| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Economou-Eliopoulos | + 2288 word(s) | 2288 | 2021-02-08 08:59:21 | | | |
| 2 | Bruce Ren | -21 word(s) | 2267 | 2021-03-01 07:09:24 | | |
Video Upload Options
Chromium concentrations in seawater are less than 0.5 μg/L, but the Cr(VI) in contaminated coastal groundwater affected by Cr-bearing rocks/ores and/or human activities, coupled with the intrusion of seawater may reach values of hundreds of μg/L. A potential explanation for the sta-bility of the harmful Cr(VI) in contaminated coastal aquifers is still unexplored.
1. Introduction
The scientific interest on the sustainable management of water resources and the effect on groundwater aquifers by either nature processes and/or extensive application in industry, is an attractive and fundamental subject to global food security. Among heavy metals, chromium (Cr) has become widespread in the environment. It appears in several oxidation states, the trivalent Cr(III) and hexavalent forms [Cr(VI)] being the most thermodynamically stable Cr forms in nature [1][2]. Cr(III) is necessary for lipid and sugar metabolism, and it is an essential trace element for human and animal health, whereas Cr(VI) in the food chain (groundwater, soil and plants), has created an alarming situation for human life and ecosystems [3][4][5]. Potential sources for the relatively high Cr contents in soil and groundwater may be the widespread Cr-bearing peridotites, which are parts of ophiolite complexes, covering more than 1% of the earth, along orogenetic zones [6], intense ore mining/smelting, industrial and agriculture activities (fertilizers and pesticides) [7][8]. The weathering processes of ultramafic rocks, depending mainly on the climate conditions and morphology, may result in the formation of laterites, both releasing significant amounts of Cr among other heavy metals (Ni, Co, Mn, Fe). Groundwater from sites characterized by the extensive presence of ultramafic rocks contain more than 10 μg/L Cr(VI), reaching values up to hundreds of μg/L of Cr(VI) [9][10][11][12][13][14][15][16]. The contamination of groundwater by Cr may be derived from industrial activities, such as in the Czech Republic (a highly industrialized country in Central Europe) [17], at the area of Friuli Venezia Giulia (northern Italy) [18], the Assopos Basin (Oinofyta or Inofyta, near the Assopos river) in Greece, exhibiting as high as 8000 μg/L Cr(VI) in shallow groundwater [19]. Additionally, ferrochromium (FeCr), which is a critical alloy in the production of stainless steel, is produced in several European countries (Finland, France, Italy, Norway, Sweden, the nations formerly comprising Yugoslavia, Germany, Italy, Switzerland, and the U.K.) generating Cr-bearing wastes [20].
In coastal groundwater that is contaminated by either Cr-bearing rocks/ores or industrial wastes, Cr(VI) often exceeds the maximum acceptable level for Crtotal in drinking water (50 μg/L) [21]. Such groundwater, at the interface zone between land and sea, is continually influenced by both marine and terrestrial processes, and it often contains elevated concentrations of Na, Cl, B, Li, Se, As, S, Ca, and Mg, which is characteristic of the seawater composition [22][23]. Although about one quarter of the global population lives in the vicinity of the world’s coastlines [24], the potential role of the presence of seawater components, which may significantly facilitate the Cr(VI) stability, inhibiting the Cr(VI) reduction to Cr(III), is unexplored in groundwater. Additionally, because high technology metals such as the rare earth elements (REEs) have become contaminants in the environment [25][26], this study presents new data on the composition of coastal water, including REEs, from the industrial zone of the Assopos and other Neogene Basins. They are combined with available data from previous studies, and the delineated relationships are presented. A major aim was the interpretation of the Cr(VI) stability, as a function of the salinity in groundwater resulting from the intrusion of seawater, the prevention of groundwater from further degradation, and protection of the valuable water resources.
2. Chromium Background
Structure of Cr(III) and Cr(VI) and Soluble Products
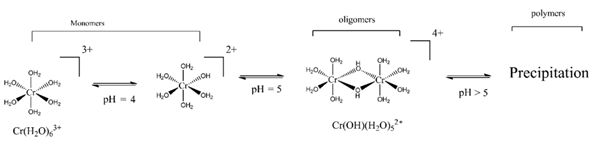
Assuming that Cr(III) forms hexa-coordinate complexes with the octahedral arrangement of ligands, [Cr(H2O)63+] is the main Cr species in solutions of inorganic Cr(III) salts under strongly acidic pH, while at pH ≥ 4, Cr(III)-bound H2O molecules undergo hydrolysis, resulting in the formation of soluble oligomeric products, whilst polymeric products are insoluble (Figure 1) [27].
