
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paola Letardi | + 1831 word(s) | 1831 | 2021-02-13 03:05:59 | | | |
| 2 | Vicky Zhou | Meta information modification | 1831 | 2021-02-19 02:33:08 | | | | |
| 3 | Vicky Zhou | Meta information modification | 1831 | 2021-02-19 02:33:26 | | |
Video Upload Options
Coatings to be applied on metallic heritage should satisfy complex requirements. This overview presents the main issues to be considered both from a Materials Science view and from a conservation-restoration one. The corrosion mechanism for outdoor bronze monuments is outlined with some of the research project which addressed the need for more affective treatments.
1. Introduction
Metal objects represents a very broad category in heritage conservation. It is something easy to realise just by having a look at the corrosion identification booklet published by Parks Canada [1] to “provide descriptions and helpful tips, accompanied by photographs, to anyone in charge of metal collections”. Selwyn [2] provided a deeper discussion about the known chemical and physical characteristics of “metals and alloys of interest for conservation professionals, along with the different form of corrosion problems indoors, outdoors, and in archaeological settings”. In her book, one can find an extensive discussion of metals and alloys of interest for cultural heritage (Table 1), main information on construction steps (Table 2)—which may influence the conservation—and on corrosion basic principles. Specific bibliographic references are provided there for each item.
Table 1. Most used metals in heritage objects, with common minerals from which they are extracted, historical data and typical appearance (adapted from Tables 1.1, 1.2 and 1.5 in Ref. [2]).
| Metal | Mineral | Formula | Approximate Date of First Widespread Use | Typical Colours of Corrosion Products |
|---|---|---|---|---|
| aluminium | gibbsite | Al(OH)3 | 1800–1900 A.D. (Europe/USA) | colourless or white |
| copper | chalcocite | Cu2S | ~7000 B.C. (Near East) for native copper~ 5000 B.C. (Near East) for smelted copper |
Cu(I): red, black, colourless Cu(II): green, blue |
| gold | (native) | Au | 5000–4000 B.C. (Balkans) | – |
| iron | hematite | Fe2O3 | 1000–0 B.C. (Near East) | Fe(I,III): black Fe(III): red, yellow, orange |
| lead | galena | PbS | 6000–5000 B.C. (Near East/Balkans) | white, red yellow |
| nickel | pentlandite | (Ni,Fe)9S8 | 2000–1000 B.C. (Near East) for copper/nickel alloys | green |
| silver | argentite | Ag2S | 4000–3000 B.C. (Balkans/Near East) | black, white |
| tin | cassiterite | SnO2 | 4000–3000 B.C. (Near East) | black, white |
| zinc | smithsonite | ZnCO3 | 100–200 A.D. (Rome) for copper/zinc alloys 900–1000 A.D. (India) for zinc metal |
colourless or white |
Table 2. Basic stages of metal objects construction techniques (adapted from Table 1.4 in Ref. [2]).
| Construction Step | Description |
|---|---|
| Forming and Shaping | production by pouring liquid metals into moulds (casting) and by mechanical deformation (forging, rolling; working such as milling, turning, spinning, grinding, stamping, cutting, drilling |
| Assembling | fitting components by welding, soldering, brazing, rivetting, bolting, crinping, gluing |
| Finishing | Completing surface appearance by plating, burnishing, polishing, etching, sand-blasting, painting, lacquering, engraving, chasing, embossing, enameling, patinating |
As a rule, the inherent instability of metallic heritage offers similar preservation challenges to those faced in civil engineering, automotive and construction industries [3]. The main source of this instability is the energy required to extract metals from their ores (smelting), which leaves metals in a high energy state with the tendency to return to the lower energy mineral state [2]. The actual behaviour of every metallic item is the result of complex interactions between the chemical–physical properties of the object and the particular environment around it [4]. These interactions may reach a static or dynamical equilibrium over time. It is thus essential to consider the specific environments all along the lifetime of a metallic heritage object in order to develop treatments and identify realistic conservation goals [4]. Through a deeper understanding of the complex chemical, thermodynamic and kinematic factors, several choices to mitigate the adverse effects of corrosion can be developed by modifying the environment or the surface finishing of the object, as addressed by Corrosion Science [5]. Corrosion is one of the major issues for metal heritage objects conservation, and methodologies and principles of corrosion science have been slowly entering conservation practice from the second half of the 20th century [3][6][7][8][9][10].
