
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sybele Saska | + 1906 word(s) | 1906 | 2021-03-04 07:13:17 | | | |
| 2 | Camila Xu | Meta information modification | 1906 | 2021-03-18 03:41:08 | | |
Video Upload Options
The hydrogel is a hydrophilic scaffold composed of covalent and non-covalent polymeric chains bonds, providing a 3D shape environment similar to the native extra-cellular matrix (ECM).
1. Printable Hydrogels
The hydrogel is a hydrophilic scaffold composed of covalent and non-covalent polymeric chains bonds, providing a 3D shape environment similar to the native extra-cellular matrix (ECM) [1][2]. Its cross-linked polymers form a porous 3D structure with a high hydration level (they swell up to 99% (w/w) concerning their dry weight) without dissolving, allowing the network to retain proteins and growing factors, as well as providing an environment for gaseous and nutrients exchange, being essential for cell growth and survival [3][4][5][6][7]. Furthermore, hydrogel 3D scaffolds are beneficial for cell transplantation and tissue engineering [8][9].
2. Methods used for fabrication of hydrogel scaffolds
The methods used for fabrication of hydrogel scaffolds include solvent casting/leaching, gas foaming/leaching, photo-lithography, electrospinning, and 3D printing [7]. Regarding the development of printable hydrogels, the most challenging approach are the physicochemical and mechanical properties, which allow the hydrogel to hold minimally adequate mechanical properties after printing and quick gelation to ensure fidelity of form of the structure to be rebuilt [1][7][8]. The printed shape maintenance depends on the hydrogel’s rheological properties, which is related to its composition (polymer and crosslinking) [7][10].
Bioresorbability and biodegradability are required to allow scaffold degradation within the implantation site during tissue regeneration [11]. Bioresorbable polymers present four degradation stages in biological systems: hydration, strength decrease, loss of mass integrity, and solubilization via phagocytosis [12]. The degradation rate relies on polymeric nature, quantity, pH, and environment temperature [13]. The resorption of polymers is desirable for biomedical applications once they perform their function, the polymer chain tend to break into small pieces that will be reabsorbed or eliminated from the body [14][15]. Additionally, the scaffold’s gradual degradation promotes an increase in pore size, allowing a higher rate of cell proliferation and migration [16] for subsequent replacement of newly formed tissue.
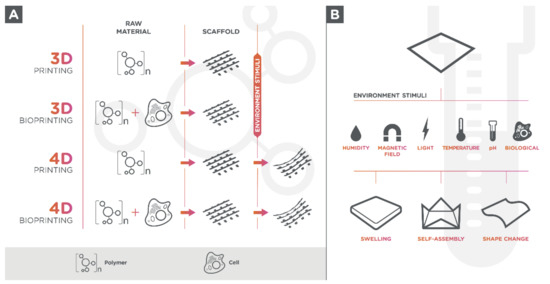
Living cells can be seeded onto 3D-printed hydrogel-scaffold or can be used in a bio ink formulation since hydrogels are biologically active components for 3D printing (bioprinting) (Figure 1A) [17]. The use of tissue-specific cells in materials for 3D printing allows the creation of multifaceted and 3D-mimicked tissues, which facilitates cell adhesion due to its cell-containing products, proliferation, and differentiation once they are seeded within the structure [18][19][20].
Bioink selection for 3D bioprinting relies on several requirements, including printability, viscoelasticity, biocompatibility with living cells, tissue regeneration, resorption, shear-thinning, permeability to oxygen, nutrients, and metabolic wastes [21][22]. Furthermore, other crucial characteristics for bioinks based on hydrogels include the reversibility of gelation (relevant for pre-culture before delivery), fast gelling, and the absence of volume modification during gelling [23]. The bioink’s rheological, mechanical, and biological properties will directly impact the functionality of the final printed tissues and organs [24].
The most common 3D bioprinting techniques are the inkjet printing, microextrusion, laser-assisted printing (SLS or SLM), and stereolithography (SLA). Inkjet printing (also known as a drop-on-demand printer and direct-writing) is a fast and low-cost method in which drops of bioink liquid are ejected through thermal, electrostatic or piezoelectric actuation onto a substrate to form 3D structures in a discontinuous process [21][25][26][27]. In the microextrusion method, the bioink is extruded by a pneumatic or mechanical (piston or screw) dispensing system (needles or nozzle) in a continuous process [28][29]. In the laser-assisted printing system, a focused laser is pulsed in an absorbing layer (titanium or gold) forcing a drop of the bioink layer to deposit on substrate and form the desired structure [30][31]. The SLA technique is based on photosensitive polymers (photopolymers), acting as feedstock, which are polymerized through a UV laser light in a layer-by-layer process [32].