Figure 1. Soluble oligomeric product, Cr(OH)2(H2O)84+ by hydrolysis and polymerization of [Cr(H2O)63+] (modified after [27].
Surface waters commonly contain a mixture of soluble monomeric and oligomeric Cr-products [28]. Additionally, in the presence of organic matter, the formation of stable Cr(III) complexes with small organic molecules can increase their mobility at the source of contamination sites and maintain the solubility of Cr(III) even at neutral pH [27].
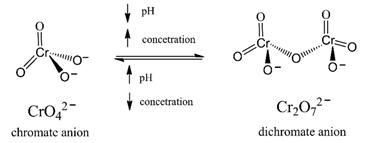
Chromate and dichromate have tetrahedral arrangements of coordinated oxygen (Figure 2). The chromate anion (CrO42−) is the predominant form of Cr(VI) in dilute solutions at neutral pH, co-existing in equilibrium with its protonated form [HCrO4]− in an approximately 3:1 ratio at these conditions [29].
Figure 2. Structures of tetrahedral chromate, and dichromate (modified from ref [27]).
3. Geological and Hydrological Outline
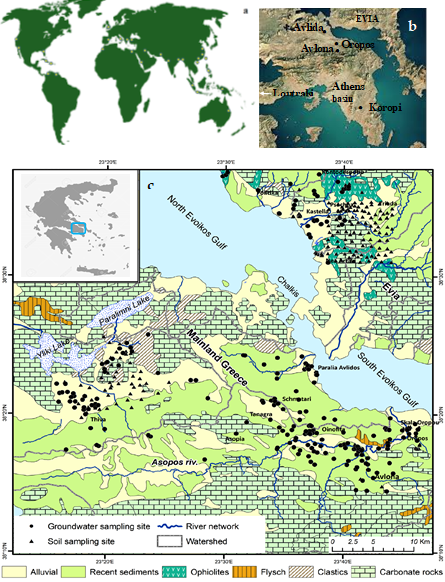
Detailed studies in Greece have shown that the main aquifers in the Greece are developed in alluvial formations, such as Quaternary and Neogene unconsolidated deposits (porous aquifers) covering the lowlands and semi-mountainous area of Greece and in carbonate rocks (karstic aquifers) [30][31][32][33][34][35][36]. Specifically, the Neogene Assopos–Thiva Basin (Figure 3c) is mainly composed of tertiary and quaternary sediments of more than 400 m thickness, and expands over an area of approximately 700 km2. Alternations of marls and marly limestone occurs in the lowest parts of the basin sequences, and continental sediments consisting of conglomerates with small intercalations of marls, marly limestones, schists, sandstones, clays and flysch are dominant in the upper parts. A sharp tectonic contact between the sediment types, due to the intense neotectonic deformation, is a characteristic feature of the entire area [30]. The morpho-tectonic structure and evolution of this basin are the result of E–W to WNW–ESE trending fault systems, with peridotites and a Ni-laterite over-thrusted on the Triassic–Jurassic carbonates [30]. Quaternary sediments cover large parts of the Assopos valley and host two types of aquifers: (i) aquifers within Neogene conglomerates, sandstones and marly limestone to a depth of approximately 150 m; and (ii) karst type aquifers within the Triassic–Jurassic limestones at deeper levels of the basin, such as the Mavrosouvala aquifer [31][32][33]. The Neogene Basin of the Central Evia is characterized by strong geomorphological contrast and is built up mainly of Pleistocene to Holocene sediments hosting the most productive aquifers in this area. Strongly tectonized ultramafic rocks (harzburgites and lherzolites), over-thrusted onto Upper Cretaceous limestones and flysch sediments, are widespread in C. Evia. Alluvial deposits are the host rocks to the aquifer, which is probed by many shallow wells (10–180 m) or agricultural activities.
Figure 3. Sketch map showing locations of contaminated groundwater by Cr(VI) coasts (a) examples such as in California, Pakistan, India, Northern China, the Mediterranean Sea, etc. [23]; (b) Karstic-type aquifer developed in Neogene lacustrine formations of the Mesogeia Basin in Attica [37]; (c) simplified geological map presenting the sites of the groundwater well and soil samples at the C. Evia and Assopos–Thiva Basins [36].
