| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chang Geun Yoo | + 1388 word(s) | 1388 | 2021-02-08 04:53:00 | | | |
| 2 | Nicole Yin | Meta information modification | 1388 | 2021-02-25 07:09:38 | | |
Video Upload Options
Hemp is a type of Cannabis sativa plant and has multiple applications in food, construction, pharmaceuticals, and materials. Hemp was used in fabrics, twine, and paper products in the 1800s and 1900s; however, its production and applications were regulated with the Marijuana Tax Act in 1937 and the Controlled Substances Act in 1970 by the US Congress. In the 2014 Farm Bill, the US Congress defined industrial hemp depending on the level of THC (less than 0.3% THC on a dry weight basis) and allowed its agricultural pilot program by research institution and department of agriculture if the state laws allow.
1. Introduction
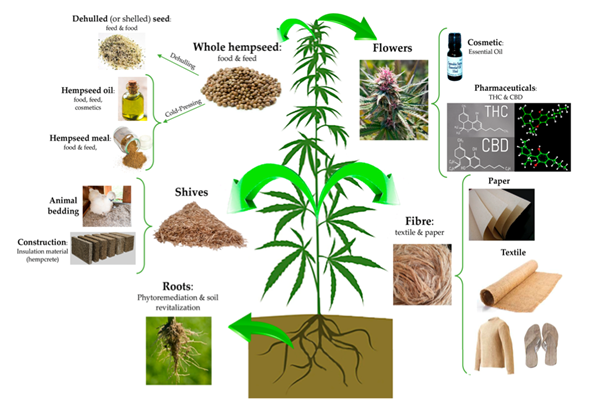
Hemp is a type of the Cannabis sativa plant and has multiple applications in food, construction, pharmaceuticals, and materials like textile and paper (Figure 1)[1]. Whole hempseed can be used as food after dehulling and also produce hempseed oil and meal by cold-pressing[2][3][4]. Hemp flowers are used for the production of cannabidiol (CBD) and ∆-9-tetrahydrocannabinol (THC)[5][6], and roots can be used for phytoremediation[7]. The stem of hemp is composed of fiber and shiv and covered by bark[8]. The fiber and shiv have been used for paper and textile products and in the applications of animal bedding and construction materials, respectively[9][10][11]; however, the development of its applications in biofuels and biochemical applications were also studied due to its high carbohydrate contents[12][13].
Figure 1. Industrial applications of hemp plant. (Reproduced with permission from Farinon et al., Nutrients; published by MDPI, 2020[1]).
Hemp has a long association with human life. It was used in fabrics, twine, and paper products in the 1800s and 1900s; however, its production and applications were regulated with the Marijuana Tax Act in 1937 and the Controlled Substances Act in 1970 by the US Congress[14].
2. Characteristics
2.1. Chemical Composition of Industrial Hemp
Chemical composition is one of the crucial biomass properties to evaluate its potential as a feedstock in its biofuels and biochemicals production[15]. It provides the contents of carbohydrate fractions including cellulose and hemicellulose for the production of fermentable sugars, fermentation products, as well as furan-based chemicals and material composition for paper, biocomposites, and others[15][16][17][18]. Lignin is a crucial recalcitrance factor as a physical barrier, non-productive binder, and toxic inhibitor in biological conversion processes of biomass[19]. Also, ash plays a role in some thermochemical biomass conversion processes as a catalyst[20][21]. Table 1 summarizes the chemical composition of industrial hemp biomass in the previous studies. The chemical composition of different industrial hemp cultivars was studied[13][22][23]. Each component showed wide ranges of composition: 32.6–51.1% of cellulose, 10.6–16.6% of hemicellulose, 14.6–29.4% of lignin, 2.6–7.6% of ash, 3.7–20.0% of extractives, and 0.3–23.1% of others. Viswanathan et al. investigated five different hemp varieties cultivated at the same field and condition but originally from different plant locations (Seward County, York County, Loup County, 19m96136, and CBD Hemp)[22]. The authors reported that 32.6–44.5% of cellulose, 10.6–15.5% of xylan, 17.0–21.5% of lignin, 2.6–7.6% of ash, and 5.3–20.0% of