| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yosra A Helmy | + 1973 word(s) | 1973 | 2021-02-18 03:57:43 | | | |
| 2 | Bruce Ren | -21 word(s) | 1952 | 2021-02-19 09:50:25 | | |
Video Upload Options
Emerging infectious diseases present great risks to public health. The novel severe acute respira-tory syndrome coronavirus 2 (SARS-CoV-2), causing coronavirus disease 2019 (COVID-19), has become an urgent public health issue of global concern. It is speculated that the virus first emerged through a zoonotic spillover. Basic research studies have suggested that bats are likely the ancestral reservoir host. Nonetheless, the evolutionary history and host susceptibility of SARS-CoV-2 remains unclear as a multitude of animals has been proposed as potential intermedi-ate or dead-end hosts. SARS-CoV-2 has been isolated from domestic animals, both companion and livestock, as well as in captive wildlife that were in close contact with human COVID-19 cases. Currently, domestic mink is the only known animal that is susceptible to a natural infection, develop severe illness, and can also transmit SARS-CoV-2 to other minks and humans.
1. Introduction
Coronaviruses (CoVs) (order: Nidovirales, family: Coronaviridae, subfamily: Coronavirinae) are enveloped, positive-stranded RNA viruses [1][2][3][4]. CoVs can infect birds (Gammacoronaviruses and Deltacoronaviruses) or mammals (predominantly Alpacoronaviruses and Betacoronaviruses) [5][6]. For over 80 years, animal coronaviruses, such as transmissible gastroenteritis virus (TGEV) of swine or bovine CoV (BCoV), have been known to infect wildlife and livestock species [7]. To date, seven CoVs have been identified in humans: HCoV-OC53, HCoV-229E, HCoV-NL63, HCoV-HKU1, MERS-CoV, SARS-CoV, and SARS-CoV-2. The first reports of endemic human CoVs (HCoVs) were documented in the 1960s when HCoV-OC53 and HCoV-229E were described [8][9]. It was not until 2004 and 2005 that HCoV-NL63 and HCoV-HKU1 were detected, respectively [10][11]. Endemic human coronaviruses most likely evolved from ancestral viruses of animal reservoirs [12].
In 2003, severe acute respiratory syndrome coronavirus (SARS-CoV) was reported as the first CoV of global health importance, which originated from several horseshoe bat species before transmission into human populations [13]. At least 8000 infections and 774 mortalities were linked to SARS-CoV [14]. Less than a decade later, the Middle Eastern respiratory syndrome (MERS) illness caused by a coronavirus (MERS-CoV) became an endemic disease throughout the Middle East, Africa, and Southeast Asia [15]. The zoonotic origins of MERS-CoV remain unclear, but it is speculated that the virus was transmitted from bat species to dromedary camels in the distant past [15][16].
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first detected in the Huanan South seafood market (HSSM), a large market that also traded live animals, within Wuhan City, Hubei province, China [17]. In addition to fish and shellfish, a diverse selection of live wildlife, including hedgehogs, badgers, snakes, and poultry, was marketed at the time when the outbreak occurred [18]. Aside from live wildlife, animal-food products such as carcasses and meat were also available [19]. Here, it is suggested that several clusters of pneumonia cases were linked to the HSSM [20][21]. Phylodynamic analysis reveals that SARS-CoV-2 most likely emerged as early as October 2019 [22][23], suggesting that the HSSM was mainly a super spreading location and not an index spillover event. Shortly after, SARS-CoV-2 spread globally and the World Health Organization (WHO) declared it a pandemic on 11 of March 2020 [24]. As of 4 of January 2021, approximately 1,839,660 million people have died from the novel coronavirus disease 2019 (COVID-19) and more than 83,910,386 million have been infected worldwide [24]. Following 229E-CoV, NL63-CoV, OC43-CoV, HKU1-CoV, SARS-CoV, and MERS-CoV, SARS-CoV-2 is the seventh coronavirus to infect humans.
SARS-CoV and SARS-CoV-2 belong to the subgenus Sarbecoviruses, characterized by frequent recombination events [25][26]. To date, research indicates that SARS-CoV-2 is not an outcome of a recombination event of any known Sarbecoviruses [27]. It is hypothesized that SARS-CoV-2 originated from an unknown animal reservoir [28][29][30]. Currently, the closest related sequences originated from horseshoe bat (96%) and pangolin CoVs (91%) [31][32]. Although the receptor-binding domain (RBD) between pangolin CoV is structurally identical to SARS-CoV-2 [33][34], it is unclear if pangolins function as intermediate or dead-end hosts [35][36][37]. Moreover, a diverse array of mammalian, avian, and reptilian species have been proposed as other potential intermediate hosts [38][39][40][41] Narrow genomic variation in CoVs can lead to wide host diversity as demonstrated by the similarity of SARS-CoV-2 to SARS-CoV and MERS-CoV, sharing 99.8% [36] and 99.5% [42] similarity to that from civet cats and dromedary camels, respectively. Consequently, minimal genetic variation is needed for CoVs to exhibit unique host specificity. Therefore, numerous mammalian, avian, and reptilian species have been proposed as potential hosts of SARS-CoV-2 [43][44][45].
