| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Federico Massi | + 1047 word(s) | 1047 | 2021-02-07 10:22:24 | | | |
| 2 | Nicole Yin | Meta information modification | 1047 | 2021-02-26 03:38:25 | | |
Video Upload Options
Plasmopara viticola, the causal agent of grapevine downy mildew, is a high risk pathogen associated with the development of fungicide resistance.
1. Introduction
Downy mildew, caused by the oomycete Plasmopara viticola, is one of the major threats for grapevine production, due to the quantitative and qualitative yield losses that are associated with severe disease epidemics[1]. P. viticola is an obligate parasite of grapevine, causing the main damage to the Eurasian grapevine species (Vitis vinifera), which is the most cultivated species worldwide due to the high quality of its grapes.
2. Characteristics and Management
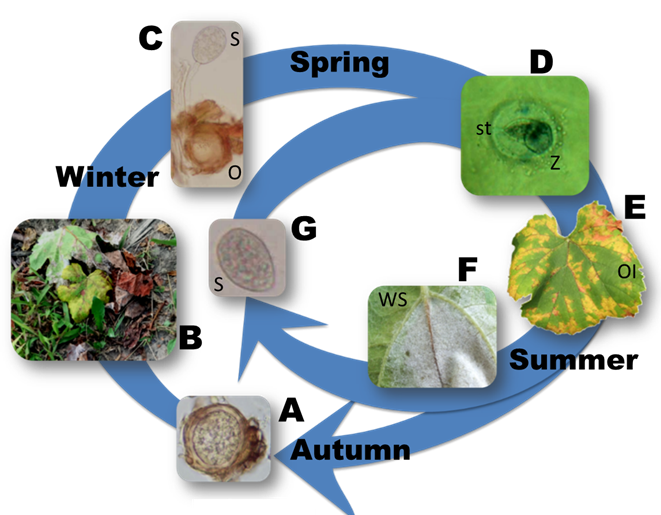
Most of the V. vinifera cultivars are highly susceptible to the pathogen, and only recently have sources of resistance been found in the center of origin of viticulture, which is located in Georgia (South Caucasus)[2][3]. This high susceptibility makes chemical control of the pathogen the most important measure to ensure an adequate yield. The timing of fungicide application depends on pathogen features and on weather conditions. P. viticola is a polycyclic pathogen, able to undergo numerous infection cycles during a single grapevine growing season. It overwinters as oospores (Figure 1A), which are sexual structures found in dead leaves on the vineyard floor (Figure 1B). In spring, with favorable weather conditions, oospores produce a single macrosporangium (Figure 1C), where the asexual spores (the zoospores) are formed. The zoospores infect the receptive grapevine tissues through stomata (Figure 1D) in the presence of free water, provided by rain or dew, at temperatures below 32 °C. Consequently, frequent fungicide applications are needed in vineyards located in areas with frequent rainfall and moderate temperatures during the grapevine growing season[4].
Figure 1. Disease cycle of P. viticola: the pathogen survives the winter period as oospores, i.e., the overwintering structures differentiated by sexual reproduction in autumn (A), embedded in dead leaves on the vineyard floor (B). With favorable weather conditions, oospores typically produce sporangia (C) that, in turn, produce zoospores (D). Zoospores are splashed by rain onto leaves and other receptive tissues of the grapevines, originating the primary infections through stomata penetration (D). Disease symptoms, visible as yellow discoloration (oil spots, Ol) on the upper side of the leaves (E), appear at the end of the incubation period and are followed, in high humidity conditions, by the emission of sporangiophores (F) with sporangia (G) that will cause secondary infections through the emission of new zoospores. O = oospore; S = sporangium; st = stoma; Z = zoospore; OI = oil spot symptom on the upper side of the leaf; WS = white sporulation, consisting of sporangiophores and sporangia, on the underside of the leaf.