extractives. Das et al. studied with five fiber-only industrial hemp samples and six dual-purpose (for fiber and grain) samples[13]. A large variation was observed in lignin content (15.4–29.4%) associated with its biological conversion. The authors also discussed the potential value of the hemp lignin upgrading. Similarly, the composition of industrial hemp in other studies also ranges 36.5–46.4% of cellulose, 13.3–20.1% of hemicellulose, 14.8–22.9% of lignin, 2.4–4.2% of ash, and 13.3–14.4% of extractives[24][25][26][27][28]. The chemical composition of the separated industrial hemp fractions such as hurds[29][30], fiber and shives[31], and woody core[32] were also tested. Singh et al. reported that the hemp fibers have higher cellulose (57.5%), arabinan (1.2%), and ash (2.9%) content, while the hemp shives have higher xylan (19.9%) and Klason lignin (23.9%) content[31]. Kuglarz et al. compared the composition of industrial hemp Felina 32 variety under conventional and organic cultivation[33]. There was no significant difference in the cultivation methods, while the lignin content of the hemp increased by the pretreatment (diluted acid and steam pretreatment) reaction severity, and the pretreated conventional cultivated hemp has shown significantly higher lignin content compared to organically cultivated hemp. The authors explained the lignin content changes by the decomposition of polysaccharides and the formation of pseudo-lignin. In general, lower lignin content and higher carbohydrate content are ideal in the biological conversion of biomass. Overall, carbohydrate contents of industrial hemp are similar or higher compared to other agricultural residues such as corn stover (34.2% glucan, 22.3% xylan), rice straw (37.7% glucan, 19.8% xylan), barley straw (41% glucan, 22.4% xylan), and sugarcane bagasse (40.4% glucan, 22.4% xylan)[34][35][36][37]. Therefore, currently developed pretreatment strategies are readily applied for its conversion.
Table 1. Chemical composition of industrial hemp biomass.
|
Biomass Samples |
Cellulose [%] |
Hemicellulose [%] |
Lignin [%] |
Ash [%] |
Others [%] |
Ref |
|
Industrial hemp cultivars |
32.6–44.5 |
10.6–15.5 a |
17.0–21.5 |
2.6–7.6 |
5.3–20.0 (Extractives) |
[22] |
|
Industrial hemp |
36.5 |
17.0 a |
21.9 |
- |
13.3 (Extractives) |
[25] |
|
11.3 (Protein, ash) |
||||||
|
Industrial hemp cultivars |
43.8–51.1 |
11.6–14.2 a |
15.4–29.4 |
- |
3.7–11.9 (Extractives) |
[13] |
|
0.3–23.1 (others) |
||||||
|
Industrial hemp cultivars |
40.1–42.7 |
12.5–16.6 a |
14.6–17.8 |
- |
11.8–17.7 (Extractives) |
[23] |
|
Industrial hemp (conventional vs. organic) |
39.8–42.0 |
15.4–15.7 |
13.2–15.0 |
4.7–5.8 |
3.1–3.8 (Protein) |
[33] |
|
0.6–0.8 (Lipids) |
||||||
|
Industrial hemp hurds |
42.4 |
28.0 |
17.5 b |
- |
- |
[29] |
|
Industrial hemp |
46.4 |
20.1 a |
15.0 |
2.4 |
- |
[24] |
|
Industrial hemp |
42.3 |
18.2 |
22.9 |
4.2 |
- |
[26] |
|
Industrial hemp fiber and shives |
42.9–57.5 |
5.1–20.4 |
16.2–23.9 |
0.0–2.9 |
0.6–0.8 (Formic acid) |
[31] |
|
2.0–6.2 (Acetic acid) |
||||||
|
6.0–15.5 (Residuals) |
||||||
|
Industrial hemp woody core |
37.3 |
19.8 |
12.4 |
- |
- |
[32] |
|
Industrial hemp |
40.7 |
13.3 a |
15.7 |
- |
14.4 (Extractives) |
[27] |
|
Industrial hemp hurds |
75.0 (Holocellulose) |
23.0 |
1.2 |
1.1 (Oil-CH2Cl2) |
[30] |
|
|
|
0.8 (Oil-Acetone) |
|||||
|
44.0 (α-Cellulose) |
25.0 (Hemicellulose) |
0.6 (Pectin-Acidic water) |
||||
|
|
|
1.6 (Protein and amino acid-basic water) |
||||
|
Industrial hemp |
40.1 |
16.0 a |
14.8 |
- |
- |
[28] |
a Xylan content, b Acid insoluble lignin content.