Here, we provide an overview of the host diversity SARS-CoV-2 to veterinary and public health interventions. Evidence in support of reverse zoonotic transmission has been reported in numerous settings where infected humans have engaged in close contact with domestic and captive zoo animals [46]. Mink is the only animal to date that has been shown to transmit SARS-CoV-2 to humans, however, we cannot exclude a SARS-CoV-2 transmission potential from cats, dogs, and ferrets to humans. Further studies are needed to elucidate this hypothesis. Moreover, in selected animal groups, there is evidence that animals were infected by SARS-CoV-2 from humans, followed by a subsequent zoonotic transmission of SARS-CoV-2 from these same animals back to human populations [46][47]. This review aims to provide a cross-disciplinary, “One Health” approach to evaluate the SARS-CoV-2 emergence and spread at the intersection of humans and animals [38]. Based on the definition from the Centers for Disease Control and Prevention (CDC): “One Health is a collaborative, multisectoral, and transdisciplinary approach—working at the local, regional, national, and global levels—with the goal of achieving optimal health outcomes recognizing the interconnection between people, animals, plants, and their shared environment” [48]. Furthermore, these findings might support future surveillance programs to unravel the complex evolutionary histories of SARS-CoV-2 and those of SARS-CoV-like CoV viruses of other animal host species.
2. Epidemiology of Human SARS-CoV-2 Infections
Contextual understanding of the epidemiology of the virus is essential to properly study the epidemiology of SARS-CoV-2. Since the initial outbreak in Wuhan, most research on SARS-CoV-2 transmission has been collected through human-to-human transmission studies [49]. Initial studies of SARS-CoV-2 have indicated that the reproductive number (R0) in humans varies from 1.4 to 3.9 [50][51][52][53][54], and approximately 40 to 50% of SARS-CoV-2 human cases are asymptomatic [55][56][57][58][59]. The incubation period for COVID-19 is speculated to be 14 days alongside a median time of 4–5 days from exposure to symptoms onset [60][61][62]. Global disease trends suggest that women exhibit stronger immune responses than men and they have lower mortality rates [63][64]. Moreover, living at high altitudes has been suggested as a potential natural protective effect for lower mortality [65][66]. Additionally, viral transmission varies by geographic region due to differences in cases’ demographics, genetics, and health behavior practices [67].
At the population level, systematic health and socioeconomic inequalities have placed many marginalized groups at increased risk of high morbidity and mortality of SARS-CoV-2 infections [68][69]. Previous studies documented that racial and ethnic minorities are disproportionately higher affected by SARS-CoV-2 infections [70][71]. In many of these cases, social determinants have historically limited these groups from accessing fair opportunities for economic, physical, and emotional health [72]. Moreover, socioeconomic status has been linked to the availability of housing and housing conditions (i.e., the number of individuals per household) [73][74]. Living conditions, such as homelessness and crowded living environments (e.g., prisons, nursing homes, and orphanages) have been reported to be associated with increased SARS-CoV-2 infections [75][76].
At the individual level, older adults and people with underlying medical conditions are at higher risk for a severe SARS-CoV-2 illness [77]. In contrast to these groups, most infected children that express symptoms, if any, are generally mild and require only supportive care [78]. According to the CDC, some examples of underlying medical and physical conditions that could increase the risk of severe SARS-CoV-2 illness include cancer, chronic kidney disease, heart conditions, obesity (body mass index (BMI) of 30 kg/m2 or higher but <40 kg/m2), severe obesity (BMI ≥ 40 kg/m2), and diabetes mellitus [77]. Other individual-level risk factors include people with disabilities, developmental and behavioral disorders, and drug and substance use disorders [77].
3. Transmission Pathways of SARS-CoV-2 in Humans
Transmission of SARS-CoV-2 among humans is thought to occur via three primary pathways: (1) inhaling respiratory droplets from an infected individual, (2) inhaling infected airborne particles, (3) or contact with infected environmental surfaces also known as fomites [79]. Indirect or direct contact with infected people can facilitate the exposure to infected saliva and other respiratory secretions, commonly excreted when an infected individual coughs, sneezes, talks, or sings [80][81][82][83]. It is important to note that the diameter of respiratory droplets (>5–10 μm) is typically larger than that of nuclei or aerosols (>5 μm) [84]. Therefore, the transmission of infected respiratory droplets can occur when a susceptible individual is within 1 m of an infected case [85].
In contrast to droplet transmission, airborne transmission can occur mostly indoors through the dissemination of infectious aerosols that can be suspended in the air for long distances (usually greater than 2 m) and periods (typically hours) [86][87]. Experimental studies that have created infectious aerosols in controlled laboratory settings demonstrated that SARS-CoV-2 can persist in the air from 3 to 16 h [87][88]. Additionally, respiratory excretions from infected individuals can contaminate a variety of surfaces, thus creating fomites that can infect other individuals in the immediate environment upon contact followed by touching the mouth, nose, or eyes [85].
In general, microenvironmental characteristics such as ambient temperature, pH, and humidity greatly impact the persistence of SARS-CoV-2 on surfaces [89]. Similar to other human and animal CoVs [89], SARS-CoV-2 also exhibits low persistence on copper, latex, and other limited porosity surfaces compared to metals, glass, and highly porous fabrics [90][91]. Although SARS-CoV-2 has been reported to survive in environments at 40 °C for up to several hours [92], CoVs survive best at lower environmental temperatures and lower relative humidity [89]. While at room temperature, SARS-CoV-2 is stable at a wide range of pH values (pH 3–10) [93]. Despite evidence of SARS-CoV-2 contamination of surfaces and persistence on various substrates, there is no specific study that directly associates SARS-CoV-2 transmission through fomites [85]. Therefore, it is suggested that fomite transmission has lower importance compared to transmission via inhaling infected respiratory droplets or airborne particles [93][94].
