
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Günter Emons | + 2448 word(s) | 2448 | 2021-02-16 09:44:37 | | | |
| 2 | Vivi Li | Meta information modification | 2448 | 2021-02-18 09:35:58 | | |
Video Upload Options
Endometrial cancer (EC) is one of the most common gynecological malignancies. Gonadotropin releasing hormone (GnRH) is a decapeptide first described to be secreted by the hypothalamus to regulate pituitary gonadotropin secretion. In this systematic review, we analyze and summarize the data indicating that most EC express GnRH and its receptor (GnRH-R) as part of an autocrine system regulating proliferation, the cell cycle, and apoptosis. We analyze the available data on the expression and function of GnRH-II, its putative receptor, and its signal transduction. GnRH-I and GnRH-II agonists, and antagonists as well as cytotoxic GnRH-I analogs, have been shown to inhibit proliferation and to induce apoptosis in human EC cell lines in pre-clinical models. Treatment with conventional doses of GnRH-agonists that suppress pituitary gonadotropin secretion and ovarian estrogen production has become part of fertility preserving therapy of early EC or its pre-cancer (atypical endometrial hyperplasia). Conventional doses of GnRH-agonists had marginal activity in advanced or recurrent EC. Higher doses or more potent analogs including GnRH-II antagonists have not yet been used clinically. The cytotoxic GnRH-analog Zoptarelin Doxorubicin has shown encouraging activity in a phase II trial in patients with advanced or recurrent EC, which expressed GnRH-R. In a phase III trial in patients with EC of unknown GnRH-R expression, the cytotoxic GnRH doxorubicin conjugate was not superior to free doxorubicin. Further well-designed clinical trials exploiting the GnRH-system in EC might be useful.
1. Introduction
Endometrial cancer (EC), derived from the epithelial lining of the uterine cavity, is one of the most common female cancers. Worldwide, 382,069 new cases were diagnosed in 2018 and 89,929 women died of this disease [1]. In Western Europe and North America, EC is the fourth most common malignancy in women and the most common malignant tumor of the female genital organs [2].
The prognosis of EC is rather favorable, as about 75% of cases are diagnosed in an early stage and can be cured by surgery and in more advanced cases by additional radiotherapy and/or chemotherapy [2] resulting in a general five-year cancer-specific survival of around 80% for all stages and histological types [1][2]. The majority of EC (85%) known as the so-called type 1 cancers develop due to prolonged exposure to endogenous or exogenous estrogens in the absence of sufficient progestogen activity [3][4][5]. These type 1 EC are hormone-dependent and can be treated in early stages without surgery by endocrine manipulation, including estrogen withdrawal and/or high dose progestogens in premenopausal women who have not yet completed their families. Later in their development, these hormone-dependent EC dedifferentiate, lose expression of estrogen receptors and/or progesterone receptors, and are no longer amenable to estrogen withdrawal, anti-estrogens, or progestogens [2]. Type 2 EC do not express estrogen receptors or progesterone receptors, are not dependent on these steroids, and have a poor prognosis. They are responsible for the majority of EC-related deaths [2].
Gonadotropin releasing hormone (GnRH, also called luteinizing hormone releasing hormone, LHRH) is a hypothalamic decapeptide, regulating secretion of gonadotropins by the pituitary. By the end of the 1980s, super-active analogs of GnRH and respective depot preparations became widely available and were used for medical hypophysectomy (suppression of secretion of luteinizing hormone, LH, and follicle-stimulating hormone, FSH) leading to suppression of gonadal function in both sexes. This strategy of reversible medical castration was successfully introduced into the treatment of a variety of sex hormone-dependent diseases including prostate cancer and premenopausal breast cancer as well as endometriosis and uterine fibroids [6]. Estrogen withdrawal due to reversible medical castration through GnRH-agonists was also established as a conservative treatment of early EC and its pre-cancers in young women who wished to preserve their fertility [6].
In a variety of malignant tumors including breast, prostate, ovarian, and endometrial cancers, the expressions of GnRH and its receptors (GnRH-R) were discovered. These GnRH-R mediated direct anti-proliferative effects of agonistic and antagonistic analogs of GnRH in vitro and in nude mouse models xeno-transplanted with human cancers. Since GnRH-R are also expressed in type 2 EC, it was speculated that the treatment with GnRH-analogs might be an efficacious endocrine therapy with low toxicity for patients with EC that does not express estrogen receptors or progesterone receptors [6]. Respective clinical trials showed some activity of this approach. Finally, GnRH-analogs coupled with cytotoxic molecules were developed for targeted therapies through the GnRH-R on the surface of cancer cells. This approach has provided encouraging results in patients with EC (see below).