2.2. Morphological Properties of Industrial Hemp
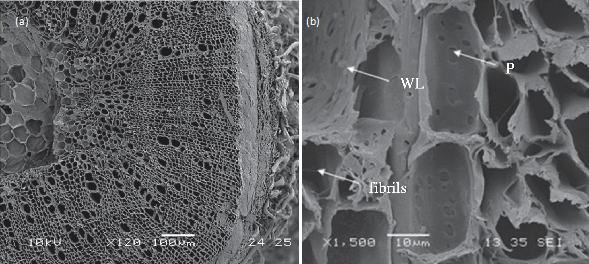
Hemp stem is composed of multiple layers including epidermis, phloem, xylem, and pith layers. The cell arrangement in the vascular cambium is presented in Figure 2a[38]. Between cells, the closed cell structures were observed with some voids at the pith, and a boundary was shown between phloem and xylem. Fibers were observed at the external surface of the hemp. These fibers are mechanically robust and used as yarns and fabrics[39][40]. Also, they have high cellulose content and low lignin content compared to xylem and pith layers[12][41][42][43]. Figure 2b shows the scanning electron microscope image of the fibrils in vessels[38]. The secondary cell walls are covered with a warty layer (WL) and several fibrils in the vessels of hemp shiv. Several pits are observed on the secondary cell walls. The morphological information of hemp can be used to explain the modification of hemp during the conversion process like pretreatment.
Figure 2. Images of (a) cross section of hemp stem and (b) the secondary walls of vessels in hemp shiv. WL, warty layer; P, simple pits. (Reproduced with permission from Jiang et al., Royal Society Open Science; published by The Royal Society, 2018.[38]).
2.3. Other Characteristics of Industrial Hemp: Crystallinity and Degree of Polymerization of Cellulose
Besides the chemical composition and morphological properties, crystallinity and degree of polymerization of cellulose are important factors for the evaluation of biomass in material applications and are also considered as a possible recalcitrance factor in their biological conversion[15]. Table 2 presents the crystallinity and degree of polymerization (DP) of cellulose in the previous studies. Most studies analyzed these characteristics to evaluate the material properties of hemp. Stevulova et al. measured the crystallinity of hemp hurds using Fourier-transform infrared spectroscopy (FTIR) and X-ray powder diffraction (XRD) [8]. Although the values differently ranged (35.7–49.2 by XRD; 55.6–90.2 by FTIR), they showed a good correlation (R2 = 0.9647) between the values. The authors indicated that chemical modification using sodium hydroxide, ethylenediaminetetraacetic acid, and calcium hydroxide increased the crystallinity. These chemical modifications also affected the DP of cellulose. By the chemical transformation, the DP of cellulose was decreased (untreated: 1302; chemically modified: 585–929). This research group also reported the DP of cellulose in hemp hurds after several treatments with ultrasound treatment[44]. The DP of cellulose was decreased further by chemical treatments (sodium hydroxide and hot-water) with ultrasound treatment. Industrial hemp fibers were also treated with sodium hydroxide, acetic anhydride, maleic anhydride, and silane and their crystallinities were reported to explain the influence of treatment on the fiber structure and tensile properties[45]. The DP and crystallinity of unbleached and bleached nanofibers were also reported in each step of the chemical treatment[46]. These properties in hemp bast and shiv fiber were also analyzed to explain the changes in their mechanical properties[47][48]. Although there were no reports to use these properties for explaining the recalcitrance of the industrial hemp in their biological conversion yet, these factors can be correlated to the conversion performance in terms of the abundance of reducing ends and structural rigidity.
Table 2. Crystallinity and degree of polymerization of cellulose in industrial hemp biomass.
|
Biomass |
Crystallinity |
DP of Cellulose |
Ref |
|
Untreated and chemically modified hemp hurds |
35.7–49.2 |
585–1302 |
[8] |
|
55.6–90.2 a |
|||
|
Tempo-oxidized hemp bast |
- |
560–1100 b |
[48] |
|
Natural and treated hemp hurds |
- |
200–1300 |
[44] |
|
Chemically treated industrial hemp fibers |
84.8–91.6 |
- |
[45] |
|
Untreated and chemically treated hemp fibers |
57.4–71.2 |
1138–1155 b |
[46] |
|
Hemp Shiv Fiber |
38.8–50.1 |
- |
[47] |
a Measured by FTIR, b Viscosity-average degree of polymerization (DPv).