The most important aquifers in the Mesogeia Basin in Attica, which is part of the Attica–Cyclades zone, are constituted by Triassic–Jurassic limestones, Upper Cretaceous limestones, marly limestones, and travertine limestones (permeable rocks) [34][35][36][37][38]. The main part of the Mesogeia Basin, including the Koropi area (Figure 2), is occupied by Quaternary formations (gravels, sand, and clays) which characterize a low productive phreatic aquifer [35]. The alluvial deposits form a phreatic aquifer displaying limited permeability and poor hydraulic characteristics, due to fine grain compositions; they feed a large number of wells and boreholes, with depths ranging from 10 to 20 m [35][36][37].[39]
4. Global Distribution of Coastal Groundwater
Climate change, due to increasing concentrations of greenhouse gases, may affect the saltwater intrusion through changes in precipitation and temperature [40]. Coastal aquifers within the zones of influence are threatened by rising sea levels worldwide (Figure 3).
The phenomenon of seawater intrusion has been recorded in many coastal areas, such as in Texas, Florida, Indonesia, Syria, in South Asia (Bangkok) in Africa (Nigeria, Egypt and Tunisia), China, Australia, and Europe (Cyprus, Turkey, Estonia, Italy, and Greece). Global review publications provide an extensive array of field, laboratory, and computer-based techniques for the investigation of the seawater intrusion, its composition, and prediction of freshwater–saltwater interfaces over regional scales [41][42][43][44][45][46].
5. Geochemical Characteristics and Salinization of Coastal Groundwater
A common feature of the groundwater from the Neogene lacustrine formations of the Attica Basin, which has been affected by occasionally enclosed (tectonically) serpentinized peridotites, and the C. Evia, Assopos–Thiva Basins and the Loutraki area (Figure 3b,c) is the effect of the seawater intrusion [41], although they have been classified in various water types: the water samples from Attica as a Ca–HCO3 water type [37], while those from C. Evia and Assopos–Thiva Basins and the Loutraki area are of Mg–HCO3 type, due to the CO2-driven dissolution of dominant minerals, such as serpentine and Mg-carbonates/hydroxides. In order to better understanding the hydrochemistry of the above aquifers and the driving force of the Cr(VI) stability in contaminated coastal water, representative groundwater samples from the Attica, Assopos–Thiva, and C. Evia Neogene Basins were analyzed in the present study for major and trace elements, including rare earth elements (REEs) and platinum group elements (PGEs) (Table 1), which were combined with the relative literature data.
Major and trace elements (Table 1) are in a good agreement with those in previous studies, often exhibiting Cr(VI) concentrations over the maximum acceptable level for Crtotal in drinking water (50 μg/L), although the water contaminated by industrial activities in the area of Oinofita (or Inofyta), near the Assopos river (Assopos Basin) has shown concentrations higher than 8000 μg/L. Besides the elevated B, Br, Se, Li, As, V, U, S, Na, K, Ca and Mg concentrations, the concentrations for the majority of REEs are lower than the detection limit of the method (<0.01 μg/L), except values of 0.02 μg/L La and 0.01 to 0.02 μg/L Nd measured in the Assopos and C. Evia aquifers, and 0.02 μg/L Gd and 0.02 μg/L Dy measured in the Attica aquifers. The measured range for Sc of lanthanides was 2–3 μg/L in the Attica aquifers and 4–14 μg/L Sc in the C. Evia and Assopos Basins (Table 1). With respect to platinum-group elements, Pd was lower than the detection limit of the method (0.2 μg/L). The Pt was lower than detection limit (0.01 μg/L) in the Attica aquifers, while 0.01 values were measured in the C. Evia and Assopos aquifers. The Ru concentrations ranged from 0.06 to 0.3 μg/L in Attica, and from 0.06 to 0.19 μg/L in the C. Evia and Assopos Basins (Table 1). Additionally, these water samples were analyzed for the elements Au, Ag, Al, Ti, W, Tl, Te, Ta, Sn, In, Hf, Ge, Pb, Cs, Hg, Be, Bi, Cd, Ga and Y, but their concentrations were lower than the detection limit of the method. The measured concentrations for REEs and PGEs in water leachates for altered peridotites were also too low (Table 1). The available data on groundwater from previous studies exhibiting the lowest B and Cr(VI) concentrations have been recorded in the Mavrosouvala wells of karst type (Table 2), which are used for the municipal water supply of Athens city.