2. Discussion
2.1. GnRH-Receptors in EC
The data reviewed above clearly suggest that most human EC cell lines and primary EC express high affinity/low-capacity receptors for GnRH. It is true that some groups were not able to find these GnRH-R [7][8][9]. However, most researchers worldwide detected them either by binding assay, RT-PCR, restriction enzyme analysisand Southern blot analysis or immunohistochemistry [10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26]. The sequence of the cDNA of the GnRH-R in HEC-1A and Ishikawa human EC cell lines is identical to that of the GnRH-R in a human pituitary gland [27]. Approximately 50–95% of primary human EC express GnRH-R [10][12][16][17][18][21][22][23][26].
2.2. Expression of GnRH in EC
The production of GnRH by EC-cell lines and in 75–100% of primary tumor samples was shown by immunoassay, bioassay, high performance liquid chromatography, immunohistochemistry, RT-PCR, restriction enzyme analysis, and Southern blot analysis [8][22][28][29], so that this finding is also well accepted.
2.3. Effects of GnRH, GnRH-Agonists, and GnRH Antagonists on the Proliferation of EC Cells
In established human EC cell lines as well as in primary cultures of EC, most researchers found a growth inhibition induced by treatment with GnRH-agonists [11][13][9][14][16][18][30][31]. Comparable anti-proliferative effects were seen, when GnRH-antagonists were used [11][9][16][32]. Under serum free conditions, IGF-1, EGF, and estrogen induced proliferation of EC cell lines was reduced by GnRH analogs [32][33][34]. Significant anti-proliferative effects of GnRH analogs were seen at 10 pM to 1 nM concentrations [11], but relevant growth inhibition was observed at higher concentrations (1 µM, 10 µM) [11][13][9][14][16][18][33][34][30][31].
A common finding made by all groups was that GnRH-antagonists had the same anti-proliferative effects on EC cells as GnRH-agonists, suggesting that the dichotomy between GnRH-agonists and antagonists, known from the pituitary, is not valid in EC cells [6]. To elucidate the effects of GnRH produced and secreted by the tumor cells, we treated cell cultures of human ovarian cancer cell lines EFO-21 and EFO-27 that had been shown to express high affinity GnRH-R and to secrete GnRH with neutralizing concentrations of antiserum to GnRH. This resulted in a significant stimulation of proliferation. Native GnRH in low concentrations had no or little anti-proliferative effect [35].
Thus, it seems reasonable to conclude that the majority of human EC express GnRH and GnRH-R as an autocrine system reducing their proliferation. Both GnRH agonists and antagonists have dose-dependent anti-proliferative effects.
2.4. Signal Transduction and Intracellular Actions of GnRH in EC
In the pituitary gonadotrope GnRH-R that have bound the GnRH couple to G-protein αq and induce activation of phospholipase (PLC), protein kinase (PKC), and adenylyl cyclase (AC) [8][9][33]. These enzymes and pathways are present in human EC cell lines but are not involved in the mediation of anti-proliferative effects of GnRH agonists [33]. Upon binding of a GnRH agonist or antagonist, the GnRH-R rather couples to G-protein αi and activates a phosphotyrosine phosphatase that reduces EGF induced tyrosine phosphorylation of the EGF-R [27][33][36][37][38][39]. This results in a suppression of the Ras/MAPK/ERK pathway and an inhibition of c-fos expression [27][33][36] (Figure 1). This mechanism has been shown in human breast and ovarian cancer cell lines [40]. In addition, through this mechanism, GnRH-agonists can inhibit E2 induced cell proliferation of ERα-positive human endometrial, ovarian, and breast cancer cell lines (Figure 1) [41]. In breast cancers, signalling of membrane-bound G-Protein-coupled estrogen receptor 1 (GPER) through transactivation of EGF-R could also be inhibited by GnRH-agonist treatment (Figure 1) [40]. Thus, E2-induced proliferation of ERα-negative but GPER-positive breast cancer cells could be prevented by treatment with GnRH agonists.