3. Others
The biopolymers (alginate, hyaluronic acid, collagen, fibrin, fibroin, gelatin, and chitosan), are the most used polymers for the production of printable hydrogels and hydrogel-based bioinks in addition to the synthetic polymers, which include the polyethylene glycol (PEG), the polylactide acid (PLA), the poly(lactic-co-glycolic acid) (PLGA), the polycaprolactone (PCL), and the poloxamers.
Agarose is a linear polysaccharide polymer derived from red algae, and its gelation arises through the formation of intermolecular hydrogen bonds upon cooling [33][34]. Agarose hydrogels’ viscoelastic properties depend on the source, the purification method employed, the molecular weight, and in the solution concentration [35]. These hydrogels can elicit unfavored in vivo reactions [36] and are usually used as a fugitive ink or sacrificial material in tissue engineering [37][38].
Alginate is a water-soluble polysaccharide and consists of a linear (1–4)-linked β-d-mannuronic acid (M blocks) and its C5-epimer α-l-guluronic acid (G blocks) residues [39]. The gel’s viscosity and elasticity depend on the alginate source, concentration, and the G block content [40][41][42]. It can be extracted from a brown seaweed or can be synthesized using bacterial Pseudomonas or Azotobacter [43]. This polyanionic hydrophilic polysaccharide presents a relatively short cross-linking time and is compatible with several cell types [44][45]. The hydrogel is formed when multivalent cations (usually Ca2+) are added to an aqueous alginate solution. Although it is mechanically unstable for a prolonged culture, alginate hydrogels present low degradation rates and cannot be used alone [46]. Alginate-based hydrogels can be applied for vascular and cartilage tissues and are extensively studied in the area of tissue engineering. [47][48][49].
Chitosan is a deacetylated form of chitin derived from shells of crustaceans [50][51]. This natural cationic polysaccharide is insoluble in water and needs to be solubilized in acid solutions [52]. The hydrogel presents relatively good mechanical stability and may be easily mixed with other hydrogels. Due to its acidity, it needs to be neutralized and can reduce cell viability. In addition, presents a limited printability due to its low mechanical strength and low gelation speed and for that reason cannot be printed alone. There are only few studies of chitosan-based hydrogels for tissue engineering [53].
Collagen, the most abundant protein in the mammalian species and marine organisms, is the primary studied natural polymer for biomaterials [54]. Collagen hydrogels are considered a suitable cell carrier that may be easily mixed with other hydrogel materials; therefore, it presents low mechanical stability and a prolonged cross-linking time (slow gelation). Likewise, it is not indicated to be used alone, and it better performs when used in polymeric composites. Type I collagen (Col-I) can self-assemble to form fibrous hydrogels at 37 °C [55]. These hydrogels have been reported in various tissue engineering applications, but mainly have been mainly utilized in cartilage and skin tissues [56][57][58].
Gelatin is a partially hydrolyzed polypeptide and is considered a form of collagen. Its gelling property depends on its source. Gelatin hydrogels present a good cell viability, a low mechanical stability, and a high solubility at a physiological temperature [59]. The thermo-responsive property functions as a cell carrier and fugitive ink, making it a good choice to be used in tissue engineering. Gelatin methacryloyl (GelMA) is a modified gelatin with a low mechanical stability [60]. Although, GelMA is compatible to many cell types, cell viability in GelMA hydrogels depends on the photocrosslinking time, which is the intensity of light and photoinitiator used to induce polymerization [61]. There are several studies of vascular, cartilage, and liver tissue engineering using gelatin and GelMA based-hydrogels [62][63][64][65][66][67][68].
The hyaluronic acid is a linear non-sulfated glycosaminoglycan (GAG) polysaccharide that requires association to other polymers as a consequence of its low mechanical stability [69]. It is commonly used to increase cell viability through cell proliferation enhancement. Due to their properties, hyaluronic acid-based hydrogels have been studied for cardiovascular and cartilage tissue engineering [70][71].