Table 2. Average composition of groundwater from the Assopos, Thiva, Attica Basins and C. Evia and seawater. Data from refs [46][47][48][49].
|
|
|
|
|
μg/L |
|
|
|
|
|
mg/L |
|
|
|
g/L |
‰ |
|
Location |
n |
Cr |
Cr(VI) |
B |
Li |
As |
Se |
Ca |
Mg |
S |
Na |
K |
pH |
TDS |
δ53Cr |
|
Groundwater |
|||||||||||||||
|
Karst type |
|||||||||||||||
|
Mavrosouvala |
3 |
<1 |
<1 |
18 |
2.5 |
2.4 |
<0.5 |
90.3 |
17 |
4 |
14 |
0.6 |
7.4 |
0.31 |
|
|
Attica, Athens |
19 |
9.7 |
9 |
43 |
6.4 |
3.5 |
1.9 |
120 |
20 |
15 |
44 |
2.5 |
7.3 |
0.43 |
|
|
Attica, Koropi |
31 |
12 |
10 |
130 |
8 |
8.4 |
10 |
135 |
55 |
37 |
217 |
8.8 |
7.4 |
0.48 |
|
|
Assopos basin |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Avlida |
10 |
70 |
64 |
440 |
16 |
4 |
9.3 |
36 |
99.8 |
37 |
310 |
11 |
7.4 |
1.13 |
|
|
Oropos |
3 |
61 |
57 |
120 |
23 |
3 |
3 |
38.5 |
55.3 |
15 |
109 |
1.6 |
7.5 |
0.59 |
|
|
Oropos |
As.K.W. |
900 |
850 |
130 |
32 |
4.5 |
8.8 |
40.2 |
140 |
60 |
408 |
2.5 |
7.7 |
1.42 |
1.16 ± 0.09 |
|
Avlona |
2 |
72 |
66 |
36 |
0.8 |
2.3 |
1.6 |
64 |
72 |
6 |
28 |
0.8 |
7.4 |
0.41 |
1.01 ± 0.02 |
|
C. Evia |
7 |
63 |
62 |
70 |
5.3 |
0.6 |
1.2 |
78 |
73 |
20 |
33.4 |
1.9 |
7.4 |
0.4 |
1.42 ± 0.37 |
|
C.Evia |
E7 |
260 |
250 |
40 |
7.3 |
0.7 |
2.2 |
64 |
13 |
44 |
32 |
2.4 |
7.34 |
0.28 |
0.98 ± 0.08 |
|
Water leachates (altered peridotites) |
|
||||||||||||||
|
C. Evia |
R2BA |
35 |
36 |
124 |
3.2 |
1.7 |
<0.5 |
26 |
1.9 |
<1 |
3.4 |
0.7 |
8.1 |
|
0.56 ± 0.04 |
|
R2BB |
64 |
63 |
98 |
0.2 |
<0.5 |
<0.5 |
26 |
6.1 |
<1 |
2.8 |
0.6 |
8.1 |
|
0.86 ± 0.06 |
|
|
R3 |
30 |
29 |
78 |
15 |
0.8 |
<0.5 |
25 |
2.9 |
<1 |
2.4 |
0.6 |
8.3 |
|
0.96 ± 0.06 |
|
|
Seawater |
|||||||||||||||
|
Mediterranean |
4 |
0.55 |
<1 |
4200 |
160 |
84 |
360 |
400 |
1200 |
1200 |
6380 |
485 |
|
|
1.13 ± 0.09 |
|
Assopos Estuary |
Ass.c. |
0.26 |
<1 |
4500 |
190 |
88 |
360 |
392 |
1250 |
1260 |
6400 |
500 |
|
|
0.79 ± 0.14 |
|
Pacific Ocean |
6 |
0.3 |
|
|
|
|
|
|
|
|
|
|
|
|
0.72 ± 0.14 |
|
Atlantic Ocean |
15 |
0.22 |
|
|
|
|
|
|
|
|
|
|
|
|
0.76 ± 0.24 |
|
Baltic sea |
12 |
0.16 |
|
|
|
|
|
|
|
|
|
|
|
|
0.42 ± 0.20 |
|
Antarctica |
3 |
0.29 |
|
|
|
|
|
|
|
|
|
|
|
|
0.57 ± 0.03 |
|
Geothermal water |
|||||||||||||||
|
Aedipsos, Evia |
9 |
n.d. |
|
9500 |
1400 |
68 |
380 |
1080 |
310 |
480 |
9800 |
300 |
6.6 |
27 |
|
|
K. Vourla, C. Greece |
5 |
n.d. |
|
3400 |
380 |
29 |
145 |
540 |
350 |
200 |
45,800 |
110 |
6.1 |
9.0 |
|
|
Vendenheim, France |
2 |
2.7 |
|
37,000 |
155,000 |
960 |
3450 |
62 |
180 |
27,400 |
3700 |
7.5 |
96 |
|
|
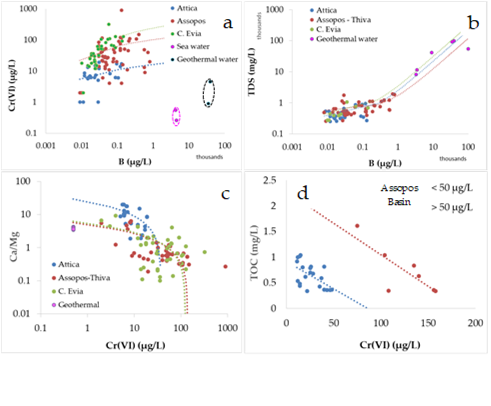