Other modes of SARS-CoV-2 transmission could potentially include fecal-oral, bloodborne, and zoonotic transmission. To date, there have been no published reports indicating SARS-CoV-2 transmission through feces or urine. However, SARS-CoV-2 has been found in the feces of COVID-19 patients [95][96], leading to successful cultures of SARS-CoV-2 from stool specimens [97][98]. Additionally, levels of SARS-CoV-2 RNA concentrations in municipal wastewater parallel trends in local COVID-19 outbreaks, supporting an additional methodology for tracking SARS-CoV-2 levels in local human populations [99][100].
Previous studies have detected low concentrations of SARS-CoV-2 in plasma or serum [101]. The potential for bloodborne transmission remains unclear but it is unlikely given the low concentration of viral RNA detected from blood [102][103].
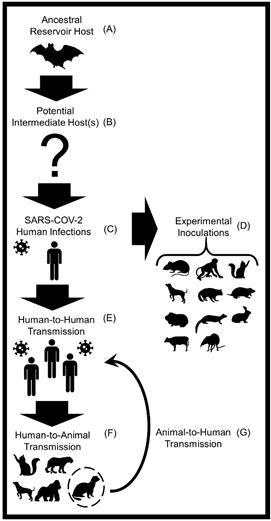
The most recent novel SARS-CoV-2 transmission pathway was described at the human-animal intersection, as current findings suggest a spillback and spillover potential of SARS-CoV-2, especially between humans and domestic mink and between humans and companion cats [104][105]. This synthesis review serves to further evaluate the animal host diversity and zoonotic transmission potential of SARS-CoV-2 (Figure 1).
Figure 1. A conceptual diagram displaying the transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among humans and various animal hosts. (A) Horseshoe bats (Rhinolophus affinis) are the most likely animal reservoir and ancestral hosts of the SARS-like CoV that gave rise to SARS-CoV-2 [105]. (B) A multitude of animals including mammals, birds, and reptiles have been proposed as potential intermediate hosts . (C) SARS-CoV-2 was first reported in humans in December 2019 in Wuhan, China [106]. (D) Successful laboratory infections of SARS-CoV-2 have been reported in the following mammals: domestic dogs, domestic cats, ferrets, rabbits, raccoon dogs, hamsters, mice, tree shrews, cattle, and several species of non-human primates [107][108]. (E) In January 2020, the World Health Organization (WHO) first reported that human-to-human transmission of SARS-CoV-2 is feasible [109][110]. (F) Natural infections of SARS-CoV-2 in animals transmitted from humans (i.e., reverse zoonosis or anthroponosis) have been detected in domestic dogs and cats, domestic mink, ferrets, mice, hamsters, captive gorillas, and captive large cats (e.g., tigers and lions) [111][112]. (G) Evidence of SARS-CoV-2 spillback from domestic minks to humans and intraspecies transmission of SARS-CoV-2 among minks has been detected. At this time these are the described transmission pathways and animals.
References
- Mousavizadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2020, 10, doi:10.1016/j.jmii.2020.03.022.
- Ramadan, N.; Shaib, H. Middle East respiratory syndrome coronavirus (MERS-CoV): A review. Germs 2019, 9, 35–42, doi:10.18683/germs.2019.1155.
- Miłek, J.; Domanska-Blicharz, K. Coronaviruses in avian species—Review with focus on epidemiology and diagnosis in wild birds. J. Vet. Res. 2018, 62, 249–255, doi:10.2478/jvetres-2018-0035.
- Chu, D.K.W.; Leung, C.Y.H.; Gilbert, M.; Joyner, P.H.; Ng, E.M.; Tse, T.M.; Guan, Y.; Peiris, J.S.M.; Poon, L.L.M. Avian Coronavirus in Wild Aquatic Birds. J. Virol. 2011, 85, 12815–12820, doi:10.1128/jvi.05838-11.
- Tiwari, R.; Dhama, K.; Sharun, K.; Yatoo, M.I.; Malik, Y.S.; Singh, R.; Michalak, I.; Sah, R.; Bonilla-Aldana, D.K.; Rodri-guez-Morales, A.J. COVID-19: Animals, veterinary and zoonotic links. Vet. Q. 2020, 40, 169–182, doi:10.1080/01652176.2020.1766725.
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Hosts and Sources of Endemic Human Coronaviruses. Adv. Virus Res. 2018, 100, 163–188, doi:10.1016/bs.aivir.2018.01.001.
- Saif, L. Animal coronaviruses: What can they teach us about the severe acute respiratory syndrome? Rev. Sci. Tech. l’OIE 2004, 23, 643–660, doi:10.20506/rst.23.2.1513.
- McIntosh, K.; Dees, J.H.; Becker, W.B.; Kapikian, A.Z.; Chanock, R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc. Natl. Acad. Sci. USA 1967, 57, 933–940, doi:10.1073/pnas.57.4.933.
- Hamre, D.; Procknow, J.J. A New Virus Isolated from the Human Respiratory Tract. Exp. Biol. Med. 1966, 121, 190–193, doi:10.3181/00379727-121-30734.
- Woo, P.C.Y.; Lau, S.K.P.; Chu, C.-M.; Chan, K.-H.; Tsoi, H.-W.; Huang, Y.; Wong, B.H.L.; Poon, R.W.S.; Cai, J.J.; Luk, W.-K.; et al. Characterization and Complete Genome Sequence of a Novel Coronavirus, Coronavirus HKU1, from Patients with Pneumonia. J. Virol. 2005, 79, 884–895, doi:10.1128/jvi.79.2.884-895.2005.