References
- Barbara Farinon; Romina Molinari; Lara Costantini; Nicolò Merendino; The seed of industrial hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935, 10.3390/nu12071935.
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 282–308.
- Zając, M.; Guzik, P.; Kulawik, P.; Tkaczewska, J.; Florkiewicz, A.; Migdał, W. The quality of pork loaves with the addition of hemp seeds, de-hulled hemp seeds, hemp protein and hemp flour. LWT 2019, 105, 190–199.
- Devi, V.; Khanam, S. Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J. Cleaner Prod. 2019, 207, 645–657.
- RES. The Species Problem in Cannabis: Science and Semantics; JSTOR: New York, NY, USA, 1979; Volume 1.
- Cherney, J.H.; Small, E. Industrial hemp in North America: Production, politics and potential. Agronomy 2016, 6, 58.
- Ahmad, R.; Tehsin, Z.; Malik, S.T.; Asad, S.A.; Shahzad, M.; Bilal, M.; Shah, M.M.; Khan, S.A. Phytoremediation Potential of Hemp (Cannabis sativaL.): Identification and Characterization of Heavy Metals Responsive Genes. CLEAN-Soil Air Water 2016, 44, 195–201.
- Nadezda Stevulova; Julia Cigasova; Adriana Estokova; Eva Terpakova; Anton Geffert; Frantisek Kacik; Eva Singovszka; Marian Holub; Properties Characterization of Chemically Modified Hemp Hurds. Materials 2014, 7, 8131-8150, 10.3390/ma7128131.
- Van der Werf, H.M.; van der Veen, J.H.; Bouma, A.; Ten Cate, M. Quality of hemp (Cannabis sativa L.) stems as a raw material for paper. Ind. Crops Prod. 1994, 2, 219–227.
- Wang, H.; Postle, R.; Kessler, R.; Kessler, W. Removing pectin and lignin during chemical processing of hemp for textile applications. Text. Res. J. 2003, 73, 664–669.
- Nguyen, T.-T.; Picandet, V.; Amziane, S.; Baley, C. Influence of compactness and hemp hurd characteristics on the mechanical properties of lime and hemp concrete. Eur. J. Environ. Civ. Eng. 2009, 13, 1039–1050.
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Roozeboom, K.; Wang, D. Bioconversion of industrial hemp biomass for bioethanol production: A review. Fuel 2020, 281, 118725.
- Das, L.; Li, W.; Dodge, L.A.; Stevens, J.C.; Williams, D.W.; Hu, H.; Li, C.; Ray, A.E.; Shi, J. Comparative Evaluation of Industrial Hemp Cultivars: Agronomical Practices, Feedstock Characterization, and Potential for Biofuels and Bioproducts. ACS Sustainable Chem. Eng. 2020, 8, 6200–6210.
- Coit, M. The Fate of Industrial Hemp in the 2018 Farm Bill-Will Our Collective Ambivalence Finally be Resolved? J. Food L. Pol’y 2018, 14, 12.
- Chang Geun Yoo; Yongil Yang; Yunqiao Pu; Xianzhi Meng; Wellington Muchero; Kelsey L. Yee; Olivia A. Thompson; Miguel Rodriguez; Garima Bali; Nancy L. Engle; et al.Erika LindquistVasanth SinganJeremy SchmutzStephen P. DiFazioTimothy J. TschaplinskiGerald A. TuskanJin-Gui ChenBrian DavisonArthur J. Ragauskas Insights of biomass recalcitrance in natural Populus trichocarpa variants for biomass conversion. Green Chemistry 2017, 19, 5467-5478, 10.1039/c7gc02219k.
- Chheda, J.N.; Dumesic, J.A. An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates. Catal. Today 2007, 123, 59–70.
- Yoo, C.G.; Zhang, S.; Pan, X. Effective conversion of biomass into bromomethylfurfural, furfural, and depolymerized lignin in lithium bromide molten salt hydrate of a biphasic system. RSC Adv. 2017, 7, 300–308.