Present analytical data (Table 1) combining those from previous studies (Table 2) indicated that the best pronounced positive trend between B and Cr(VI) was recorded in C. Evia and Attica (Figure 4a). Limited data from the neighboring Mediterranean seawater (Assopos river estuary and Evoic Gulf) were plotted in a separate field characterized by much higher B and lower Cr(VI), comparable to those for geothermal water (Figure 4a). In addition, a good positive correlation between total dissolved solids (TDS) and B concentrations is a common feature of those water types (Figure 4b). A negative trend between the mass ratio Ca/Mg and the Cr(VI) concentrations recorded in groundwater from the studied Neogene Basins was followed by geothermal water (Figure 4c). In addition, a negative trend was clear between total organic carbon and Cr(VI) concentrations, for both lower and higher Cr(VI)concentrations of 50 μg/L in groundwater from the Assopos Basin (Figure 4d).
Figure 4. Plots of the Cr(VI) and total dissolved solids (TDS) versus B concentrations (a,b); Ca/Mg mass ratio and total organic carbon (TOC) versus Cr(VI) concentrations (c,d) for coastal groundwater from Attica, C. Evia), the Assopos–Thiva Basins, sea and geothermal water. Data from Tables 1 and 2 .
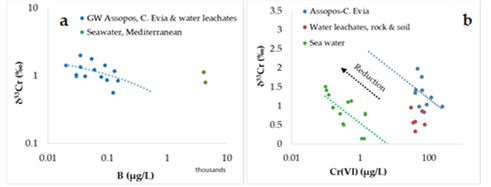
The plots of available stable chromium isotopes expressed as δ53Cr values versus B and Cr(VI) concentrations for coastal groundwater from the C. Evia, Assopos Basin and water leachates for peridotites showed a negative trend (Figure 5a). Moreover, plots of δ53Cr values versus Cr(VI) concentrations for seawater from the Pacific and Atlantic oceans, and Mediterranean and Baltic seas, showed a negative trend as well (Figure 5b).
Figure 5. Plot of the δ53Cr values versus B (a), and Cr(VI) (b) concentrations for coastal groundwater (GW) from the C. Evia, Assopos–Thiva Basins, water leachates for rocks (serpentinized peridotites) and soils, and seawater (Mediterranean Sea, Pacific and Atlantic oceans, and Baltic Sea). Data from refs [50].
References
- Shock, E.L.; Sassani, D.C.; Willis, M.; Sverjensky, D.A. Inorganic species in geologic fluids: Correlations among standard molal thermodynamic properties of aqueous ions and hydroxide complexes. Geochim. Cosmochim. Acta 1997, 61, 907–950, doi:10.1016/s0016-7037(96)00339-0.
- Dabizha, A.; Kersten, M. Exothermic adsorption of chromate by goethite. Appl. Geochem. 2020, 123, 104785, doi:10.1016/j.apgeochem.2020.104785.
- Keesstra, S.D.; Bouma, J.; Wallinga, J.; Tittonell, P.; Smith, P.; Cerdà, A.; Montanarella, L.; Quinton, J.N.; Pachepsky, Y.; Van Der Putten, W.H.; et al. The significance of soils and soil science towards realization of the United Nations Sustainable De-velopment Goals. Soil 2016, 2, 111–128, doi:10.5194/soil-2-111-2016.
