace [25][26]. Fourth, Zn fluctuation can cause the node collapse in the BF and block the gas pipeline [27]. Fifth, Zn expands the coke reactivity list and diminishes the coke’s quality after reaction [28]. Sixth, the Zn enhancement and development in furnace bricks are the fundamental factors that prompt a rising bull in the furnace. The behavior of Zn inside the furnace brick structure is changed from thick to free when Zn is already inside, improved, and extended [17]. Lastly, BFS cannot be directly recycled because Zn is present at a maximum acceptable concentration (approximately 0.5%) [13][29] and might form crusts inside the furnace, thereby affecting the operation. The Zn vapor penetrates the lining of the furnace and causes damage [29].
2.3. Effect of Alkaline Elements on the Pb and Zn Present during the Ironmaking Process
In a complex furnace environment, the destructive effect of Pb and Zn on the brick’s body is not only caused by both chemicals alone, but also by many factors, such as the effect of alkali metals on the penetration of Pb and Zn and on the reduction reaction. K and Na enter the furnace from coke and ore in the form of oxides, silicates, and carbonates [5].
Alkali metals, Pb, and Zn not only affect the structure of the BF body, but also present other hazards. For example, when Na2O or K2O increases from 1 to 3 kg/t HM, the coke ratio increases by 13.13 and 8.51 kg/t HM, the gas utilization decreases by 4.04% and 2.67%, respectively, thereby reducing the Fe content and affecting the Pb and Zn [23].
When the temperature in the BF is lower than 450 K, the deposition of Zn will lead to C deposition, which may be due to the decomposition of ZnO; that is, a small part of ZnS and ZnO.Al2O3 as decomposing agents of CO into CO2 and C and the infiltration of the deposited C into the cracks of the lining bricks aggravate the damage in the bricks [11]. The circulation and strengthening of the alkali metals in the furnace will adversely affect the size and density of the coke, consequently reducing the Fe, Pb, and Zn contents [30].
Figure 1 and Figure 2 show the effect of harmful elements on gas utilization and coke rate, respectively. Figure 3 and Figure 4 show the variation of the coke rate under different Zn and Pb and Na2O and K2O loads, respectively.
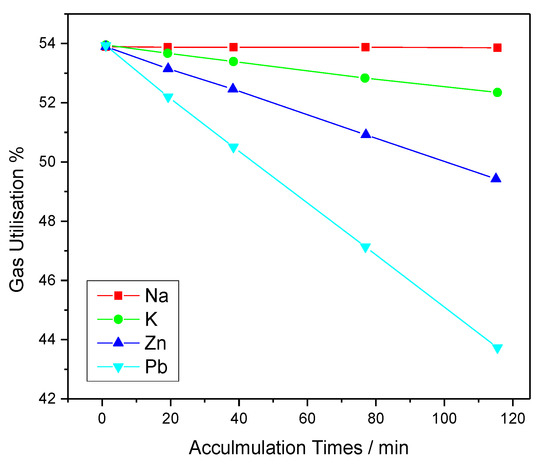
Figure 1. Effect of harmful elements on the gas utilization under various accumulation times.
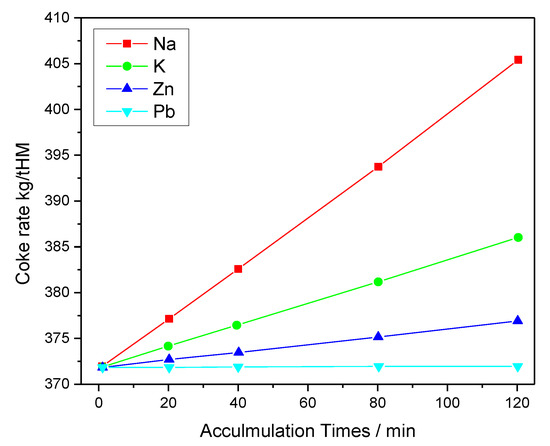
Figure 2. Effect of harmful elements on the coke rate under various accumulation times.
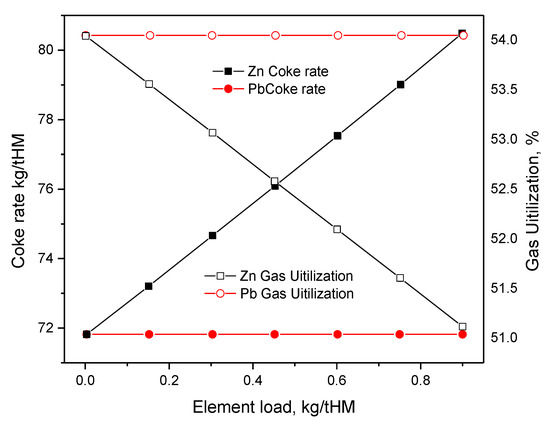
Figure 3. Variation of the coke rate under various Zn and Pb loads.