Polyethylene glycol (PEG) is the most used synthetic polymer to produce biomedical hydrogels [72]. This hydrophilic polymer can be transformed into a gel by photopolymerization [73]. PEG hydrogels present good mechanical stability and their properties may be easily manipulated using chemical modification techniques, however, they do not provide biological cues for cell proliferation [74]. The photocrosslinking time, the intensity of the light, and the photoinitiator have a great influence on cell viability. PEG-based hydrogels can be applied in different approaches to tissue regeneration, such as vascular, bone, and cartilage tissues [38][75][76][77][78].
Poly(lactic acid) (PLA) is a biocompatible synthetic hydrophobic aliphatic polyester [79] commonly used in bone tissue engineering [76][77][78][79]. The stereoisomers distribution within the polymers chains (L/D ratios) and molecular weights determines the thermal stability and the degradation properties [80].
Poly(lactic-co-glycolic acid) (PLGA) is a synthetic copolymer composed of lactic acid (LA) and glycolic acid (GA), which polymerizes through the ester linkage of their monomers [81][82]. This copolymer can be degraded by hydrolysis and the degradation time is determined by the monomer’s ratio. Considering its good mechanical strengths and structural versatility, it is often used as support structures for cartilaginous and osteochondral tissue regeneration [83][84][85][86][87]. Nonetheless, it is commonly associated with other polymers (polymeric composites) [88][89][90][91][92] since it presents poor bioactivity characteristics.
Polycaprolactone (PCL) is a thermoplastic polyester obtained by ring-opening polymerization of ε-caprolactone monomers via anionic, cationic, coordination, or radical polymerization mechanism [93]. It is a bioresorbable polymer that degrades by hydrolysis of their ester linkages. PCL may be produced with different molecular weights and shape, impacting on the degradation rate and mechanical strength [94]. PCL hydrogels present good rheological and viscoelastic properties, regulable resorption, and controllable mechanical properties; nevertheless, PCL does not have biofunctional groups to promote better surface chemistry and favor a better cell adhesion in comparison to other bioactive polymers; hence, the PCL present a low biocompatibility [95][96]. It is consequently an excellent choice of use as a supporting device, especially for hard tissues. There are several reports of its use in cardiac, bone, and cartilage engineering [96][97][98][99][100][101][102][103].
Pluronic acid (or polaxamer) is a tri-block thermoplastic copolymer consisting of a hydrophobic poly(propylene oxide) (PPO) portion and two hydrophilic poly(ethylene oxide) (PEO) portions arranged in a PEO-PPO-PEO configuration. The non-ionic surfactant gelation temperature is dependent on its concentration and structure [104]. The main characteristics of this gel form are good biocompatibility, low cytotoxicity, weak mechanical properties, quick degradation rates, rapid dissolution in aqueous solutions, and poor cell viabilities [105][106]. In the area of tissue engineering, polaxamer hydrogels have been studied for diverse approaches in tissue regeneration [104][107][108][109][110].
Biocompatible and bioresorbable polymers can also be used to produce bio-based aerogels. Aerogels are materials synthesized from gels by replacement of the solvent with a gas [111]. This replacement is carried out, after gelation step, during a supercritical fluid drying process [112]. The result is a material with a high porosity (90–99%), comprising meso and micropores (50 nm), which provides a high internal surface area and low densities [113][114][115][116][117][118]. These scaffolds can be used for tissue engineering applications due to its nanofibrous structure that are suitable for cell adhesion, proliferation and migration [119]. However the traditional technologies for aerogel production lack reproducible customization of the 3D structures and do not allow the fabrication of complex structures [111]. 3D printing of aerogel can overcome the above-mentioned shortcomings, but it requires printable sol or gel with suitable viscosity and mechanical strength. Only a few studies have been reported using 3D printing techniques [111][120][121][122][123][124][125]. Maleki et al. [125] formulated a hybrid silica–silk fibroin aerogel with an excellent printability in the wet state using a micro-extrusion based 3D printing approach. Cheng et al. [111] described a new technique that integrates direct ink writing and freeze-casting with non-toxic solvent-based inks followed by special drying techniques. Taken together, these polymers, biopolymers, or synthetic polymers, could be divided into conventional and advanced (smart) polymers according to their response to environmental [126].
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 54, 3–12.