Although the basic laws underlying corrosion processes in the field of cultural heritage are the same as in industry, the criteria and priorities behind operational choices to mitigate the adverse effects of corrosion are deeply different [4][5][7][11][12][13]. The vast possibilities of interaction between distinct metals and types of environment give rise to unlimited combinations of corrosion forms, making it useless for conservation purposes to gather occurrences following traditional corrosion classifications. Since the exchange with its surroundings is so intensive, metallic heritage can be better understood and conserved when its context is considered. Accordingly, broad categories of heritage metal objects can be identified depending on the environment where they have been (or still are) [14]:
- Archaeological metals are characterised mainly by long burial in soil [15], waterlogged [12][15][16] or underwater [17]; they bear information on very long-term corrosion of metals [18][19]; the equilibrium state reached during burial may be broken when excavated, giving rise to new corrosion process if not properly treated [20].
- A large variety of historic objects (such as scientific instruments, fine arts, historic pieces, ethnographic specimens, etc.) is conserved indoors (museums, monumental buildings, collections); the main preservation strategy in this environment is preventive conservation [4][21], such as humidity control; critical parameter to consider are dangers from “off-gassing” materials used to build display cases and rooms, as well as air pollution introduced by visitors [21].
- Outdoors monumental and architectural items (sculptures, roofs and decorative objects, functional artifacts and industrial heritage) are mainly subject to weather conditions, pollution and climate change [4][6][14][22][23][24][25][26][27].
2. Coatings for Outdoor Bronze Monuments
In order to apply correctly the principles of Corrosion Science to the conservation of metal heritage objects, each specific metal or alloy, construction technique, and particular corrosion problem encountered in a museum, outdoor, or archaeological setting should be carefully considered [2][3][4][15][18][22]. The specific characteristics of heritage objects are the manifold result of a complex and often not completely known history. This can lead to inappropriate treatments when coatings checked for a specific context are adopted in another one without taking into account all the relevant features [28]. For this reason, the topic of protective treatments for metallic cultural heritage can be wide and complex and oversimplified research data may be useless for the conservation of metal heritage objects purposes. Accordingly, from now on the discussion will be restricted to outdoors artifacts. Within this category, the distinctive features relative to bronze sculptures will be considered in order to enlighten the specific characteristics required for conservation treatments [29].
Environmental changes produced by the industrial era raised problems in many fields. Increased pollution and acid rain effects on materials were widely recognised [30] and many research efforts addressed this topic, which is the field of Atmospheric Corrosion [31]. Outdoor copper and copper alloy statues started suffering from a sharp decrease in surface stability and localised corrosion endangering their artistic and aesthetic content [22]. This triggered a growing interaction between conservator-restorers and the scientific community [6][7][8][9][10][25][32]. Over the last decades, this allowed a number of improvements: A growing understanding of the electrochemical processes typical of outdoor bronze monuments [33][34][35][36][37][38][39]; the dissemination of the “Theory of Restoration” by Brandi as a tool to lead choices on new conservation practices [7][11][40][41]; more awareness about possible strategies to deal with the changing equilibria and available resources [3][42].
The present understanding of copper and copper alloys atmospheric corrosion ([31], chapters 8, 13, 14; appendix E, K), can be roughly described as the formation of a passivating cuprite layer in clean humid air which evolves into a more complex surface layer (patina) according to the main pollutants present in the surrounding atmosphere [3][25][34]. Green basic copper hydroxysulfates (mainly brochantite) form in SO2 rich atmosphere, while basic hydroxychlorides (atacamite, clinoatacamite) dominate marine environments [43]. Alloying elements play a key role in the corrosion mechanism, which differs from pure copper [34][39][44][45] A decuprification process was identified [37][44][45], along with cyclic corrosion similar to the “bronze disease” traditionally associated with archaeological copper alloys [2][35][45][46]. The growth of this surface layer on outdoor bronze sculptures and architectonic elements exposed to different weather and pollution conditions is the result of a specific timeline. Several factors determine its local composition and texture, such as the solubility of corrosion products, sheltered and unsheltered exposure, the changing weather (relative humidity, temperature, light and Ultra Violet (UV) radiation, time of wetness), the composition and concentration of air pollutants (Figure 1).