- Muensri, P.; Kunanopparat, T.; Menut, P.; Siriwattanayotin, S. Effect of lignin removal on the properties of coconut coir fiber/wheat gluten biocomposite. Composites Part A 2011, 42, 173–179.
- Chang Geun Yoo; Xianzhi Meng; Yunqiao Pu; Arthur J. Ragauskas; The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresource Technology 2020, 301, 122784, 10.1016/j.biortech.2020.122784.
- Wei, J.; Guo, Q.; Ding, L.; Gong, Y.; Yu, J.; Yu, G. Understanding the effect of different biomass ash additions on pyrolysis product distribution, char physicochemical characteristics, and char gasification reactivity of bituminous coal. Energy Fuels 2019, 33, 3068–3076.
- Jeong, K.; Jeong, H.J.; Lee, G.; Kim, S.H.; Kim, K.H.; Yoo, C.G. Catalytic effect of alkali and alkaline earth metals in lignin pyrolysis: A density functional theory study. Energy Fuels 2020, 34, 9734–9740.
- Viswanathan, M.B.; Park, K.; Cheng, M.H.; Cahoon, E.B.; Dweikat, I.; Clemente, T.; Singh, V. Variability in structural carbohydrates, lipid composition, and cellulosic sugar production from industrial hemp varieties. Ind. Crops Prod. 2020, 157.
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Wang, D. Conversion of liquid hot water, acid and alkali pretreated industrial hemp biomasses to bioethanol. Bioresour. Technol. 2020, 309, 123383.
- Mariusz Kuglarz; Merlin Alvarado-Morales; Dimitar Borisov Karakashev; Irini Angelidaki; Integrated production of cellulosic bioethanol and succinic acid from industrial hemp in a biorefinery concept. Bioresource Technology 2016, 200, 639-647, 10.1016/j.biortech.2015.10.081.
- Das, L.; Liu, E.; Saeed, A.; Williams, D.W.; Hu, H.; Li, C.; Ray, A.E.; Shi, J. Industrial hemp as a potential bioenergy crop in comparison with kenaf, switchgrass and biomass sorghum. Bioresour. Technol. 2017, 244, 641–649.
- Gunnarsson, I.B.; Kuglarz, M.; Karakashev, D.; Angelidaki, I. Thermochemical pretreatments for enhancing succinic acid production from industrial hemp (Cannabis sativa L.). Bioresour. Technol. 2015, 182, 58–66.
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Wang, D. High Ethanol Concentration (77 g/L) of Industrial Hemp Biomass Achieved Through Optimizing the Relationship between Ethanol Yield/Concentration and Solid Loading. ACS Omega 2020, 5, 21913–21921.
- Kuglarz, M.; Grübel, K. Integrated Production of Biofuels and Succinic Acid from Biomass after Thermochemical Pretreatments. Ecol. Chem. Eng. S 2018, 25, 521–536.
- Moxley, G.; Zhu, Z.; Zhang, Y.-H.P. Efficient Sugar Release by the Cellulose Solvent-Based Lignocellulose Fractionation Technology and Enzymatic Cellulose Hydrolysis. J. Agric. Food Chem. 2008, 56, 7885–7890.
- Gandolfi, S.; Ottolina, G.; Riva, S.; Fantoni, G.P.; Patel, I. Complete Chemical Analysis of Carmagnola Hemp Hurds and Structural Features of Its Components. BioResources 2013, 8, 2641–2656.
- Priyanka Singh; Santosh Pandit; Jørgen Garnæs; Sanja Tunjic; Venkata Rss Mokkapati; Abida Sultan; Anders Thygesen; Aiga Mackevica; Ramona Valentina Mateiu; Anders Egede Daugaard; et al.Anders BaunIvan Mijakovic Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. International Journal of Nanomedicine 2018, 13, 3571-3591, 10.2147/ijn.s157958.
- Chunliang Xie; Wenbing Gong; Qi Yang; Zuohua Zhu; Li Yan; Zhenxiu Hu; Yuande Peng; White-rot fungi pretreatment combined with alkaline/oxidative pretreatment to improve enzymatic saccharification of industrial hemp. Bioresource Technology 2017, 243, 188-195, 10.1016/j.biortech.2017.06.077.