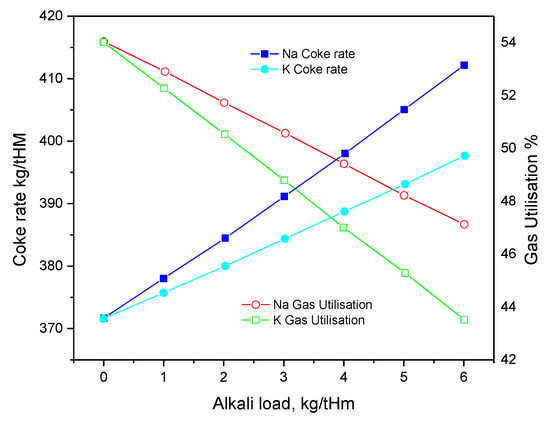
Figure 4. Variation of the coke rate under various Na2O and K2O loads.
A rise in loading times increases the coke rate, thereby leading to the accumulation of harmful elements. The loading of Na, K, Zn, and Pb into the BF increases the coke rate by 13.99, 6.25, 3.63, and 0.02 kg/t HM, respectively. The increased coke rate and the decreased gas utilization under various loads and accumulation times of each element was also estimated [23].
2.4. Environmental Effect of Pb and Zn Impurities
In steel wastes, the main constituents that are classified under the hazardous classification are potentially toxic elements (e.g., Pb and Zn) [13] and alkali metals (e.g., K) because of their concentration and potential environmental impact [31][32]. Large amounts of BF dust are landfilled, some of which contain several harmful elements [29]. The environmental impacts of these constituents strongly depend on their mineralogical and chemical forms. The analytical identification of solid matrices is a difficult and complex process [32]. The harmfulness of these elements to the environment is summarized in three aspects: (1) the leaching of Zn and Pb contaminates the groundwater [4][33][34], (2) the presence of harmful elements (e.g., Pb, Cd, Zn, and As) in a landfill violates environmental laws [20], and (3) recycling is hampered by the high Zn ratio, which causes several issues in the furnace operation because of the increase in Zn accumulation [31].
Further research should focus on the relevance of the process conditions (e.g., different counter pressure at the blast furnace top) and input composition with respect to the damage mechanism of the elements present in the BF lining. Such information can lead to the generation of the limits for these elements in the BF, as well as to the development of an improved internal recycling strategy (e.g., additional knowledge about the maximum chargeable Zn content, which can improve the amount of top gas dust internally recycled at the sinter plant) [18].
References
- Haldar, S.K. Chapter 13—Mineral Processing. In Mineral Exploration, 2nd ed.; Haldar, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 259–290.
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags—A review. Resour. Conserv. Recycl. 2019, 146, 244–255.
- Xu, R.; Dai, B.; Wang, W.; Schenk, J.; Xue, Z. Effect of iron ore type on the thermal behaviour and kinetics of coal-iron ore briquettes during coking. Fuel Process. Technol. 2018, 173, 11–20.
- Van Herck, P.; Carlo, V.; Rudy, V.; Ronald, M. Zinc and lead removal from blast furnace sludge with a hydrometallurgical process. Environ. Sci. Technol. 2000, 34, 3802–3808.
- Yang, Y.; Raipala, K.; Holappa, L. Chapter 1.1—Ironmaking. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 2–88.
- Ma, N. Recycling of basic oxygen furnace steelmaking dust by in-process separation of zinc from the dust. J. Clean. Prod. 2016, 112, 4497–4504.
- Bailong, L. Recovery of gold and iron from the cyanide tailings by magnetic roasting. Rare Met. Mater. Eng. 2013, 42, 1805–1809.
- Forster, J.; Pickles, C.A.; Elliott, R. Microwave carbothermic reduction roasting of a low grade nickeliferous silicate laterite ore. Miner. Eng. 2016, 88, 18–27.
- Zhang, Y.; Li, H.; Yu, X. Recovery of iron from cyanide tailings with reduction roasting–water leaching followed by magnetic separation. J. Hazard. Mater. 2012, 213, 167–174.
- Peng, N.; Peng, B.; Li, C.; Li, M.; Wang, J.; Yan, H.; Yuan, Y. Recovery of iron from zinc calcines by reduction roasting and magnetic separation. Miner. Eng. 2012, 35, 57–60.
- Han, J.; Liu, W.; Qin, W.; Peng, B.; Yang, K.; Zheng, Y. Recovery of zinc and iron from high iron-bearing zinc calcine by selective reduction roasting. J. Ind. Eng. Chem. 2015, 22, 272–279.
- Gupta, S.; French, D.; Sakurovs, R.; Grigore, M.; Sun, H.; Cham, T.; Hilding, T.; Hallin, M.; Lindblom, B.; Sahajwalla, V. Minerals and iron-making reactions in blast furnaces. Prog. Energy Combust. Sci. 2008, 34, 155–197.
- Li, Y.; Yuan, Y.; Liu, H.; Peng, B.; Liu, Z. Iron extraction from lead slag by bath smelting. Trans. Nonferrous Met. Soc. China 2017, 27, 1862–1869.