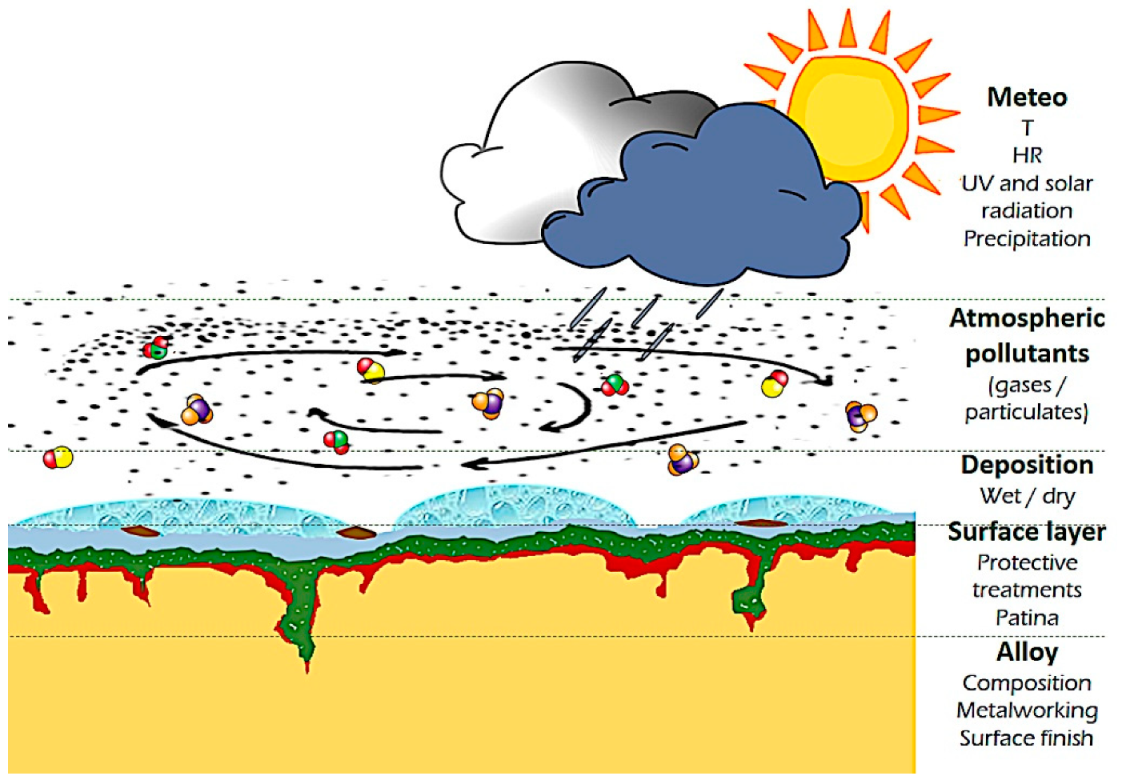
On a single monument, different conditions are often present, and the evolution of the overall result can be detrimental for aesthetic reasons if not for the material loss [42][45]. This leads to the necessity of restoration. The first step consists of cleaning [47][48][49]. The cleaning methodologies adopted should selectively remove only water-soluble compounds [47], atmospheric particulate deposits, hydrocarbon compounds coming from environmental pollution and other organic compounds from past treatments [50] or failed coatings [51]. At the same time, it should preserve the part of the patina valuable for aesthetic, historical and conservation reasons [48][52]. Afterward, the common practice consists in the use of treatments to prevent or reduce detrimental corrosion, which for outdoor bronze consist in the application of coatings that avoid the contact of the metal/patina layer with the actively corroding agents in the atmosphere (water, corroding ions) and/or other treatments (inhibitors) to reduce the electrochemical reaction rates [4][5]. Sooner or later, cleaning and application of a protective treatment should be repeated, according to environmental conditions, maintenance programs, etc. (Figure 2).
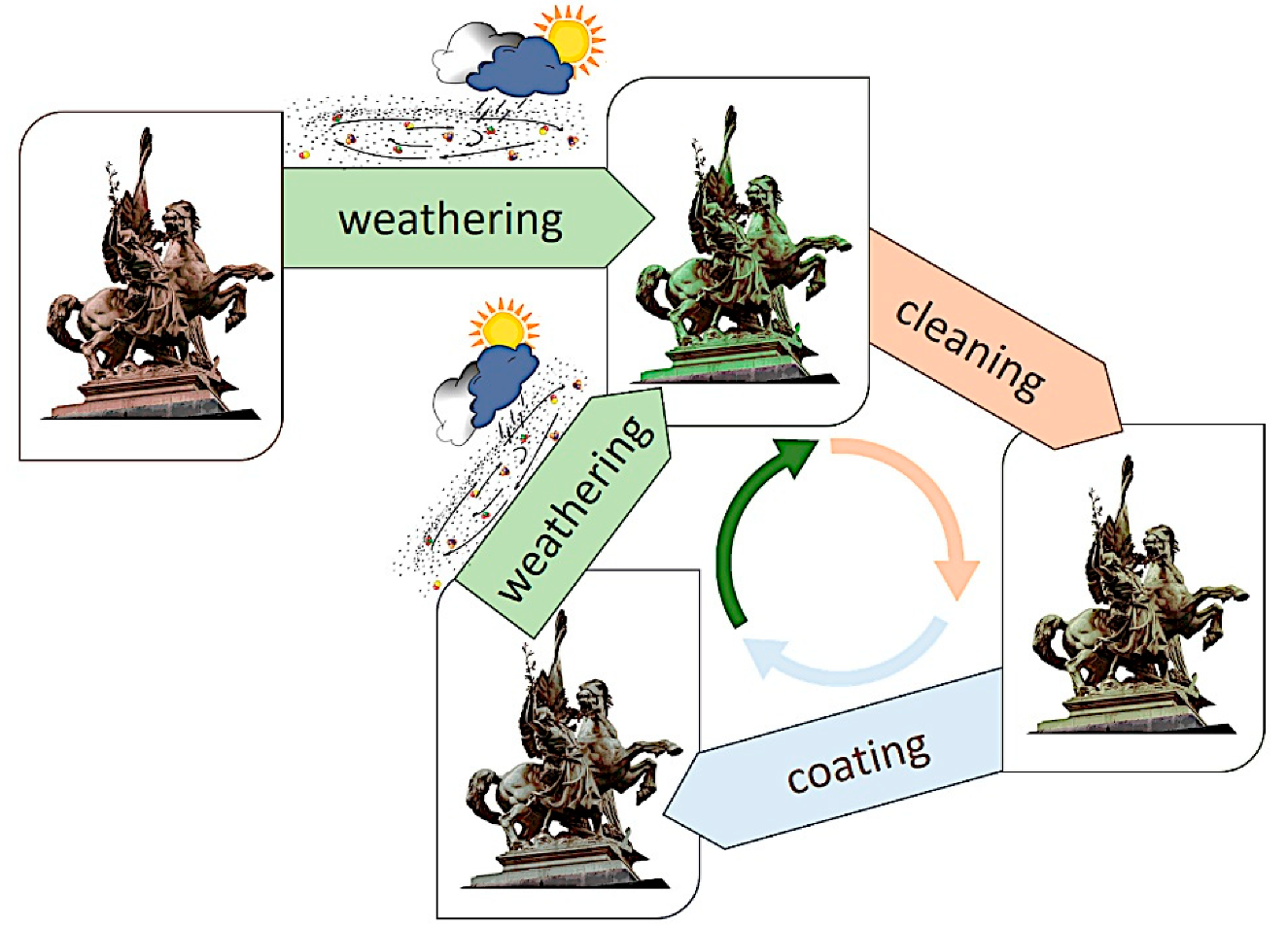
Since the end of the 20th Century, several projects have addressed the need for new/more effective protective treatments to be applied on metallic heritage objects.
The EU-FP3-funded project “New Conservation Methods for Outdoor Bronze Sculptures” [53] considered a new class of sol–gel derived coatings-organic inorganic copolymers, called ormocers (ORganically MOdified CERamics); addressed requirements were the use in outdoor bronze conservation with good protection against corrosion, and at the same time the right compromise between stability and reversibility of the cured coating [54].