3. The History of the Chemical Control of P. viticola
From the end of the Nineteenth Century, when the first agrochemical compounds were tested against P. viticola, until now, the panorama of phytoiatric practices has changed greatly, especially because of the availability of new active substances. Although agronomic practices represent a useful tool for disease management and the development of resistant varieties has made great progress, the use of chemical products still represents today the only effective means to control this fungal disease[5]. The growing of traditional varieties of Vitis vinifera is not conceivable without the use of fungicide applications[6]. The first documented attempts to control downy mildew using chemicals dates back to 1882, when the French botanist Pierre-Marie-Alexis Millardet noticed that the grapevine plants cultivated along the roadside did not show P. viticola symptoms. In the field, only these plants were treated, with a mush made with copper sulphate and lime, to discourage people from eating the grapes. This observation led to the development of the “Bordeaux mixture” to control downy mildew[7]. Its strong efficacy in inhibiting multiple metabolic processes in the fungal pathogen, together with a robust fastness and persistence, made the Bordeaux mixture quickly popular first in Europe, then in Australia and the USA [8]. Among protectant fungicides, copper still represents the most traditional and used chemical. However, intensive use of copper can cause serious environmental problems such as accumulation in the soil and adverse negative effects on beneficial organisms.
The use of the Bordeaux mixture in agriculture was greatly reduced during the Second World War, because copper was preferentially needed by the weapon industries[9] [9], and its availability for agriculture became secondary. Alternative control compounds were evaluated, but the results were always disappointing[10]. Experiments were conducted using zinc, aluminum, magnesium sulphates, and other metal salts, such as iron, silver, cadmium, and chromium. After several years of testing, the conclusion was that there were no better alternatives to the Bordeaux mixture[11]. Because of the scarcity of copper and the absence of options, growers started preparing the Bordeaux mixture with a lower concentration of copper sulphate. Despite the lower dose, disease control was still acceptable in many cases, if the fungicide was employed at the right time during the epidemics. This highlighted the importance of correct and timely applications[12].
After the Second World War, the first organic fungicides were synthesized by the chemical industry to control downy mildew. The dithiocarbamates and phthalimides were the first chemical classes employed against P. viticola. Members of these classes (e.g., zineb and captan), showed similar or higher control than the Bordeaux mixture[13][14]. The success of these fungicides was mainly caused by the higher return on investment and the absence of phytotoxicity, the latter often observed when using copper compounds[15][16]. However, intensive use of dithiocarbamates induced an excessive vegetative growth, favoring infections by other pathogens such as Botrytis cinerea, the grey mold agent[5][6][17][18]. Environmental toxicity and interference with natural competitors of spider mites like Tetranychus urticae and Panonychus ulmi [19][20] were reported as well.
A second wave in the development of control solutions occurred between the 1970s and the 1980s, when target-site fungicides were introduced into the market. Target-site fungicides inhibit a single biochemical pathway within the fungal cell[21] and generally have a more favorable toxicological profile compared to previous, multisite solutions, which interfere with numerous metabolic processes of the fungus[22][23][24]. Many of the newly discovered fungicide classes were systemic or cytotropic, i.e., able to penetrate and redistribute in the plant tissues, ensuring a better rain fastness and curative activity[25]. The substantial difference between systemic and cytotropic active ingredients is that the former can translocate inside the tissues of the plant (mainly through xylem vessels) and protect the newly formed vegetation, whereas the latter redistribute only locally[23].
References
- Gessler, C.; Pertot, I.; Perazzolli, M.; Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 2011, 50, 3–44.
- Toffolatti, S.L.; De Lorenzis, G.; Costa, A.; Maddalena, G.; Passera, A.; Bonza, M.C.; Pindo, M.; Stefani, E.; Cestaro, A.; Casati, P.; et al. Unique resistance traits against downy mildew from the center of origin of grapevine (Vitis vinifera). Sci. Rep. 2018, 8, 12523.
- Toffolatti, S.L.; De Lorenzis, G.; Brilli, M.; Moser, M.; Shariati, V.; Tavakol, E.; Maddalena, G.; Passera, A.; Casati, P.; Pindo, M.; et al. Novel aspects on the interaction between grapevine and Plasmopara viticola: Dual-rna-seq analysis highlights gene expression dynamics in the pathogen and the plant during the battle for infection. Genes (Basel) 2020, 11, 261.