- Van der Hoek, L.; Pyrc, K.; Jebbink, M.F.; Vermeulen-Oost, W.; Berkhout, R.J.M.; Wolthers, K.C.; Dillen, P.M.E.W.-V.; Kaan-dorp, J.; Spaargaren, J.; Berkhout, B. Identification of a new human coronavirus. Nat. Med. 2004, 10, 368–373, doi:10.1038/nm1024.
- Cummings, D.A.; Radonovich, L.J.; Gorse, G.J.; Gaydos, C.A.; Bessesen, M.T.; Brown, A.C.; Gibert, C.L.; Hitchings, M.D.T.; Lessler, J.; Nyquist, A.-C.; et al. Risk Factors for Healthcare Personnel Infection With Endemic Coronaviruses (HKU1, OC43, NL63, 229E): Results from the Respiratory Protection Effectiveness Clinical Trial (ResPECT). Clin. Infect. Dis. 2020, doi:10.1093/cid/ciaa900.
- Wang, L.-F.; Shi, Z.; Zhang, S.; Field, H.; Daszak, P.; Eaton, B.T. Review of Bats and SARS. Emerg. Infect. Dis. 2006, 12, 1834–1840, doi:10.3201/eid1212.060401.
- World Health Organization (WHO). Summary Table of SARS Cases by Country, 1 November 2002–August 2003. Summary Table of SARS Cases by Country N-A; World Health Organisation (WHO): Geneva, Switzerland. Available online: https://covid19.who.int/ (accessed on 1 December 2020).
- World Health Organization (WHO). Middle East Respiratory Syndrome Coronavirus (MERS-CoV); WHO: Geneva, Switzerland, 2013.
- Lu, G.; Wang, Q.; Gao, G.F. Bat-to-human: Spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015, 23, 468–478, doi:10.1016/j.tim.2015.06.003.
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473, doi:10.1016/s0140-6736(20)30185-9.
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new corona-virus associated with human respiratory disease in China. Nature 2020, 579, 265–269 doi:10.1038/s41586-020-2008-3.
- Ashour, H.M.; Elkhatib, W.F.; Rahman, M.; Elshabrawy, H.A. Insights into the Recent 2019 Novel Coronavirus (SARS-CoV-2) in Light of Past Human Coronavirus Outbreaks. Pathogens 2020, 9, 186, doi:10.3390/pathogens9030186.
- Abdel-Moneim, A.S.; Abdelwhab, E.M. Evidence for SARS-CoV-2 Infection of Animal Hosts. Pathogens 2020, 9, 529, doi:10.3390/pathogens9070529.
- Gortázar, C.; de la Fuente, J. COVID-19 is likely to impact animal health. Prev. Vet. Med. 2020, 180, 105030, doi:10.1016/j.prevetmed.2020.105030.
- Lai, A.; Bergna, A.; Acciarri, C.; Galli, M.; Zehender, G. Early phylogenetic estimate of the effective reproduction number of SARS‐CoV‐2. J. Med. Virol. 2020, 92, 675–679, doi:10.1002/jmv.25723.
- Nie, Q.; Li, X.; Chen, W.; Liu, D.; Chen, Y.; Li, H.; Li, D.; Tian, M.; Tan, W.; Zai, J. Phylogenetic and phylodynamic analyses of SARS-CoV-2. Virus Res. 2020, 287, 198098, doi:10.1016/j.virusres.2020.198098.
- World Health Organization (WHO). Coronavirus Disease (COVID-19) Dashboard. 2020. Available online: https://covid19.who.int/ (accessed on 29 September 2020).
- Bobay, L.-M.; O’Donnell, A.C.; Ochman, H. Recombination events are concentrated in the spike protein region of Betacoro-naviruses. PLoS Genet. 2020, 16, e1009272, doi:10.1371/journal.pgen.1009272.
- Zhang, Y.; Zhao, W.; Mao, Y.; Chen, Y.; Wang, S.; Zhong, Y.; Su, T.; Gong, M.; Du, D.; Lu, X.; et al. Site-specific N-glycosylation Characterization of Recombinant SARS-CoV-2 Spike Proteins. Mol. Cell. Proteom. 2020, 120 002295, doi:10.1074/mcp.ra120.002295.
- Boni, M.F.; Lemey, P.; Jiang, X.; Lam, T.T.-Y.; Perry, B.W.; Castoe, T.A.; Rambaut, A.; Robertson, D.L. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. 2020, 5, 1–10, doi:10.1038/s41564-020-0771-4.
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799, doi:10.1056/nejmoa2001282.
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286, doi:10.1007/s12098-020-03263-6.
- Helmy, Y.A.; Fawzy, M.; Elaswad, A.; Sobieh, A.; Kenney, S.P.; Shehata, A.A. The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. J. Clin. Med. 2020, 9, 1225, doi:10.3390/jcm9041225.
- Flores-Alanis, A.; Sandner-Miranda, L.; Delgado, G.; Cravioto, A.; Morales-Espinosa, R. The receptor binding domain of SARS-CoV-2 spike protein is the result of an ancestral recombination between the bat-CoV RaTG13 and the pangolin-CoV MP789. BMC Res. Notes 2020, 13, 1–6, doi:10.1186/s13104-020-05242-8.
- Lam, T.T.-Y.; Jia, N.; Zhang, Y.-W.; Shum, M.H.-H.; Jiang, J.-F.; Zhu, H.-C.; Tong, Y.-G.; Shi, Y.-X.; Ni, X.-B.; Liao, Y.-S.; et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature 2020, 583, 282–285, doi:10.1038/s41586-020-2169-0.