After the public engagement following the refurbishment of the Statue of Liberty [55], some sponsors supported the research “Coating strategies for the protection of outdoor bronze art and ornamentation” [56], where the performance of 29 coatings on different copper alloy substrates was addressed and the importance of considering a coating system as a whole, and not only by its part, was pointed out.
In the mainframe of EU-FP6-funded project “EU-ARTECH”, traditional treatments performances (Incralac, waxes, Benzotriazole) were compared to innovative treatments ones on coupons with natural and artificial patina [56]; different commercial organo-silanes were tested, the use of limewater was considered to adjust pH toward alkalinity to inhibit bronze disease, and the possible use of fungi to transform unstable corrosion patinas to insoluble copper oxalate was investigated [40]; on copper lamina with natural green patina (mainly brochantite) the Dynasylan F8263 and SIVOClear showed a protective behaviour comparable to Incralac but without perceivable chromatic alterations [57][58]. Biopatina was further investigated in other projects [40][59].
Other EU-funded projects addressed the development of new and more effective protective treatments for outdoor bronze artworks [60][61]. Several efforts were performed to deepen the understanding of the forms and properties of outdoor bronze surface layers [36][39][44][48][62]. Increasing attention was paid to the characterisation and comparison of surface layers and treatments on artworks [63][64][65][66][67].
Until now, the transfer of innovative treatments in the practice of restoration of outdoor bronzes was quite poor, despite the considerable amount of laboratory studies in this field since the 1990s.
References
- Ankersmit, B.; Griesser-Stermscheg, M.; Selwyn, L.; Sutherland, S. Rust Never Sleeps: Recognizing Metals and Their Corrosion Products; Parks Canada: Gatineau, QC, Canada, 2008.
- Selwyn, L. Metals and Corrosion: A Handbook for the Conservation Professional; Canadian Conservation Institute: Ottawa, ON, Canada, 2004.