- Xu, B.; Li, Y.; Deng, B.; Liu, X.; Wang, L.; Zhu, Q. Chitosan hydrogel improves mesenchymal stem cell transplant survival and cardiac function following myocardial infarction in rats. Exp. Ther. Med. 2017, 13, 588–594.
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242.
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746.
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539.
- Yue, K.; Santiago, G.T.-D.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271.
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292.
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 38.
- Guadalupe, E.; Ramos, D.; Shelke, N.B.; James, R.; Gibney, C.; Kumbar, S.G. Bioactive polymeric nanofiber matrices for skin regeneration. J. Appl. Polym. Sci. 2015, 132.
- Qasim, M.; Baipaywad, P.; Udomluck, N.; Na, D.; Park, H. Enhanced therapeutic efficacy of lipophilic amphotericin B against Candida albicans with amphiphilic poly(N-isopropylacrylamide) nanogels. Macromol. Res. 2014, 22, 1125–1131.
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11.
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576.
- Warrer, K.; Karring, T.; Nyman, S.; Gogolewski, S. Guided tissue regeneration using biodegradable membranes of polylactic acid or polyurethane. J. Clin. Periodontol. 1992, 19, 633–640.
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. B Polym. Phys. 2011, 49, 832–864.
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344.
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479.
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2019, 11, 013001.
- Majeed, S. Advancement of Bio inks in three Dimensional Bioprinting. Biomed. J. Sci. Tech. Res. 2018, 11, 4.
- Guillemot, F.; Mironov, V.; Nakamura, M. Bioprinting is coming of age: Report from the International Conference on Bioprinting and Biofabrication in Bordeaux (3B’09). Biofabrication 2010, 2, 10201.
- Ozbolat, I.T. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015, 33, 395–400.
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239.
- Dorishetty, P.; Dutta, N.K.; Choudhury, N.R. Bioprintable tough hydrogels for tissue engineering applications. Adv. Colloid Interface Sci. 2020, 281, 102163.
- You, F.; Wu, X.; Zhu, N.; Lei, M.; Eames, B.F.; Chen, X. 3D Printing of Porous Cell-Laden Hydrogel Constructs for Potential Applications in Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1200–1210.
- Carrow, J.K.; Kerativitayanan, P.; Jaiswal, M.K.; Lokhande, G.; Gaharwar, A.K. Polymers for Bioprinting. In Essentials of 3D Biofabrication and Translation; Atala, A., Yoo, J.J., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 229–248. ISBN 978-0-12-800972-7.
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536.
- Dogan, E.; Bhusal, A.; Cecen, B.; Miri, A.K. 3D Printing metamaterials towards tissue engineering. Appl. Mater. Today 2020, 20, 100752.
- Bedell, M.L.; Navara, A.M.; Du, Y.; Zhang, S.; Mikos, A.G. Polymeric Systems for Bioprinting. Chem. Rev. 2020, 120, 10744–10792.
- Khalil, S.; Sun, W. Biopolymer deposition for freeform fabrication of hydrogel tissue constructs. Mater. Sci. Eng. C 2007, 27, 469–478.
- Kesti, M.; Müller, M.; Becher, J.; Schnabelrauch, M.; D’Este, M.; Eglin, D.; Zenobi-Wong, M. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015, 11, 162–172.
- Ringeisen, B.R.; Kim, H.; Barron, J.A.; Krizman, D.B.; Chrisey, D.B.; Jackman, S.; Auyeung, R.Y.C.; Spargo, B.J. Laser Printing of Pluripotent Embryonal Carcinoma Cells. Tissue Eng. 2004, 10, 483–491.
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48.
- Sinha, S.K. Chapter 5—Additive manufacturing (AM) of medical devices and scaffolds for tissue engineering based on 3D and 4D printing. In 3D and 4D Printing of Polymer Nanocomposite Materials; Sadasivuni, K.K., Deshmukh, K., Almaadeed, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–160. ISBN 978-0-12-816805-9.
- Velasco, D.; Tumarkin, E.; Kumacheva, E. Microfluidic Encapsulation of Cells in Polymer Microgels. Small 2012, 8, 1633–1642.
- Fu, X.T.; Kim, S.M. Agarase: Review of Major Sources, Categories, Purification Method, Enzyme Characteristics and Applications. Mar. Drugs 2010, 8, 200–218.