- Lau, S.K.P.; Woo, P.C.Y.; Yip, C.C.Y.; Fan, R.Y.Y.; Huang, Y.; Wang, M.; Guo, R.; Lam, C.S.F.; Tsang, A.K.L.; Lai, K.K.Y.; et al. Isolation and Characterization of a Novel Betacoronavirus Subgroup A Coronavirus, Rabbit Coronavirus HKU14, from Domestic Rabbits. J. Virol. 2012, 86, 5481–5496, doi:10.1128/jvi.06927-11.
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nat. Cell Biol. 2020, 581, 221–224, doi:10.1038/s41586-020-2179-y.
- Frutos, R.; Serra-Cobo, J.; Chen, T.; Devaux, C.A. COVID-19: Time to exonerate the pangolin from the transmission of SARS-CoV-2 to humans. Infect. Genet. Evol. 2020, 84, 104493, doi:10.1016/j.meegid.2020.104493.
- Guan, Q.; Sadykov, M.; Mfarrej, S.; Hala, S.; Naeem, R.; Nugmanova, R.; Al-Omari, A.; Salih, S.; Al-Mutair, A.; Carr, M.J.; et al. A genetic barcode of SARS-CoV-2 for monitoring global distribution of different clades during the COVID-19 pandemic. Int. J. Infect. Dis. 2020, 100, 216–223, doi:10.1016/j.ijid.2020.08.052.
- Xiao, K.; Zhai, J.; Feng, Y.; Zhou, N.; Zhang, X.; Zou, J.-J.; Li, N.; Guo, Y.; Li, X.; Shen, X.; et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nat. Cell Biol. 2020, 583, 286–289, doi:10.1038/s41586-020-2313-x.
- Konda, M.; Dodda, B.; Konala, V.M.; Naramala, S.; Adapa, S. Potential Zoonotic Origins of SARS-CoV-2 and Insights for Preventing Future Pandemics Through One Health Approach. Cureus 2020, 12, e8932, doi:10.7759/cureus.8932.
- Zhao, J.; Cui, W.; Tian, B.-P. The Potential Intermediate Hosts for SARS-CoV-2. Front. Microbiol. 2020, 11, 11, doi:10.3389/fmicb.2020.580137.
- Kiros, M.; Andualem, H.; Kiros, T.; Hailemichael, W.; Getu, S.; Geteneh, A.; Alemu, D.; Abegaz, W.E. COVID-19 pandemic: Current knowledge about the role of pets and other animals in disease transmission. Virol. J. 2020, 17, 1–8, doi:10.1186/s12985-020-01416-9.
- Pandey, K.; Acharya, A.; Mohan, M.; Ng, C.L.; Reid, S.P.; Byrareddy, S.N. Animal models for SARS‐CoV‐2 research: A com-prehensive literature review. Transbound. Emerg. Dis. 2020, 13907, doi:10.1111/tbed.13907.
- Raj, V.S.; Farag, E.A.; Reusken, C.B.; Lamers, M.M.; Pas, S.D.; Voermans, J.; Smits, S.L.; Osterhaus, A.D.; Al-Mawlawi, N.; Al-Romaihi, H.E.; et al. Isolation of MERS Coronavirus from a Dromedary Camel, Qatar, 2014. Emerg. Infect. Dis. 2014, 20, 1339–1342, doi:10.3201/eid2008.140663.
- El-Zowalaty, M.E.; Järhult, J.D. From SARS to COVID-19: A previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans—Call for a One Health approach. One Heal. 2020, 9, 100124, doi:10.1016/j.onehlt.2020.100124.
- Tazerji, S.S.; Duarte, P.M.; Rahimi, P.; Shahabinejad, F.; Dhakal, S.; Malik, Y.S.; Shehata, A.A.; Lama, J.; Klein, J.; Safdar, M.; et al. Transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to animals: An updated review. J. Transl. Med. 2020, 18, 1–11, doi:10.1186/s12967-020-02534-2.
- McNamara, T.; Richt, J.A.; Glickman, L. A Critical Needs Assessment for Research in Companion Animals and Livestock Following the Pandemic of COVID-19 in Humans. Vector Borne Zoonotic Dis. 2020, 20, 393–405, doi:10.1089/vbz.2020.2650.
- Munnink, B.B.O.; Sikkema, R.S.; Nieuwenhuijse, D.F.; Molenaar, R.J.; Munger, E.; Molenkamp, R.; van der Spek, A.; Tolsma, P.; Rietveld, A.; Brouwer, M.; et al. Transmission of SARS-CoV-2 on mink farms between humans and mink and back to hu-mans. Science 2021, 371, 172–177, doi:10.1126/science.abe5901.
- Enserink, M. Coronavirus rips through Dutch mink farms, triggering culls. Science 2020, 368, 1169, doi:10.1126/science.368.6496.1169.
- Centers for Disease Control (CDC). One Health Basics. Available online: https://www.cdc.gov/onehealth/basics/index.html (accessed on 28 January 2021).
- Phelan, A.L.; Katz, R.; Gostin, L.O. The Novel Coronavirus Originating in Wuhan, China. JAMA 2020, 323, 709, doi:10.1001/jama.2020.1097.
- Chowdhury, M.A.; Hossain, N.; Kashem, M.A.; Shahid, A.; Alam, A. Immune response in COVID-19: A review. J. Infect. Public Heal. 2020, 13, 1619–1629, doi:10.1016/j.jiph.2020.07.001.
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489–493, doi:10.1126/science.abb3221.
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374, doi:10.1038/s41577-020-0311-8.
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science 2020, 368, 1016–1020, doi:10.1126/science.abb7015.