- Bhateria, R.; Singh, R. A review on nanotechnological application of magnetic iron oxides for heavy metal removal. J. Water Process Eng. 2019, 31, 100845.
- Zhang, D.; Zhang, X.; Yang, T.; Rao, S.; Hu, W.; Liu, W.; Chen, L. Selective leaching of zinc from blast furnace dust with mono-ligand and mixed-ligand complex leaching systems. Hydrometallurgy 2017, 169, 219–228.
- Min, X.; Xie, X.; Chai, L.; Liang, Y.; Li, M.; Ke, Y. Environmental availability and ecological risk assessment of heavy metals in zinc leaching residue. Trans. Nonferrous Met. Soc. China 2013, 23, 208–218.
- Yang, X.; Chu, M.; Shen, F.; Zhang, Z. Mechanism of zinc damaging to blast furnace tuyere refractory. Acta Metall. Sin. (Engl. Lett.) 2009, 22, 454–460.
- Trinkel, V.; Aschenbrenner, P.; Thaler, C.; Rechberger, H.; Mallow, O.; Fellner, J. Distribution of Zn, Pb, K, and Cl in blast furnace lining. Steel Res. Int. 2017, 88, 7.
- Zuo, H.B.; Wang, C.; Zhang, J.L.; Zhao, Y.G.; Jiao, K.X. Application of carbon composite bricks for blast furnace hearth. In 6th International Symposium on High-Temperature Metallurgical Processing; Jiang, T., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 595–602.
- Mikhailov, I.; Komarov, S.; Levina, V.; Gusev, A.; Issi, J.; Kuznetsov, D. Nanosized zero-valent iron as Fenton-like reagent for ultrasonic-assisted leaching of zinc from blast furnace sludge. J. Hazard. Mater. 2017, 321, 557–565.
- Esezobor, D.E.; Balogun, S.A. Zinc accumulation during recycling of iron oxide wastes in the blast furnace. Ironmak. Steelmak. 2006, 33, 419–425.
- Moradi, S.; Monhemius, A.J. Mixed sulphide–oxide lead and zinc ores: Problems and solutions. Miner. Eng. 2011, 24, 1062–1076.
- Jiao, K.X.; Zhang, J.; Liu, Z.; Chen, C.; Liu, F. Circulation and accumulation of harmful elements in blast furnace and their impact on the fuel consumption. Ironmak. Steelmak. 2017, 44, 344–350.
- Asadi Zeydabadi, B.; Mowla, D.; Shariat, M.; Kalajahi, J. Zinc recovery from blast furnace flue dust. Hydrometallurgy 1997, 47, 113–125.
- Yin, H.; Zhang, J. Study on the law of zinc absorption and change of metallurgical property of sinter and pellet. Iron Steel 2010, 45, 15–18.
- Kelebek, S.; Yörük, S.; Davis, B. Characterization of basic oxygen furnace dust and zinc removal by acid leaching. Miner. Eng. 2004, 17, 285–291.
- Li, Q.; Rao, X.; Xu, B.; Yang, Y.; Liu, T.; Jiang, T.; Hu, L. Extraction of manganese and zinc from their compound ore by reductive acid leaching. Trans. Nonferrous Met. Soc. China 2017, 27, 1172–1179.
- Wang, W.; Wang, J.; Xu, R.; Yu, Y.; Jin, Y.; Xue, Z. Influence mechanism of zinc on the solution loss reaction of coke used in blast furnace. Fuel Process. Technol. 2017, 159, 118–127.
- Bakkar, A.; Neubert, V. Recycling of cupola furnace dust: Extraction and electrodeposition of zinc in deep eutectic solvents. J. Alloy. Compd. 2019, 771, 424–432.
- Li, K.; Khanna, R.; Zhang, J.; Liu, Z.; Sahajwalla, V.; Yang, T.; Kong, D. The evolution of structural order, microstructure and mineral matter of metallurgical coke in a blast furnace: A review. Fuel 2014, 133, 194–215.
- Wang, J.; Wang, Z.; Zhang, Z.; Zhang, G. Removal of zinc from basic oxygen steelmaking filter cake by selective leaching with butyric acid. J. Clean. Prod. 2019, 209, 1–9.
- Rodgers, K.J.; Hursthouse, A.; Cuthbert, S. The potential of sequential extraction in the characterisation and management of wastes from steel processing: A prospective review. Int. J. Environ. Res. Public Health 2015, 12, 11724–11755.
- Pan, D.A.; Li, L.; Tian, X.; Wu, Y.; Cheng, N.; Yu, H. A review on lead slag generation, characteristics, and utilization. Resour. Conserv. Recycl. 2019, 146, 140–155.
- Li, Y.; Liu, Z.; Liu, H.; Peng, B. Clean strengthening reduction of lead and zinc from smelting waste slag by iron oxide. J. Clean. Prod. 2017, 143, 311–318.