- Meena, R.; Siddhanta, A.K.; Prasad, K.; Ramavat, B.K.; Eswaran, K.; Thiruppathi, S.; Ganesan, M.; Mantri, V.A.; Rao, P.V.S. Preparation, characterization and benchmarking of agarose from Gracilaria dura of Indian waters. Carbohydr. Polym. 2007, 69, 179–188.
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463.
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917.
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211.
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133–161.
- Mancini, M.; Moresi, M.; Rancini, R. Mechanical properties of alginate gels: Empirical characterisation. J. Food Eng. 1999, 39, 369–378.
- Drury, J.L.; Dennis, R.G.; Mooney, D.J. The tensile properties of alginate hydrogels. Biomaterials 2004, 25, 3187–3199.
- Gomez, C.G.; Rinaudo, M.; Villar, M.A. Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr. Polym. 2007, 67, 296–304.
- Remminghorst, U.; Rehm, B.H.A. Bacterial alginates: From biosynthesis to applications. Biotechnol. Lett. 2006, 28, 1701–1712.
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976.
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53.
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R.; et al. Engineering alginate as bioink for bioprinting. Acta Biomater. 2014, 10, 4323–4331.
- Attalla, R.; Ling, C.; Selvaganapathy, P. Fabrication and characterization of gels with integrated channels using 3D printing with microfluidic nozzle for tissue engineering applications. Biomed. Microdevices 2016, 18, 17.
- Markstedt, K.; Mantas, A.; Tournier, I.; Ávila, H.M.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496.
- Poldervaart, M.T.; Wang, H.; van der Stok, J.; Weinans, H.; Leeuwenburgh, S.C.G.; Öner, F.C.; Dhert, W.J.A.; Alblas, J. Sustained Release of BMP-2 in Bioprinted Alginate for Osteogenicity in Mice and Rats. PLoS ONE 2013, 8, e72610.
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184.
- Unagolla, J.M.; Alahmadi, T.E.; Jayasuriya, A.C. Chitosan microparticles based polyelectrolyte complex scaffolds for bone tissue engineering in vitro and effect of calcium phosphate. Carbohydr. Polym. 2018, 199, 426–436.
- Park, S.Y.; Lee, B.I.; Jung, S.T.; Park, H.J. Biopolymer composite films based on κ-carrageenan and chitosan. Mater. Res. Bull. 2001, 36, 511–519.
- Dong, L.; Wang, S.-J.; Zhao, X.-R.; Zhu, Y.-F.; Yu, J.-K. 3D-Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 13412.
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827.
- Xing, R.; Liu, K.; Jiao, T.; Zhang, N.; Ma, K.; Zhang, R.; Zou, Q.; Ma, G.; Yan, X. An Injectable Self-Assembling Collagen–Gold Hybrid Hydrogel for Combinatorial Antitumor Photothermal/Photodynamic Therapy. Adv. Mater. 2016, 28, 3669–3676.
- Park, J.Y.; Choi, J.-C.; Shim, J.-H.; Lee, J.-S.; Park, H.; Kim, S.W.; Doh, J.; Cho, D.-W. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014, 6, 35004.
- Rhee, S.; Puetzer, J.L.; Mason, B.N.; Reinhart-King, C.A.; Bonassar, L.J. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805.
- Yanez, M.; Rincon, J.; Dones, A.; De Maria, C.; Gonzales, R.; Boland, T. In Vivo Assessment of Printed Microvasculature in a Bilayer Skin Graft to Treat Full-Thickness Wounds. Tissue Eng. Part A 2014, 21, 224–233.
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Demitri, C.; Ambrosio, L. Bioactivation Routes of Gelatin-Based Scaffolds to Enhance at Nanoscale Level Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2019, 7, 27.
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38.
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407.
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264.
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319.
- Skardal, A.; Devarasetty, M.; Kang, H.-W.; Mead, I.; Bishop, C.; Shupe, T.; Lee, S.J.; Jackson, J.; Yoo, J.; Soker, S.; et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015, 25, 24–34.
- Chen, Y.-C.; Lin, R.-Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039.
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.-R.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell’Erba, V.; et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 2016, 110, 45–59.
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.-S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211.
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-Methacrylamide Hydrogels as Potential Biomaterials for Fabrication of Tissue-Engineered Cartilage Constructs. Macromol. Biosci. 2013, 13, 551–561.
- Prestwich, G.D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J. Control. Release 2011, 155, 193–199.
- Gaetani, R.; Feyen, D.A.M.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.F.M.; Sluijter, J.P.G. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339–348.