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavail-ability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533, doi:10.1016/j.chemosphere.2017.03.074.
- Kanellopoulos, C. Influence of ultramafic rocks and hot springs with travertine depositions on geochemical composition and baseline of soils. Application to eastern central Greece. Geoderma 2020, 380, 114649, doi:10.1016/j.geoderma.2020.114649.
- Oze, C.; Bird, D.K.; Fendorf, S. Genesis of hexavalent chromium from natural sources in soil and groundwater. Proc. Natl. Acad. Sci. USA 2007, 104, 6544–6549, doi:10.1073/pnas.0701085104.
- Wazne, M.; Jagupilla, S.C.; Moon, D.H.; Christodoulatos, C.; Koutsospyros, A. Leaching Mechanisms of Cr(VI) from chro-mite ore processing residue. J. Environ. Qual. 2008, 37, 2125–2134, doi:10.2134/jeq2007.0443.
- Mateo-Sagasta, J.; Marjani, S.; Turral, H.; Burke, J. Water pollution from agriculture: A global review executive summary. FAO IWMI, Rome, 2017, 35p.
- Oze, C.; Sleep, N.H.; Coleman, R.G.; Fendorf, S. Anoxic oxidation of chromium. Geol. 2016, 44, 543–546, doi:10.1130/g37844.1.
- Fantoni, D.; Brozzo, G.; Canepa, M.; Cipolli, F.; Marini, L.; Ottonello, G.; Zuccolini, M.V. Natural hexavalent chromium in groundwaters interacting with ophiolitic rocks. Environ. Geol. 2002, 42, 871–882, doi:10.1007/s00254-002-0605-0.
- Megremi, I. Controlling Factors of the Mobility and Bioavailability of Cr and Other Metals at the Environment of Ni-Laterites. Ph.D. Thesis, University of Athens, Athens, Greece, 2010.
- Megremi, I.; Vasilatos, Ch.; Atsarou, A.; Theodoratou, Ch.; Economou-Eliopoulos, M.; Mitsis, I. Geochemical evidences for the sources of the Cr(VI) contamination in groundwater in central Euboea and Assopos-Thiva basins, Greece. Natural ver-sus anthropogenic origin. Eur. Water 2013, 41, 23–34.
- Kaprara, E.; Kazakis, N.; Simeonidis, K.; Coles, S.; Zouboulis, A.I.; Samaras, P.; Mitrakas, M. Occurrence of Cr(VI) in drink-ing water of Greece and relation to the geological background. J. Hazard. Mater. 2015, 281, 2–11, doi:10.1016/j.jhazmat.2014.06.084.
- Pyrgaki, K.; Argyraki, A.; Kelepertzis, E.; Paraskevopoulou, V.; Botsou, F.; Dassenakis, E.; Mitsis, I.; Skourtsos, E. Occurence of hexavalent chromium in the ophiolite related aquifers of Loytraki and Schinos areas. Bull. Geol. Soc. Greece 2017, 50, 2261–2270, doi:10.12681/bgsg.14292.
- Vithanage, M.; Kumarathilaka, P.; Oze, C.; Karunatilake, S.; Seneviratne, M.; Hseu, Z.-Y.; Gunarathne, V.; Dassanayake, M.; Ok, Y.S.; Rinklebe, J. Occurrence and cycling of trace elements in ultramafic soils and their impacts on human health: A crit-ical review. Environ. Int. 2019, 131, 104974, doi:10.1016/j.envint.2019.104974.
- Paternoster, M.; Rizzo, G.; Sinisi, R.; Vilardi, G.; Di Palma, L.; Mongelli, G. Natural hexavalent chromium in the Pollino Massif groundwater (Southern Apennines, Italy): Occurrence, geochemistry and preliminary remediation tests by means of innovative adsorbent nanomaterials. Bull. Environ. Contam. Toxicol. 2020, 1–7, doi:10.1007/s00128-020-02898-7.
- Novák, M.; Chrastný, V.; Čadková, E.; Farkaš, J.; Bullen, T.D.; Tylcer, J.; Szurmanova, Z.; Cron, M.; Prechova, E.; Curik, J.; et al. Common occurrence of a positive δ53Cr shift in central European waters contaminated by geogenic/industrial chromi-um relative to source values. Environ. Sci. Technol. 2014, 48, 6089–6096, doi:10.1021/es405615h.