GnRH agonists stimulated via G-protein αi the JNK/AP-1 pathway, leading to inhibition of the cell cycle (Figure 1) [42]. Native GnRH, GnRH-agonists, and GnRH-antagonists did not induce apoptosis but rather protected endometrial cancer cells from apoptosis induction by UV-light or the cytotoxic agent doxorubicin through activation of NF-κB (Figure 1) [43]. Thus, we conclude from our data and those of others, that the expression of GnRH and GnRH-R in human EC is part of an autocrine system, counteracting the proliferative effects of growth factors and estrogens, and increasing AP-1 expression, leading to cell cycle arrest. In addition, this GnRH system reduces apoptosis (Figure 1). Other authors, however, found induction of apoptosis caused by GnRH-agonists and/or antagonists, the activation of protein kinase C, and other mechanisms including FasL and FAS, telomerase transferase, telomerase activity, and annexin V [13][14][15][18][30][31][39][44].
The reasons for these discrepancies are unknown. It is not unreasonable to speculate that the GnRH-R in tumor cells can couple to multiple signal-transduction pathways depending on the cellular milieu in different cell lines and even different passages of the same cell line [40].
2.5. GnRH-II and GnRH-II-Receptors in EC
The data from our group suggest that a truncated, but functional 5-transmembrane human GnRH-II-R, is expressed in human endometrial and ovarian cancers. After binding of the GnRH-antagonist, native GnRH-II, or GnRH-II-agonist, it couples to G protein αi and activates the signal transduction mechanisms described for the GnRH-I-R in human cancers including inhibition of autophosphorylation of the EGF-receptor and phosphorylation of JNK [45][46][47][48] (Figure 2). Our findings suggest that the antiproliferative effects of GnRH-I antagonists are mediated through GnRH-II-R. The anti-proliferative effects of native GnRH-II mediated through GnRH-II-R were much more pronounced than those of GnRH-I agonist mediated through GnRH-I-R [45][46][49][47][48].
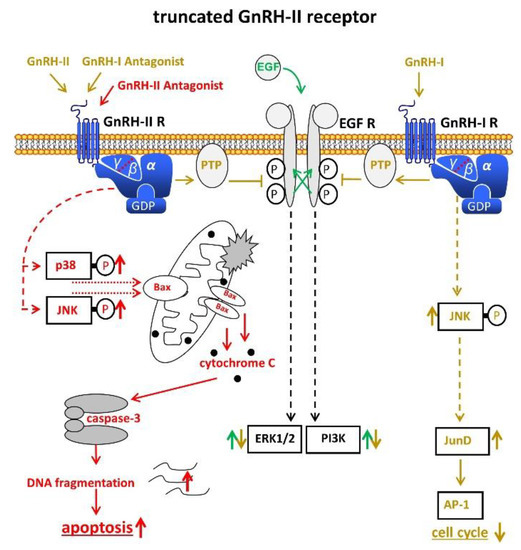
Figure 2. Signal transduction of the putative truncated GnRH-II receptor in endometrial cancer (EC). Binding of GnRH-II, GnRH-II agonists, and GnRH-I antagonists cause activation of PTP, leading to dephopsphorylation of activated EGF-R with the consequences described in Figure 1. Binding of GnRH-II antagonists induce apoptosis through activation of p38, JNK, and the intrinsic apoptotic pathway. AP-1, activvator protein-1. Bax, B-cell lymphoma 2 (Bcl-2)-associated X protein. EGF, epidermal growth factor. EGF-R, EGF receptor. ERK1/2, p44/42 mitogen-activated protein (MAP) kinase. GDP, guanosindiphosphat. GnRH, gonadotropin releasing hormone. JNK, c-Jun N-terminale kinase. JunD, transcription factor JunD. P38, mitogen-activated protein kinase P38. PI3K, phosphoinositide 3-kinase. PTP, protein tyrosine phosphatase.
GnRH-I, GnRH-I-agonists, GnRH-I-antagonists, and GnRH-II-agonists did not increase apoptosis in EC, ovarian, or breast cancer cell lines, but rather protected cells from programmed cell death [48][50]. Antagonists of GnRH-II, however, potently induced apoptosis in human endometrial, ovarian, and breast cancer cells, mediated through activation of stress-induced mitogen activated protein kinases p38 and c-JunNH2-terminal kinase, leading to activation of proapoptotic protein Bax [48][50] (Figure 2). GnRH-II antagonists potently inhibited the growth of human endometrial and ovarian cancers in nude mice [51].
Other groups provided evidence that no functional GnRH-II-R is expressed in the human and that the effects of GnRH-II are mediated through GnRH-I-R [24][25][31][42] (Figure 3).