- Watkinson, D. Conservation, corrosion science and evidence-based preservation strategies for metallic heritage artefacts. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; EFS Series; Woodhead Publishing: Cambridge, UK, 2013; pp. 9–36.
- Watkinson, D. Preservation of metallic cultural heritage. In Shreir’s Corrosion; Elsevier: Amsterdam, The Netherlands, 2010; pp. 3307–3340.
- Cano, E.; Lafuente, D. Corrosion inhibitors for the preservation of metallic heritage artefacts. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; EFS Series; Woodhead Publishing: Cambridge, UK, 2013; pp. 570–594.
- Alunno-Rossetti, V.; Marabelli, M. Analyses of the patinas of a gilded horse of StMark’s Basilica in Venice: Corrosion mechanism and conservation problems. Stud. Conserv. 1976, 21, 161–170.
- Baboian, R. A corrosion scientist’s summary of Dialogue 89. In Dialogue/89: The Conservation of Bronze Sculpture in the Outdoor Environment: A Dialogue among Conservators, Curators, Environmental Scientists, and Corrosion Engineers; Drayman-Weisser, T., Ed.; National Association of Corrosion Engineers: Houston, UK, 1992; pp. 379–386.
- Marabelli, M.; Napolitano, G. Nuovi sistemi protettivi applicabili su opere o manufatti in bronzo esposti all’aperto (New Protective Systems Applied to Outdoor Bronze Statuary or Artifacts). Mater. E Strutt. Probl. Conserv. 1991, 1, 51–58.
- Leoni, M. Un particolare fenomeno corrosivo sulla Fiorenza del Giambologna. OPD Restauro 1991, 3, 52–56.
- Scott, D.A.; Podany, J.; Considine, B.B. (Eds.) Ancient & Historic Metals: Conservation and Scientific Research—Proceedings of a Symposium Organized by the J. Paul Getty Museum and the Getty Conservation Institute November 1991; Getty Conservation Institute: Marina del Rey, CA, USA, 1994.
- Dillmann, P.; Watkinson, D.; Angelini, E.; Adriaens, A. Introduction: Conservation versus laboratory investigation in the preservation of metallic heritage artefacts. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; EFS Series; Woodhead Publishing: Cambridge, UK, 2013; pp. 1–5.
- Matthiesen, H.; Gregory, D.; Jensen, P.; Sørensen, B. Environmental monitoring at Nydam, a waterlogged site with weapon sacrifices from the Danish Iron age. I: A comparison of methods used and results from undisturbed conditions. J. Wetl. Archaeol. 2004, 4, 55–74.
- Bertholon, R. The location of the original surface: A review of the conservation literature. In Metal 2001, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Santiago, Chile, 2–6 April 2001; MacLeod, I.D., Theile, J.M., Degrigny, C., Eds.; Western Australian Museum: Perth, Australia, 2004; pp. 167–179.
- Bernard, M.C.; Joiret, S. Understanding corrosion of ancient metals for the conservation of cultural heritage. Electrochim. Acta 2009, 54, 5199–5205.
- Selwyn, L. Overview of archaeological iron: The corrosion problem, key factors affecting treatment, and gaps in current knowledge. In Metal 2004, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Canberra, Australia, 4–8 October 2004; Ashton, J., Hallam, D., Eds.; National Museum of Australia: Canberra, Australia, 2004; pp. 294–306.
- Goodburn-Brown, D. Surface studies on metals from waterlogged sites. In Metal 95, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Semur en Auxois, France, 25–28 September 1995; MacLeod, I.D., Pennec, S.L., Robbiola, L., Eds.; James & James: London, UK, 1997; pp. 61–66.
- Memet, J.B. The corrosion of metallic artefacts in seawater: Descriptive analysis. In Corrosion of Metallic Heritage Artefacts; Dillmann, P., Béranger, G., Piccardo, P., Matthiesen, H., Eds.; European Federation of Corrosion (EFC) Series; Woodhead Publishing: Cambridge, UK, 2007; pp. 152–169.
- Robbiola, L.; Blengino, J.-M.; Fiaud, C. Morphology and mechanisms of formation of natural patinas on archaeological Cu–Sn alloys. Corros. Sci. 1998, 40, 2083–2111.
- Moore, J.D. Long-term corrosion processes of iron and steel shipwrecks in the marine environment: A review of current knowledge. J. Marit. Archaeol. 2015, 10, 191–204.
- Rémazeilles, C.; Langlet-Marzloff, V.; Creus, J.; Lotte, G.; Deshayes, C.; Baleux, F.; Robbiola, L. Remarkable corrosion resumption of archaeological bronzes, induced by the oxidation of ternary Cu–Sn–S phases in atmosphere, after long-term burial with sulfides. Corros. Sci. 2020, 175, 108865.