- Silvia L Toffolatti; Giuseppe Russo; Paola Campia; Piero A Bianco; Paolo Borsa; Mauro Coatti; Stefano Ff Torriani; Helge Sierotzki; A time-course investigation of resistance to the carboxylic acid amide mandipropamid in field populations of Plasmopara viticola treated with anti-resistance strategies. Pest Management Science 2018, 74, 2822-2834, 10.1002/ps.5072.
- Jackson, R.S. Vineyard practice. In Wine Science: Principles and Applications; Academic Press: Burlington, VT, USA, 2008; pp. 108–238.
- I. Pertot; T. Caffi; V. Rossi; L. Mugnai; C. Hoffmann; M.S. Grando; C. Gary; D. Lafond; C. Duso; D. Thiery; et al.V. MazzoniG. Anfora A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Protection 2017, 97, 70-84, 10.1016/j.cropro.2016.11.025.
- Millardet, A.; Traitement du mildiou par la melange du sulfate de cuivre et de chaux. Agric. Prat. 1885, 49, 707–710.
- Lyon, A.V.; Problems of the viticultural industry. Bull. Aust. 1924, 28, 56–72.
- Liddell Hart, B.H. A History of the Second World War; Pan Books: London, UK, 1970.
- Mestbes, J.A. La lucha contra el mildiu. Agricultura 1942, 11, 139–140.
- Raucourt, M.; Vue d’ensemble sur les essais anticryptogamiques de. Ann. Epiphyt. 1943, 9, 163–167.
- Peyer, E.; Die Erfahrungen mit schwach konzentrierter Bordeauxbrühe bei der Mehltaubekampfung in den Reben der deutschen Schweiz im Sommer 1941. Schweiz. Z. Obs. Weinbau 1942, 51, 173–178.
- Gaudineau, M.; Messiaen, C.M. Mildiou de la Vigne et nouveaux produits de lutte. Agron 1953, 4, 185–208.
- Boubals, D.; Vergnes, A. Essais de fongicides organiques dans la lutte contre le mildiou de la Vigne. Prog. Agric. Vitic. 1953, 139, 90–97
- Zorbist, L. L’efficacité des fongicides organiques en viticulture. In Proceedings III Int. Congress on Phyto-pharmacologie; Comptes Rendus de l’Académie des Sciences: Paris, France, 1954; Volume 2, pp. 682–688.
- Kundert, J. Die Peronospora der rebe und ihre bekämpfung im Jahre 1955. Schweiz. Z. Obs. Weinbau 1956, 65, 135–139.
- Goshman, L.M. Clinical toxicology of commercial products. Pharm. Sci. 1985, 74, 1139.
- Ye, L.; Dinkova-Kostova, A.T.; Wade, K.L.; Zhang, Y.; Shapiro, T.A.; Talalay, P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: Pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin. Chim. Acta. 2002, 316, 43–53.
- Posenato, G. Popolazioni di amblyseius aberrans (Oud.) resistenti ad esteri fosforici e ditiocarbammati. L’informatore Agrar. 1994, 24, 41–43.
- Lorenzon, M.; Pozzebon, A.; Duso, C. Biological control of spider mites in North-Italian vineyards using pesticide resistant predatory mites. Acarologia 2018, 58, 98–118.
- Finch, H.J.S.; Samuel, A.M.; Lane, G.P.F. 6—Diseases of farm crops. In Lockhart & Wiseman’s Crop Husbandry Including Grassland, 9th ed.; Finch, H.J.S., Samuel, A.M., Lane, G.P.F., Eds.; Woodhead Publishing: Sawston, UK, 2014; pp. 119–157.
- Edwards, R.; Ferry, D.H.G.; Temple, W.A. Fungicides and related compounds. In Classes of Pesticides; Hayes, W.J., Laws, E.R., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 1409–1470.
- Rouabhi, R. Introduction and toxicology of fungicides. In Fungicides; Carisse, O., Ed.; Intech Open: London, UK, 2010.
- Hawkins, N.J.; Fraaije, B.A. Fitness penalties in the evolution of fungicide resistance. Annu. Rev. Phytopathol. 2018, 56, 339–360.
- Boubals, D.; Lafon, R.; Control of grapevine downy mildew by penetrating systemic fungicides. Bull. L’organisation Int. Vigne Vin 1981, 54, 319–355.