- Law, N.; Doney, B.; Glover, H.; Qin, Y.; Aman, Z.M.; Sercombe, T.B.; Liew, L.J.; Dilley, R.J.; Doyle, B.J. Characterisation of hyaluronic acid methylcellulose hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 77, 389–399.
- Mawad, D.; Poole-Warren, L.A.; Martens, P.; Koole, L.H.; Slots, T.L.B.; van Hooy-Corstjens, C.S.J. Synthesis and Characterization of Radiopaque Iodine-containing Degradable PVA Hydrogels. Biomacromolecules 2008, 9, 263–268.
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656.
- Zustiak, S.P.; Leach, J.B. Hydrolytically Degradable Poly(Ethylene Glycol) Hydrogel Scaffolds with Tunable Degradation and Mechanical Properties. Biomacromolecules 2010, 11, 1348–1357.
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68.
- Gao, G.; Schilling, A.F.; Yonezawa, T.; Wang, J.; Dai, G.; Cui, X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol. J. 2014, 9, 1304–1311.
- Gao, G.; Yonezawa, T.; Hubbell, K.; Dai, G.; Cui, X. Inkjet-bioprinted acrylated peptides and PEG hydrogel with human mesenchymal stem cells promote robust bone and cartilage formation with minimal printhead clogging. Biotechnol. J. 2015, 10, 1568–1577.
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355.
- Basu, A.; Kunduru, K.R.; Doppalapudi, S.; Domb, A.J.; Khan, W. Poly(lactic acid) based hydrogels. Adv. Drug Deliv. Rev. 2016, 107, 192–205.
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455.
- Samadi, N.; Abbadessa, A.; Di Stefano, A.; van Nostrum, C.F.; Vermonden, T.; Rahimian, S.; Teunissen, E.A.; van Steenbergen, M.J.; Amidi, M.; Hennink, W.E. The effect of lauryl capping group on protein release and degradation of poly(D,L-lactic-co-glycolic acid) particles. J. Control. Release 2013, 172, 436–443.
- Wang, X.; Sui, S. Pulsatile Culture of a Poly(DL-Lactic-Co-Glycolic Acid) Sandwiched Cell/Hydrogel Construct Fabricated Using a Step-by-Step Mold/Extraction Method. Artif. Organs 2011, 35, 645–655.
- Uematsu, K.; Hattori, K.; Ishimoto, Y.; Yamauchi, J.; Habata, T.; Takakura, Y.; Ohgushi, H.; Fukuchi, T.; Sato, M. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials 2005, 26, 4273–4279.
- Jazayeri, H.E.; Tahriri, M.; Razavi, M.; Khoshroo, K.; Fahimipour, F.; Dashtimoghadam, E.; Almeida, L.; Tayebi, L. A current overview of materials and strategies for potential use in maxillofacial tissue regeneration. Mater. Sci. Eng. C 2017, 70, 913–929.
- Fan, H.; Hu, Y.; Zhang, C.; Li, X.; Lv, R.; Qin, L.; Zhu, R. Cartilage regeneration using mesenchymal stem cells and a PLGA–gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials 2006, 27, 4573–4580.
- Park, K.-S.; Kim, B.-J.; Lih, E.; Park, W.; Lee, S.-H.; Joung, Y.K.; Han, D.K. Versatile effects of magnesium hydroxide nanoparticles in PLGA scaffold–mediated chondrogenesis. Acta Biomater. 2018, 73, 204–216.
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. V An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659.
- Yan, B.; Zhang, Z.; Wang, X.; Ni, Y.; Liu, Y.; Liu, T.; Wang, W.; Xing, H.; Sun, Y.; Wang, J.; et al. PLGA–PTMC–Cultured Bone Mesenchymal Stem Cell Scaffold Enhances Cartilage Regeneration in Tissue-Engineered Tracheal Transplantation. Artif. Organs 2017, 41, 461–469.
- Chang, N.-J.; Jhung, Y.-R.; Yao, C.-K.; Yeh, M.-L. Hydrophilic Gelatin and Hyaluronic Acid-Treated PLGA Scaffolds for Cartilage Tissue Engineering. J. Appl. Biomater. Funct. Mater. 2013, 11, 45–52.
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396.