- Sun, J.; He, W.-T.; Wang, L.; Lai, A.; Ji, X.; Zhai, X.; Li, G.; Suchard, M.A.; Tian, J.; Zhou, J.; et al. COVID-19: Epidemiology, Evolution, and Cross-Disciplinary Perspectives. Trends Mol. Med. 2020, 26, 483–495, doi:10.1016/j.molmed.2020.02.008.
- Oran, D.P.; Topol, E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection. Ann. Intern. Med. 2020, 173, 362–367, doi:10.7326/m20-3012.
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Chil-dren. N. Engl. J. Med. 2020, 382, 1663–1665, doi:10.1056/nejmc2005073.
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A fa-milial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523, doi:10.1016/s0140-6736(20)30154-9.
- Gudbjartsson, D.F.; Helgason, A.; Jonsson, H.; Magnusson, O.T.; Melsted, P.; Norddahl, G.L.; Saemundsdottir, J.; Sigurdsson, A.; Sulem, P.; Agustsdottir, A.B.; et al. Spread of SARS-CoV-2 in the Icelandic Population. N. Engl. J. Med. 2020, 382, 2302–2315, doi:10.1056/nejmoa2006100.
- Burki, T. Mass testing for COVID-19. Lancet Microbe 2020, 1, e317, doi:10.1016/s2666-5247(20)30205-6.
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Charac-teristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720, doi:10.1056/nejmoa2002032.
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmis-sion dynamics in Wuhan, China, of Novel Coronavirus–infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207, doi:10.1056/nejmoa2001316.
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020, 172, 577–582, doi:10.7326/m20-0504.
- Bunders, M.J.; Altfeld, M. Implications of Sex Differences in Immunity for SARS-CoV-2 Pathogenesis and Design of Thera-peutic Interventions. Immunity 2020, 53, 487–495, doi:10.1016/j.immuni.2020.08.003.
- Turan, O.; Hakim, A.; Dashraath, P.; Jeslyn, W.J.L.; Wright, A.; Abdul‐Kadir, R. Clinical characteristics, prognostic factors, and maternal and neonatal outcomes of SARS‐CoV‐2 infection among hospitalized pregnant women: A systematic review. Int. J. Gynecol. Obstet. 2020, 151, 7–16, doi:10.1002/ijgo.13329.
- Arias-Reyes, C.; Zubieta-De-Urioste, N.; Poma-Machicao, L.; Aliaga-Raduan, F.; Carvajal-Rodriguez, F.; Dutschmann, M.; Schneider-Gasser, E.M.; Zubieta-Calleja, G.; Soliz, J. Does the pathogenesis of SARS-CoV-2 virus decrease at high-altitude? Respir. Physiol. Neurobiol. 2020, 277, 103443, doi:10.1016/j.resp.2020.103443.
- Segovia-Juarez, J.; Castagnetto, J.M.; Gonzales, G.F. High altitude reduces infection rate of COVID-19 but not case-fatality rate. Respir. Physiol. Neurobiol. 2020, 281, 103494, doi:10.1016/j.resp.2020.103494.
- Chennakesavulu, K.; Reddy, G.R. The effect of latitude and PM2.5 on spreading of SARS-CoV-2 in tropical and temperate zone countries. Environ. Pollut. 2020, 266, 115176, doi:10.1016/j.envpol.2020.115176.
- Hooper, M.W.; Nápoles, A.M.; Pérez-Stable, E.J. COVID-19 and Racial/Ethnic Disparities. JAMA 2020, 323, 2466, doi:10.1001/jama.2020.8598.
- McClure, E.S.; Vasudevan, P.; Bailey, Z.; Patel, S.; Robinson, W.R. Racial Capitalism Within Public Health—How Occupa-tional Settings Drive COVID-19 Disparities. Am. J. Epidemiol. 2020, 189, 1244–1253, doi:10.1093/aje/kwaa126.
- Stokes, E.K.; Zambrano, L.D.; Anderson, K.N.; Marder, E.P.; Raz, K.M.; Felix, S.E.B.; Tie, Y.; Fullerton, K.E. Coronavirus Disease 2019 Case Surveillance—United States, January 22–May 30, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 759–765, doi:10.15585/mmwr.mm6924e2.
- Killerby, M.E.; Link-Gelles, R.; Haight, S.C.; Schrodt, C.A.; England, L.; Gomes, D.J.; Shamout, M.; Pettrone, K.; O’Laughlin, K.; Kimball, A.; et al. Characteristics Associated with Hospitalization Among Patients with COVID-19—Metropolitan Atlan-ta, Georgia, March–April 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 790–794, doi:10.15585/mmwr.mm6925e1.
- United States Department of Health and Human Services (USDHHS.) Social Determinants of Health. Available online: https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-healthexternal (accessed on 1 December 2020).
- Emeruwa, U.N.; Ona, S.; Shaman, J.L.; Turitz, A.; Wright, J.D.; Gyamfi-Bannerman, C.; Melamed, A. Associations Between Built Environment, Neighborhood Socioeconomic Status, and SARS-CoV-2 Infection Among Pregnant Women in New York City. JAMA 2020, 324, 390–392, doi:10.1001/jama.2020.11370.
- Goyal, M.K.; Simpson, J.N.; Boyle, M.D.; Badolato, G.M.; Delaney, M.; McCarter, R.; Cora-Bramble, D. Racial and/or Ethnic and Socioeconomic Disparities of SARS-CoV-2 Infection Among Children. Pediatrics 2020, 146, 2020009951, doi:10.1542/peds.2020-009951.