- Slejko, F.F.; Petrini, R.; Lutman, A.; Forte, C.; Ghezzi, L. Chromium isotopes tracking the resurgence of hexavalent chro-mium contamination in a past-contaminated area in the Friuli Venezia Giulia Region, northern Italy. Isot. Environ. Health Stud. 2019, 55, 56–69, doi:10.1080/10256016.2018.1560278.
- Michalakis, I.B. Hydrogeological research in the industrial plain of ELVAL Ltd. (Oinofita, Viotia): Implication to the con-tamination source. Rep. Inst. Geol. and Miner. Explor. (IGME) 2015, 1–161 (In Greek).
- Yu, K.P.; Zhang, H.L.; Chen, B.; Xu, H.B.; Zhang, Y. Investigation of reaction mechanism on the lime-free roasting of chromium-containing slag. Metal. Mater. Trans. B 2015, 46, 2553–2563.
- World Health Organization (WHO). Guidelines for Drinking Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017.
- Rose, W.; Hawkes, E.; Webb, S. Geochemistry in Mineral Exploration, 2nd ed.; Academic Press: Cambridge, MA, USA, 1979.
- Post, V.E.A.; Eichholz, R.M.; Brentführer, R. Groundwater Management in Coastal Zones; Bundesanstalt für Geowissenschaften und Rohstoffe (BGR): Hannover, Germany, 2018, 107 pp.
- Nicholls, R.J.; Lowe, J.A. Benefits of mitigation of climate change for coastal areas. Glob. Environ. Chang. 2004, 14, 229–244, doi:10.1016/j.gloenvcha.2004.04.005.
- Pourjabbar, A.; Grawunder, A.; Lonschinski, M.; Merten, D.; Einax, J.W.; Georg Buchel, G. Statistical evidence of REE distri-bution and effective factors in groundwater of former uranium mining site, eastern Thuringia, Germany. In Proceedings of the 1st International Applied Geological Congress, Mashad Branch, Iran, 26–28 April 2010; pp. 1797–1802.
- Guo, H.; Zhang, B.; Wang, G.; Shen, Z. Geochemical controls on arsenic and rare earth elements approximately along a groundwater flow path in the shallow aquifer of the Hetao Basin, Inner Mongolia. Chem. Geol. 2010, 270, 117–125, doi:10.1016/j.chemgeo.2009.11.010.
- Zhitkovich, A. Chromium in drinking water: Sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011, 24, 1617–1629, doi:10.1021/tx200251t.
- Stewart, I.I.; Olesik, J.W. Investigation of Cr(III) hydrolytic olymerisation products by capillary electrophoresis-inductively coupled plasma-mass spectrometry J. Chromatogr. 2000, 872, 227–246.
- Theopold, K.H. Chromium: Inorganic and Coordination Chemistry, in Encyclopedia of Inorganic Chemistry; King, B.R., Ed.; John Wiley & Sons: New York, NY, USA, 1997; Volume 2, pp. 666–678.
- Chatoupis, Th.; Fountoulis, I. The neotectonic deformation of N. Parnis Mt, (Attica, Greece). In Proceedings of the 10th Congress of the Geological Society of Greece, Thessaloniki, Greece, 15–17 April, 2004. Bull. Geol. Soc. Greece 36, pp. 1588–1597.
- Giannoulopoulos, P. Hydrogeological–hydro-chemical survey of groundwater quality in the wider area of Assopos River basin, Viotia prefecture. Inst. Geol. Miner. Explor. (IGME) 2008, 1–74. (In Greek).
- Vasilatos, Ch.; Megremi, I.; Economou-Eliopoulos, M.; Mitsis, I. Hexavalent chromium and other toxic elements in natural waters in the Thiva–Tanagra–Malakasa Basin, Greece. Hell. J. Geosci. 2008, 43, 57–66.
- Moraki, A. Assessment of groundwater contamination by hexavalent chromium and its remediation at Avlida area, Central Greece. Hell. J. Geosci. 2010, 45, 175–183.
- Pavlopoulos, K.; Chrisanthaki, I.; Economou–Eliopoulos, M.; Lekkas, S. Hydrochemical study of metals in the groundwater of the wider area of Koropi. In Advances in the Research of Aquatic Environment; Springer Nature, Berlin Heidelberg 2011; pp. 169–176.
- Stamatis, G.; Lambrakis, N.; Alexakis, D.; Zagana, E. Goundwater quality in Mesogea basin in eastern Attica (Greece). Hy-drol. Process. 2006, 20, 2803–2818.