- García-García, P.; Reyes, R.; Segredo-Morales, E.; Pérez-Herrero, E.; Delgado, A.; Évora, C. PLGA-BMP-2 and PLA-17β-Estradiol Microspheres Reinforcing a Composite Hydrogel for Bone Regeneration in Osteoporosis. Pharmaceutics 2019, 11, 648.
- Wu, S.; Zhou, R.; Zhou, F.; Streubel, P.N.; Chen, S.; Duan, B. Electrospun thymosin Beta-4 loaded PLGA/PLA nanofiber/ microfiber hybrid yarns for tendon tissue engineering application. Mater. Sci. Eng. C. Mater. Biol. Appl. 2020, 106, 110268.
- Hoskins, J.N.; Grayson, S.M. Synthesis and Degradation Behavior of Cyclic Poly(ε-caprolactone). Macromolecules 2009, 42, 6406–6413.
- Huang, Y.-T.; Wang, W.-C.; Hsu, C.-P.; Lu, W.-Y.; Chuang, W.-J.; Chiang, M.Y.; Lai, Y.-C.; Chen, H.-Y. The ring-opening polymerization of ε-caprolactone and l-lactide using aluminum complexes bearing benzothiazole ligands as catalysts. Polym. Chem. 2016, 7, 4367–4377.
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893.
- Buyuksungur, S.; Tanir, T.E.; Buyuksungur, A.; Bektas, E.I.; Kose, G.T.; Yucel, D.; Beyzadeoglu, T.; Cetinkaya, E.; Yenigun, C.; Tönük, E.; et al. 3D printed poly(ε-caprolactone) scaffolds modified with hydroxyapatite and poly(propylene fumarate) and their effects on the healing of rabbit femur defects. Biomater. Sci. 2017, 5, 2144–2158.
- Van Rie, J.; Declercq, H.; Van Hoorick, J.; Dierick, M.; Van Hoorebeke, L.; Cornelissen, R.; Thienpont, H.; Dubruel, P.; Van Vlierberghe, S. Cryogel-PCL combination scaffolds for bone tissue repair. J. Mater. Sci. Mater. Med. 2015, 26, 123.
- Bahcecioglu, G.; Hasirci, N.; Bilgen, B.; Hasirci, V. A 3D printed PCL/hydrogel construct with zone-specific biochemical composition mimicking that of the meniscus. Biofabrication 2019, 11, 25002.
- Wang, S.-J.; Zhang, Z.-Z.; Jiang, D.; Qi, Y.-S.; Wang, H.-J.; Zhang, J.-Y.; Ding, J.-X.; Yu, J.-K. Thermogel-Coated Poly(ε-Caprolactone) Composite Scaffold for Enhanced Cartilage Tissue Engineering. Polymers 2016, 8, 200.
- Srinivasa Reddy, C.; Reddy Venugopal, J.; Ramakrishna, S.; Zussman, E. Polycaprolactone/oligomer compound scaffolds for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 3713–3725.
- Pok, S.; Myers, J.D.; Madihally, S.V.; Jacot, J.G. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013, 9, 5630–5642.
- Hernandez, I.; Kumar, A.; Joddar, B. A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering. Gels 2017, 3, 26.
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing-based PCL—Alginate—chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297.
- Müller, M.; Becher, J.; Schnabelrauch, M.; Zenobi-Wong, M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication 2015, 7, 35006.
- Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers 2020, 12, 1765.
- Doğan, A.; Yalvaç, M.E.; Şahin, F.; Kabanov, A.V.; Palotás, A.; Rizvanov, A.A. Differentiation of human stem cells is promoted by amphiphilic pluronic block copolymers. Int. J. Nanomed. 2012, 7, 4849–4860.
- Temofeew, N.A.; Hixon, K.R.; McBride-Gagyi, S.H.; Sell, S.A. The fabrication of cryogel scaffolds incorporated with poloxamer 407 for potential use in the regeneration of the nucleus pulposus. J. Mater. Sci. Mater. Med. 2017, 28, 36.
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671.
- Wu, B.; Takeshita, N.; Wu, Y.; Vijayavenkataraman, S.; Ho, K.Y.; Lu, W.F.; Fuh, J.Y.H. Pluronic F127 blended polycaprolactone scaffolds via e-jetting for esophageal tissue engineering. J. Mater. Sci. Mater. Med. 2018, 29, 140.