- Hagan, L.M.; Williams, S.P.; Spaulding, A.C.; Toblin, R.L.; Figlenski, J.; Ocampo, J.; Ross, T.; Bauer, H.; Hutchinson, J.; Lucas, K.D.; et al. Mass Testing for SARS-CoV-2 in 16 Prisons and Jails—Six Jurisdictions, United States, April–May 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1139–1143, doi:10.15585/mmwr.mm6933a3.
- Mosites, E.; Parker, E.M.; Clarke, K.E.N.; Gaeta, J.M.; Baggett, T.P.; Imbert, E.; Sankaran, M.; Scarborough, A.; Huster, K.; Hanson, M.; et al. Assessment of SARS-CoV-2 Infection Prevalence in Homeless Shelters—Four U.S. Cities, March 27–April 15, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 521–522, doi:10.15585/mmwr.mm6917e1.
- Centers for Disease Control and Prevention (CDC). People at Increased Risk. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html (accessed on 1 December 2020).
- Castagnoli, R.; Votto, M.; Licari, A.; Brambilla, I.; Bruno, R.; Perlini, S.; Rovida, F.; Baldanti, F.; Marseglia, G.L. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents. JAMA Pediatr. 2020, 174, 882, doi:10.1001/jamapediatrics.2020.1467.
- Jin, Y.-H.; Cai, L.; Cheng, Z.-S.; Cheng, H.; Deng, T.; Fan, Y.-P.; Fang, C.; Huang, D.; Huang, L.-Q.; Huang, Q.; et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard ver-sion). Mil. Med. Res. 2020, 7, 4, doi:10.1186/s40779-020-0233-6.
- Burke, R.M.; Midgley, C.M.; Dratch, A.; Fenstersheib, M.; Haupt, T.; Holshue, M.; Ghinai, I.; Jarashow, M.C.; Lo, J.; McPher-son, T.D.; et al. Active Monitoring of Persons Exposed to Patients with Confirmed COVID-19—United States, January–February 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 245–246, doi:10.15585/mmwr.mm6909e1.
- Cheng, V.C.-C.; Wong, S.-C.; Chuang, V.W.-M.; So, S.Y.-C.; Chen, J.H.-K.; Sridhar, S.; To, K.K.-W.; Chan, J.F.-W.; Hung, I.F.-N.; Ho, P.-L.; et al. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 2020, 81, 107–114, doi:10.1016/j.jinf.2020.04.024.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients in-fected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506, doi:10.1016/s0140-6736(20)30183-5.
- Liu, J.; Liao, X.; Qian, S.; Yuan, J.; Wang, F.; Liu, Y.; Wang, Z.; Wang, F.-S.; Liu, L.; Zhang, Z. Community Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, Shenzhen, China, 2020. Emerg. Infect. Dis. 2020, 26, 26, doi:10.3201/eid2606.200239.
- Meselson, M. Droplets and Aerosols in the Transmission of SARS-CoV-2. New Engl. J. Med. 2020, 382, 2063, doi:10.1056/nejmc2009324.
- World Health Organization (WHO). Scientific Brief: SARS-CoV-2 and Potential Airborne Transmission. Available online: https://www.cdc.gov/coronavirus/2019-ncov/more/scientific-brief-sars-cov-2.html (accessed on December 1 2020).
- Centers for Disease Control (CDC). Animals & COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/animals.html (accessed on 1 December 2020).
- Van Doremalen, N.; Miazgowicz, K.L.; Milne-Price, S.; Bushmaker, T.; Robertson, S.; Scott, D.; Kinne, J.; McLellan, J.S.; Zhu, J.; Munster, V.J. Host Species Restriction of Middle East Respiratory Syndrome Coronavirus through Its Receptor, Dipeptidyl Peptidase 4. J. Virol. 2014, 88, 9220–9232, doi:10.1128/jvi.00676-14.
- Fears, A.C.; Klimstra, W.B.; Duprex, P.; Hartman, A.; Weaver, S.C.; Plante, K.S.; Mirchandani, D.; Plante, J.A.; Aguilar, P.V.; Fernández, D.; et al. Persistence of Severe Acute Respiratory Syndrome Coronavirus 2 in Aerosol Suspensions. Emerg. Infect. Dis. 2020, 26, 2168–2171, doi:10.3201/eid2609.201806.
- Aboubakr, H.A.; Sharafeldin, T.A.; Goyal, S.M. Stability of SARS‐CoV‐2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transbound. Emerg. Dis. 2020, 13707, doi:10.1111/tbed.13707.
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10, doi:10.1016/s2666-5247(20)30003-3.
- Pastorino, B.; Touret, F.; Gilles, M.; de Lamballerie, X.; Charrel, R.N. Prolonged Infectivity of SARS-CoV-2 in Fomites. Emerg. Infect. Dis. 2020, 26, 2256–2257, doi:10.3201/eid2609.201788.
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing Temperature and Relative Humidity Accelerates Inactivation of SARS-CoV-2 on Surfaces. mSphere 2020, 5, 00441–20, doi:10.1128/msphere.00441-20.
- Mondelli, M.U.; Colaneri, M.; Seminari, E.M.; Baldanti, F.; Bruno, R. Low risk of SARS-CoV-2 transmission by fomites in re-al-life conditions. Lancet Infect. Dis. 2020, doi:10.1016/s1473-3099(20)30678-2.
- Goldman, E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect. Dis. 2020, 20, 892–893, doi:10.1016/s1473-3099(20)30561-2.
- Dibner, J. Fecal‐oral transmission of COVID‐19: Could hypochlorhydria play a role? J. Med. Virol. 2021, 93, 166–167, doi:10.1002/jmv.26265.