- Mariusz Kuglarz; Ingólfur Bragi Gunnarsson; Sven-Erik Svensson; Thomas Prade; Eva Johansson; Irini Angelidaki; Ethanol production from industrial hemp: Effect of combined dilute acid/steam pretreatment and economic aspects. Bioresource Technology 2014, 163, 236-243, 10.1016/j.biortech.2014.04.049.
- Sporck, D.; Reinoso, F.A.M.; Rencoret, J.; Gutierrez, A.; Del Rio, J.C.; Ferraz, A.; Milagres, A.M.F. Xylan extraction from pretreated sugarcane bagasse using alkaline and enzymatic approaches. Biotechnol. Biofuels 2017, 10, 296.
- Yoo, C.G.; Nghiem, N.P.; Hicks, K.B.; Kim, T.H. Maximum production of fermentable sugars from barley straw using optimized soaking in aqueous ammonia (SAA) pretreatment. Appl. Biochem. Biotechnol. 2013, 169, 2430–2441.
- Hou, X.D.; Smith, T.J.; Li, N.; Zong, M.H. Novel renewable ionic liquids as highly effective solvents for pretreatment of rice straw biomass by selective removal of lignin. Biotechnol. Bioeng. 2012, 109, 2484–2493.
- Yoo, C.G.; Lee, C.W.; Kim, T.H. Two-stage fractionation of corn stover using aqueous ammonia and hot water. Appl. Biochem. Biotechnol. 2011, 164, 729–740.
- Y. Jiang; M. Lawrence; M. P. Ansell; A. Hussain; Cell wall microstructure, pore size distribution and absolute density of hemp shiv. Royal Society Open Science 2018, 5, 171945, 10.1098/rsos.171945.
- Kabir, M.; Wang, H.; Lau, K.; Cardona, F.; Aravinthan, T. Mechanical properties of chemically-treated hemp fibre reinforced sandwich composites. Composites Part B 2012, 43, 159–169.
- Antony, S.; Cherouat, A.; Montay, G. Experimental, analytical and numerical analysis to investigate the tensile behaviour of hemp fibre yarns. Compos. Struct. 2018, 202, 482–490.
- Shahzad, A. Hemp fiber and its composites—A review. J. Compos. Mater. 2012, 46, 973–986.
- Jankauskienė, Z.; Butkutė, B.; Gruzdevienė, E.; Cesevičienė, J.; Fernando, A.L. Chemical composition and physical properties of dew-and water-retted hemp fibers. Ind. Crops Prod. 2015, 75, 206–211.
- Shin, S.-J.; Han, S.-H.; Park, J.-M.; Cho, N.-S. Monosaccharides from industrial hemp (Cannabis sativa L.) woody core pretreatment with ammonium hydroxide soaking treatment followed by enzymatic saccharification. J. Korea TAPPI 2009, 41, 15–19.
- Nadezda Stevulova; Adriana Estokova; Julia Cigasova; Ivana Schwarzova; Frantisek Kacik; Anton Geffert; Thermal degradation of natural and treated hemp hurds under air and nitrogen atmosphere. Journal of Thermal Analysis and Calorimetry 2016, 128, 1649-1660, 10.1007/s10973-016-6044-z.
- Moyeenuddin A. Sawpan; Kim L. Pickering; Alan Fernyhough; Effect of various chemical treatments on the fibre structure and tensile properties of industrial hemp fibres. Composites Part A: Applied Science and Manufacturing 2011, 42, 888-895, 10.1016/j.compositesa.2011.03.008.
- Bei Wang; Mohini Sain; Kristiina Oksman; Study of Structural Morphology of Hemp Fiber from the Micro to the Nanoscale. Applied Composite Materials 2007, 14, 89-103, 10.1007/s10443-006-9032-9.
- Li, X.; Du, G.; Wang, S.; Meng, Y. Influence of Gender on the Mechanical and Physical Properties of Hemp Shiv Fiber Cell Wall in Dioecious Hemp Plant. BioResources 2015, 10, 2281–2288.
- Puangsin, B.; Soeta, H.; Saito, T.; Isogai, A. Characterization of cellulose nanofibrils prepared by direct TEMPO-mediated oxidation of hemp bast. Cellulose 2017, 24, 3767–3775.