- Thickett, D. Frontiers of preventive conservation. Stud. Conserv. 2018, 63 (Suppl. 1), 262–267.
- Lins, A.; Power, T. The corrosion of bronze monuments in polluted urban sites: A report on the stability of copper mineral species at different pH levels. In Ancient & Historic Metals: Conservation and Scientific Research—Proceedings of a Symposium Organized by the J. Paul Getty Museum and the Getty Conservation Institute November 1991; Getty Conservation Institute: Marina del Rey, CA, USA, 1994; pp. 119–151.
- Matero, F.G. Conservation of architectural metalwork: Historical approaches to the surface treatment of iron. In Ancient & Historic Metals: Conservation and Scientific Research—Proceedings of a Symposium Organized by the J. Paul Getty Museum and the Getty Conservation Institute November 1991; Getty Conservation Institute: Marina del Rey, CA, USA, 1994; pp. 198–228.
- Grissom, C.A. The conservation of outdoor zinc sculpture. In Ancient & Historic Metals: Conservation and Scientific Research—Proceedings of a Symposium Organized by the J. Paul Getty Museum and the Getty Conservation Institute November 1991; Getty Conservation Institute: Marina del Rey, CA, USA, 1994; pp. 279–304.
- Strandberg, H. Perspectives on Bronze Sculpture Conservation. Modelling Copper and Bronze Corrosion. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 1997.
- Shashoua, Y.; Matthiesen, H. Protection of iron and steel in large outdoor industrial heritage objects. Corros. Eng. Sci. Technol. 2010, 45, 357–361.
- Kreislova, K.; Knotkova, D.; Geiplova, H. Atmospheric corrosion of historical industrial structures. In Corrosion and Conservation of Cultural Heritage Metallic Artefacts; Dillmann, P., Watkinson, D., Angelini, E., Adriaens, A., Eds.; European Federation of Corrosion (EFC) Series; Woodhead Publishing: Cambridge, UK, 2013; pp. 311–343.
- Thickett, D.; Stanley, B. The use and mis-use of renaissance wax. In Metal 2019, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Chemello, C., Brambilla, L., Joseph, E., Eds.; ICOM-CC and HE-Arc CR: Neuchatel, Switzerland, 2019; pp. 232–239. ISBN 978-92-9012-457-3.
- Gullman, J.; Törnblom, M. Principles of Bronze Sculpture–Its Making and Unmaking: A Study of Outdoor Bronze Sculpture Conservation; The Central Board of National Antiquities (Riksantikvarieämbetet): Stockholm, Sweden, 1994; ISBN 91-7192-943-6.
- Knotkova, D.; Boschek, P.; Kreislova, K. Effect of acidification on atmospheric corrosion of structural metals in Europe. Water Air Soil Pollut. 1995, 85, 2661–2666.
- Leygraf, C.; Odnevall Wallinder, I.; Tidblad, J.; Graedel, T. Atmospheric Corrosion, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006.
- Matteini, M.; Lalli, C.; Tosini, I. Controllo dei componenti solubili delle patine mediante cromatografia ionica e assorbimento atomico. OPD Restauro 1991, 3, 40–46.
- Graedel, T.E. Copper patinas formed in the atmosphere—II. A qualitative assessment of mechanisms. Corros. Sci. 1987, 27, 721–740.
- Robbiola, L.; Fiaud, C.; Pennec, S. New model of outdoor bronze corrosion and its implications for conservation. In Proceedings of the ICOM Committee for Conservation Tenth Triennial Meeting, Washington, DC, USA, 22–27 August 1993; pp. 796–802.
- Fischer, W.R.; Wagner, B.D.; Siedlarek, H.; Füßinger, B.; Hänßel, I.; von der Bank, N. The influence of chloride ions and light on the corrosion behaviour of copper alloys in aqueous environments with special regard to bronze disease. In Metal 95, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Semur en Auxois, France, 25–28 September 1995; MacLeod, I.D., Pennec, S., Robbiola, L., Eds.; James & James: London, UK, 1997; pp. 89–94.
- Chiavari, C.; Rahmouni, K.; Takenouti, H.; Joiret, S.; Vermaut, P.; Robbiola, L. Composition and electrochemical properties of natural patinas of outdoor bronze monuments. Electrochim. Acta 2007, 52, 7760–7769.
- Bernardi, E.; Chiavari, C.; Lenza, B.; Martini, C.; Morselli, L.; Ospitali, F.; Robbiola, L. The atmospheric corrosion of quaternary bronzes: The leaching action of acid rain. Corros. Sci. 2009, 51, 159–170.
- Wu, J.; Wang, J. The effects of UV and visible light on the corrosion of bronze covered with an oxide film in aqueous solution. Corros. Sci. 2019, 154, 144–158.