- Lee, J.; Kim, G. Three-Dimensional Hierarchical Nanofibrous Collagen Scaffold Fabricated Using Fibrillated Collagen and Pluronic F-127 for Regenerating Bone Tissue. ACS Appl. Mater. Interfaces 2018, 10, 35801–35811.
- Cheng, Q.; Liu, Y.; Lyu, J.; Lu, Q.; Zhang, X.; Song, W. 3D printing-directed auxetic Kevlar aerogel architectures with multiple functionalization options. J. Mater. Chem. A 2020, 8, 14243–14253.
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449.
- Baumann, T.F.; Worsley, M.A.; Han, T.Y.-J.; Satcher, J.H. High surface area carbon aerogel monoliths with hierarchical porosity. J. Non. Cryst. Solids 2008, 354, 3513–3515.
- Kistler, S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741.
- Wang, J.; Liu, D.; Li, Q.; Chen, C.; Chen, Z.; Song, P.; Hao, J.; Li, Y.; Fakhrhoseini, S.; Naebe, M.; et al. Lightweight, Superelastic Yet Thermoconductive Boron Nitride Nanocomposite Aerogel for Thermal Energy Regulation. ACS Nano 2019, 13, 7860–7870.
- Yao, B.; Chandrasekaran, S.; Zhang, H.; Ma, A.; Kang, J.; Zhang, L.; Lu, X.; Qian, F.; Zhu, C.; Duoss, E.B.; et al. 3D-Printed Structure Boosts the Kinetics and Intrinsic Capacitance of Pseudocapacitive Graphene Aerogels. Adv. Mater. 2020, 32, 1906652.
- Hu, P.; Lyu, J.; Fu, C.; Gong, W.; Liao, J.; Lu, W.; Chen, Y.; Zhang, X. Multifunctional Aramid Nanofiber/Carbon Nanotube Hybrid Aerogel Films. ACS Nano 2020, 14, 688–697.
- Cai, B.; Eychmüller, A. Promoting Electrocatalysis upon Aerogels. Adv. Mater. 2019, 31, 1804881.
- Baldino, L.; Cardea, S.; Reverchon, E. Natural Aerogels Production by Supercritical Gel Drying. Chem. Eng. Trans. 2015, 43, 739–744.
- Zhang, Q.; Zhang, F.; Medarametla, S.P.; Li, H.; Zhou, C.; Lin, D. 3D Printing of Graphene Aerogels. Small 2016, 12, 1702–1708.
- Tang, X.; Zhou, H.; Cai, Z.; Cheng, D.; He, P.; Xie, P.; Zhang, D.; Fan, T. Generalized 3D Printing of Graphene-Based Mixed-Dimensional Hybrid Aerogels. ACS Nano 2018, 12, 3502–3511.
- Li, V.C.-F.; Dunn, C.K.; Zhang, Z.; Deng, Y.; Qi, H.J. Direct Ink Write (DIW) 3D Printed Cellulose Nanocrystal Aerogel Structures. Sci. Rep. 2017, 7, 8018.
- Li, V.C.F.; Mulyadi, A.; Dunn, C.K.; Deng, Y.; Qi, H.J. Direct Ink Write 3D Printed Cellulose Nanofiber Aerogel Structures with Highly Deformable, Shape Recoverable, and Functionalizable Properties. ACS Sustain. Chem. Eng. 2018, 6, 2011–2022.
- Saeed, S.; Al-Sobaihi, R.M.; Bertino, M.F.; White, L.S.; Saoud, K.M. Laser induced instantaneous gelation: Aerogels for 3D printing. J. Mater. Chem. A 2015, 3, 17606–17611.
- Maleki, H.; Montes, S.; Hayati-Roodbari, N.; Putz, F.; Huesing, N. Compressible, Thermally Insulating, and Fire Retardant Aerogels through Self-Assembling Silk Fibroin Biopolymers Inside a Silica Structure—An Approach towards 3D Printing of Aerogels. ACS Appl. Mater. Interfaces 2018, 10, 22718–22730.
- Luo, Y.; Engelmayr, G.; Auguste, D.T.; da Silva Ferreira, L.; Karp, J.M.; Saigal, R.; Langer, R. 3D Scaffolds. In Principles of Tissue Engineering; Lanza, R., Langer, R., Vacanti, J.B.T.-P., Atala, A., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 475–494. ISBN 978-0-12-398358-9.