- Sun, J.; Zhu, A.; Li, H.; Zheng, K.; Zhuang, Z.; Chen, Z.; Shi, Y.; Zhang, Z.; Chen, S.-B.; Liu, X.; et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg. Microbes Infect. 2020, 9, 991–993, doi:10.1080/22221751.2020.1760144.
- Wang, J.; Feng, H.; Zhang, S.; Ni, Z.; Ni, L.; Chen, Y.; Zhuo, L.; Zhong, Z.; Qu, T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020, 94, 103–106, doi:10.1016/j.ijid.2020.04.024.
- Xiao, F.; Sun, J.; Xu, Y.; Li, F.; Huang, X.; Li, H.; Zhao, J.; Huang, J.; Zhao, J. Infectious SARS-CoV-2 in Feces of Patient with Severe COVID-19. Emerg. Infect. Dis. 2020, 26, 1920–1922, doi:10.3201/eid2608.200681.
- Ahmed, W.; Angel, N.; Edson, J.; Bibby, K.; Bivins, A.; O’Brien, J.W.; Choi, P.M.; Kitajima, M.; Simpson, S.L.; Li, J.; et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveil-lance of COVID-19 in the community. Sci. Total. Environ. 2020, 728, 138764, doi:10.1016/j.scitotenv.2020.138764.
- Bhowmick, G.D.; Dhar, D.; Nath, D.; Ghangrekar, M.M.; Banerjee, R.; Das, S.; Chatterjee, J. Coronavirus disease 2019 (COVID-19) outbreak: Some serious consequences with urban and rural water cycle. npj Clean Water 2020, 3, 1–8, doi:10.1038/s41545-020-0079-1.
- Peng, L.; Liu, J.; Xu, W.; Luo, Q.; Chen, D.; Lei, Z.; Huang, Z.; Li, X.; Deng, K.; Lin, B.; et al. SARS‐CoV‐2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J. Med. Virol. 2020, 92, 1676–1680, doi:10.1002/jmv.25936.
- Chang, L.; Zhao, L.; Gong, H.; Wang, L.; Wang, L. Severe Acute Respiratory Syndrome Coronavirus 2 RNA Detected in Blood Donations. Emerg. Infect. Dis. 2020, 26, 1631–1633, doi:10.3201/eid2607.200839.
- Chang, L.; Yan, Y.; Wang, L. Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfus. Med. Rev. 2020, 34, 75–80, doi:10.1016/j.tmrv.2020.02.003.
- Hamer, S.A.; Pauvolid-Corrêa, A.; Zecca, I.B.; Davila, E.; Auckland, L.D.; Roundy, C.M.; Tang, W.; Torchetti, M.; Killian, M.L.; Jenkins-Moore, M.; et al. Natural SARS-CoV-2 infections, including virus isolation, among serially tested cats and dogs in households with confirmed human COVID-19 cases in Texas, USA. bioRxiv 2020, doi:10.1101/2020.12.08.416339.
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273, doi:10.1038/s41586-020-2012-7.
- Yang, J.; Chen, X.; Deng, X.; Chen, Z.; Gong, H.; Yan, H.; Wu, Q.; Shi, H.; Lai, S.; Ajelli, M.; et al. Disease burden and clinical severity of the first pandemic wave of COVID-19 in Wuhan, China. Nat. Commun. 2020, 11, 1–10, doi:10.1038/s41467-020-19238-2.
- Cleary, S.J.; Pitchford, S.C.; Amison, R.T.; Carrington, R.; Cabrera, C.L.R.; Magnen, M.; Looney, M.R.; Gray, E.; Page, C.P. Animal models of mechanisms of SARS‐CoV‐2 infection and COVID‐19 pathology. Br. J. Pharmacol. 2020, 177, 4851–4865, doi:10.1111/bph.15143.
- Sarkar, J.; Guha, R. Infectivity, virulence, pathogenicity, host-pathogen interactions of SARS and SARS-CoV-2 in experi-mental animals: A systematic review. Vet. Res. Commun. 2020, 44, 101–110, doi:10.1007/s11259-020-09778-9.
- Bartels, C.J.; Fakhri, A.Q.; Shams, M.H.; Briscoe, R.P.; Schreuder, B.E. Livestock mortality and offtake in sheep and goat flocks of livestock owners making use of services offered by paravets in West Afghanistan. Prev. Vet. Med. 2017, 146, 79–85, doi:10.1016/j.prevetmed.2017.07.019.
- World Health Organization (WHO). Archived: WHO Timeline—COVID-19. Available online: https://www.who.int/news/item/27-04-2020-who-timeline---covid-19 (accessed on 1 December 2020).
- De Morais, H.A.; dos Santos, A.P.; Nascimento, N.C.D.; Kmetiuk, L.B.; Barbosa, D.S.; Brandão, P.E.; Guimarães, A.M.S.; Pettan-Brewer, C.; Biondo, A.W. Natural Infection by SARS-CoV-2 in Companion Animals: A Review of Case Reports and Current Evidence of Their Role in the Epidemiology of COVID-19. Front. Vet. Sci. 2020, 7, 591216, doi:10.3389/fvets.2020.591216.
- McAloose, D.; Laverack, M.; Wang, L.; Killian, M.L.; Caserta, L.C.; Yuan, F.; Mitchell, P.K.; Queen, K.; Mauldin, M.R.; Cronk, B.D.; et al. From People to Panthera: Natural SARS-CoV-2 Infection in Tigers and Lions at the Bronx Zoo. mBio 2020, 11, doi:10.1128/mbio.02220–20.