- Chang, T.; Maltseva, A.; Volovitch, P.; Odnevall Wallinder, I.; Leygraf, C. A mechanistic study of stratified patina evolution on Sn-bronze in chloride-rich atmospheres. Corros. Sci. 2020, 166, 108477.
- Joseph, E.; Simon, A.; Prati, S.; Wörle, M.; Job, D.; Mazzeo, R. Development of an analytical procedure for evaluation of the protective behaviour of innovative fungal patinas on archaeological and artistic metal artefacts. Anal. Bioanal. Chem. 2011, 399, 2899–2907.
- Contreras-Vargas, J.; Cama-Villafranca, J. The metal patina and surface layer of El Caballito: Calling things by their name. In Metal 2019, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Chemello, C., Brambilla, L., Joseph, E., Eds.; ICOM-CC and HE-Arc CR: Neuchatel, Switzerland, 2019; pp. 322–328. ISBN 978-92-9012-457-3.
- Dajnowski, A.; Ross, T.; Craig, A.B.; Dajnowski, B. New trends in art conservation, the use of lasers to clean as well as generate an augmented reality representation of an iconic public monument in bronze: The Alma Mater. Stud. Conserv. 2015, 60 (Suppl. 1), S65–S72.
- Di Carlo, G.; Giuliani, C.; Riccucci, C.; Pascucci, M.; Messina, E.; Fierro, G.; Lavorgna, M.; Ingo, G.M. Artificial patina formation onto copper-based alloys: Chloride and sulphate induced corrosion processes. Appl. Surf. Sci. 2017, 421, 120–127.
- Chiavari, C.; Bernardi, E.; Martini, C.; Morselli, L.; Ospitali, F.; Robbiola, L.; Textier, A. Predicting the corrosion behaviour of outdoor bronzes: Assessment of artificially exposed and real outdoor samples. In Metal 2010, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Charleston, SC, USA, 11–15 October 2010; Mardikian, P., Chemello, C., Watters, C., Hull, P., Eds.; Clemson University: Clemson, SC, USA, 2011; pp. 218–226.
- Robbiola, L.; Chiavari, C.; Bernardi, E.; Bignozzi, M.C.; Martini, C. General understanding of outdoor bronze corrosion: An overview. In Métal à Ciel Ouvert: La Sculpture Métallique D’extérieur du XIXe au début du XXe Siècle: Identification, Conservation, Restauration; International Institute for Conservation of Historic and Artistic Works; Section Française: Champs-sur-Marne, France, 2014; pp. 146–153. ISBN 9782905430182.
- Bozzini, B.; Alemán, B.; Amati, M.; Boniardi, M.; Caramia, V.; Giovannelli, G.; Gregoratti, L.; Kazemian Abyaneh, M. Novel insight into bronze disease gained by synchrotron-based photoelectron spectro-microscopy, in support of electrochemical treatment strategies. Stud. Conserv. 2017, 62, 465–473.
- Selwyn, L.S.; Binnie, N.E.; Poitras, J.; Laver, M.E.; Downham, D.A. Outdoor bronze statues: Analysis of metal and surface samples. Stud. Conserv. 1996, 41, 205–228.
- Petiti, C.; Toniolo, L.; Gulotta, D.; Mariani, B.; Goidanich, S. Effects of cleaning procedures on the long-term corrosion behavior of bronze artifacts of the cultural heritage in outdoor environment. Environ. Sci. Pollut. Res. 2020, 27, 13081–13094.
- Agnoletti, S.; Brambilla, L.; Brini, A.; Cagnini, A.; Camaiti, M.; Celi, C.; Cetarini, L.; De Lapi, R.; Galeotti, M.; Goidanich, S.; et al. Formulati e metodologie per la pulitura e la protezione di superfici metalliche. Arkos 2011, 28, 34–37.
- Chiavari, G.; Colucci, A.; Mazzeo, R.; Ravanelli, M. Organic content evaluation of corrosion patinas in outdoor bronze monuments. Chromatographia 1999, 49, 35–41.
- Letardi, P.; Salvadori, B.; Galeotti, M.; Cagnini, A.; Porcinai, S.; Santagostino Barbone, A.; Sansonetti, A. An in situ multi-analytical approach in the restoration of bronze artefacts. Microchem. J. 2016, 125, 151–158.
- Degrigny, C. Survey of European experiences on cleaning procedures. In Monumenti in Bronzo All’aperto: Esperienze di Conservazione a Confronto; Letardi, P., Trentin, I., Cutugno, G., Eds.; Nardini Editore: Florence, Italy, 2004; pp. 87–93.