- Megremi, I.; Vasilatos, C.; Vassilakis, E.; Economou-Eliopoulos, M. Spatial diversity of Cr distribution in soil and ground-water sites in relation with land use management in a Mediterranean region: The case of C. In Evia and Assopos-Thiva ba-sins. Greece. Sci. Total. Environ. 2019, 651, 656–667, doi:10.1016/j.scitotenv.
- Kampouroglou, E.E.; Economou-Eliopoulos, M. Assessment of arsenic and associated metals in the soil-plant-water system in Neogene basins of Attica, Greece. Catena 2017, 150, 206–222, doi:10.1016/j.catena.2016.11.018.
- Economou-Eliopoulos, M.; Frei, R.; Atsarou, C. Application of chromium stable isotopes to the evaluation of Cr (VI) con-tamination in groundwater and rock leachates from central Euboea and the Assopos basin, (Greece). Catena 2014, 122, 216–228.
- Ellis, A.S.; Johnson, T.M.; Bullen, T.D. Chromium isotopes and the fate of hexavalent chromium in the environment. Science 2002, 295, 2060–2062.
- Arnell, N.W. Climate change and global water resources: SRES emissions and socio-economic scenarios. Glob. Environ. Chang. 2004, 14, 31–52, doi:10.1016/j.gloenvcha.2003.10.006.
- Atsarou, C.; Economou-Eliopoulos, M. Contamination by Cr(VI) and other heavy metals in groundwater, soil and crops at Avlona (Greece). In Proceedings of the Protection and Restoration of the Environment XI, Water Resources Contamination Control, Thessaloniki, Greece, July 3–6 2012; 471–480.
- Vengosh, A.; Kloppmann, W.; Marei, A.; Livshitz, Y.; Gutierrez, A.; Banna, M.; Guerrot, C.; Pankratov, I.; Raanan, H. Sources of salinity and boron in the Gaza strip: Natural contaminant flow in the southern Mediterranean coastal aquifer. Water Re-sour. Res. 2005, 41, doi:10.1029/2004wr003344.
- DiGiulio, D.C.; Jackson, R.B. Impact to underground sources of drinking water and domestic wells from production well stimulation and completion practices in the Pavillion, Wyoming, Field. Environ. Sci. Technol. 2016, 50, 4524–4536, doi:10.1021/acs.est.5b04970.
- Ranjan, P.; Kazama, S.; Sawamoto, M.; Sana, A. Global scale evaluation of coastal fresh groundwater resources. Ocean Coast. Manag. 2009, 52, 197–206, doi:10.1016/j.ocecoaman.2008.09.006.
- Papazotos, P.; Vasileiou, E.; Perraki, M. The synergistic role of agricultural activities in groundwater quality in ultramafic environments: The case of the Psachna basin, central Euboea, Greece. Environ. Monit. Assess. 2019, 191, 317, doi:10.1007/s10661-019-7430-3.
- Economou-Eliopoulos, M.; Frei, R.; Megremi, I. Potential leaching of Cr(VI) from laterite mines and residues of metallur-gical products (red mud and slag): An integrated approach. J. Geochem. Explor. 2016, 162, 40–49, doi:10.1016/j.gexplo.2015.12.007.
- Bonnand, P.; James, R.H.; Parkinson, I.; Connelly, D.; Fairchild, I.J. The chromium isotopic composition of seawater and marine carbonates. Earth Planet. Sci. Lett. 2013, 382, 10–20, doi:10.1016/j.epsl.2013.09.001.
- Kanellopoulos, C.; Mitropoulos, P.; Valsami-Jones, E.; Voudouris, P. A new terrestrial active mineralising hydrothermal system associated with ore-bearing travertines in Greece (northern Euboea Island and Sperchios area). J. Geochem. Explor. 2017, 179, 9–24, doi:10.1016/.
- Sanjuan, B.; Negrel, G.; Le Lous, M.; Poulmarch, E.; Gal, F.; Damy, P.C. Main geochemical characteristics of the deep geo-thermal brine at Vendenheim (Alsace, France) with constraints on temperature and fluid circulation. In Proceedings of the World Geothermal Congress, Rekjavik, Iceland, April 2020; ffhal-02335810f.
- Middleton, L.T.; Elliott, D.K.; Morales, M. Coconino Sandstone; Beus, S.S., Morales, M., Eds.; Oxford University Press: New York, NY, USA, 2003.