- Römich, H. New Conservation Methods for Outdoor Bronze Sculptures. 1993–1995. Available online: https://cordis.europa.eu/project/id/EV5V0107 (accessed on 25 January 2021).
- Pilz, M.; Römich, H. Sol-gel derived coatings for outdoor bronze conservation. J. Sol-Gel Sci. Technol. 1997, 8, 1071–1075.
- Norbert, B. Conservation notes: Maintenance of outdoor bronze sculpture. Int. J. Mus. Manag. Curatorship 1988, 7, 71–75.
- Brostoff, L.B. Coating Strategies for the Protection of Outdoor Bronze Art and Ornamentation. Ph.D. Thesis, University of Amsterdam-Van ’t Hoff Institute for Molecular Sciences (HIMS), Amsterdam, The Neederlands, 2003.
- Mazzeo, R.; Bittner, S.; Farron, G.; Fontinha, R.; Job, D.; Joseph, E.; Letardi, P.; Mach, M.; Prati, S.; Salta, M.; et al. Development and evaluation of new treatments for outdoor bronze monuments. In Proceedings of the International Conference Conservation Science 2007, Milan, Italy, 10–11 May 2007; Townsend, J.H., Toniolo, L., Cappitelli, F., Eds.; Archetype: Washington, DC, USA, 2008; pp. 40–48, ISBN 978-1904982340.
- Joseph, E.; Letardi, P.; Mazzeo, R.; Prati, S.; Vandini, M. Innovative treatments for the protection of outdoor bronze monuments. In Metal 07, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Amsterdam, The Neederlands, 17–21 September 2007; Degrigny, C., van Langh, R., Joosten, I., Ankersmit, B., Eds.; Rijksmuseum: Amsterdam, The Neederlands, 2007; Volume 5, pp. 71–77.
- Albini, M.; Letardi, P.; Mathys, L.; Brambilla, L.; Schröter, J.; Junier, P.; Joseph, E. Comparison of a bio-based corrosion inhibitor versus benzotriazole on corroded copper surfaces. Corros. Sci. 2018, 143, 84–92.
- Faraldi, F.; Cortese, B.; Caschera, D.; Di Carlo, G.; Riccucci, C.; de Caro, T.; Ingo, G.M. Smart conservation methodology for the preservation of copper-based objects against the hazardous corrosion. Thin Solid Films 2017, 622, 130–135.
- Masi, G.; Aufray, M.; Balbo, A.; Bernardi, E.; Bignozzi, M.C.; Chiavari, C.; Esvan, J.; Gartner, N.; Grassi, V.; Josse, C.; et al. B-IMPACT project: Eco-friendly and non-hazardous coatings for the protection of outdoor bronzes. IOP Conf. Ser. Mater. Sci. Eng. 2020, 949, 012097.
- Letardi, P.; Ramirez-Barat, B.; Albini, M.; Traverso, P.; Cano, E.; Joseph, E. Copper alloys and weathering steel used in outdoor monuments: Available online: Weathering in an urban-marine environment. In Metal 2016, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, New Delhi, India, 26–30 September 2016; Menon, R., Chemello, C., Pandya, A., Eds.; ICOM Committee for Conservation: Paris, France, 2016; pp. 320–328. ISBN 978-92-9012-418-4.
- Letardi, P. Laboratory and field test on patinas and protective coating systems for outdoor bronze Monuments. In Metal 2004, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Canberra, Australia, 4–8 October 2004; Ashton, J., Hallam, D., Eds.; National Museum of Australia: Canberra, Australia, 2004; pp. 379–387.
- Franceschi, E.; Letardi, P.; Luciano, G. Colour measurements on patinas and coating system for outdoor bronze monuments. J. Cult. Herit. 2006, 7, 166–170.
- Sansonetti, A.; Colella, M.; Letardi, P.; Salvadori, B.; Striova, J. Laser cleaning of a nineteenth-century bronze sculpture: In situ multi-analytical evaluation. Stud. Conserv. 2015, 60, S28–S33.
- Ramírez Barat, B.; Crespo, A.; García, E.; Díaz, S.; Cano, E. An EIS study of the conservation treatment of the bronze sphinxes at the Museo Arqueológico Nacional (Madrid). J. Cult. Herit. 2017, 24, 93–99.
- Bruni, T.; Mariani, B.; Salvadori, B.; Letardi, P. A multi-analytical approach to evaluate surface treatments on copper-alloy artefacts: A case study applied to the restoration of the memorial of redipuglia. In Metal 2019, Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Chemello, C., Brambilla, L., Joseph, E., Eds.; ICOM-CC and HE-Arc CR: Neuchatel, Switzerland, 2019; pp. 92–99. ISBN 978-92-9012-457-3.