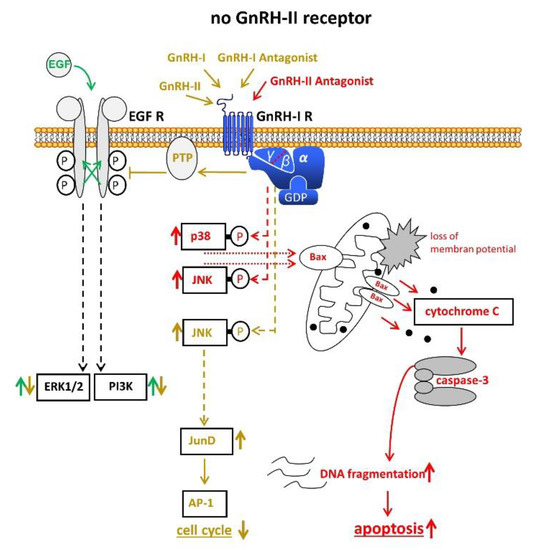
Figure 3. Signal transduction of GnRH-I and GnRH-II and their agonistic as well as antagonistic analogs through the GnRH-I-R if no functional GnRH-II-R is expressed. For details, cf. Figure 1 and Figure 2. AP-1, activvator protein-1. Bax, B-cell lymphoma 2 (Bcl-2)-associated X protein. EGF, epidermal growth factor. EGF-R, EGF receptor. ERK1/2, p44/42 mitogen-activated protein (MAP) kinase. GDP, guanosindiphosphat. GnRH, gonadotropin releasing hormone. JNK, c-Jun N-terminale kinase. JunD, transcription factor JunD. P38, mitogen-activated protein kinase P38. PI3K, phosphoinositide 3-kinase. PTP, protein tyrosine phosphatase.
2.6. GnRH-R in EC as a Target for Cytotoxic Therapy
The in vitro and in vivo experimental data available suggest that GnRH-R can be utilized as a target to specifically introduce toxic molecules into EC cells [19][52][53][54][55][56]. Of particular interest is the finding that cytotoxic GnRH-molecules can bypass the multi-drug resistance 1 system that eventually makes tumor cells refractory to chemotherapy [55].
2.7. Clinical Application of GnRH Analogs in EC and Atypical Endometrial Hyperplasia (AEH)
Estrogen withdrawal by the GnRH-agonist induced reversible medical castration, which has been used in combination with systemic or local progestogen application as a fertility sparing treatment for AEH and early EC [57][58][51][59][60][61]. Alternatively, GnRH agonists were combined with aromatase inhibitors for more intensive suppression of estrogens [60][62][61] or given alone after complete endometrial resection [63]. In these small series, complete response rates of about 70% (54–100%) were observed for early EC. Complete response rates were higher in AEH (80–100%). Pregnancy rates were about 50%. A relevant number of patients had progression during therapy or relapsed after complete remission [57][58][51][59][60][62][63][61]. Estrogen withdrawal through reversible medical castration by GnRH analogs alone or in combination with a LNG-IUD or an aromatase inhibitor seems to be preferable in obese patients [61] (Table 1).
A series of small phase II trials has assessed the efficacy of conventional doses of GnRH-agonists in the treatment of recurrent or advanced EC no longer amenable to surgery or radiotherapy, including patients with G3 tumors. Objective responses were observed in 0% to 35%, which tended to be long lasting. Toxicity was low [64][65][66][67][68][69]. It has to be considered that the serum concentrations of GnRH-agonists achieved with standard depot-preparations are in the nanomolar range and sufficient to down-regulate pituitary GnRH-R [6]. For optimal direct effects of GnRH-I-agonists or antagonists on EC cells, much higher concentrations (µM) are needed [11][13][9][14][16][18][32][33][30][31]. Clinical trials with higher doses of GnRH-I-analogs have not been performed. GnRH-II analogs, especially GnRH-II antagonists that are much more potent in vitro and in vivo [48][50], should be tested in EC patients.
Phase I and II trials with the cytotoxic GnRH analog Zoptarelin Doxorubicin resulted in an objective response rate of 23% and a clinical benefit rate of 67% in patients with advanced or recurrent EC that expressed GnRH-R [23]. Toxicity was low. In a phase III trial with patients with recurrent or metastatic EC that had failed to prior platinum chemotherapy, an equipotency of Zoptarelin Doxorubicin and free Doxorubicin was found. However, the GnRH-R-status of these tumors was unknown. Considering that Zoptarelin Doxorubicin had been shown to be ineffective in EC not expressing GnRH-R [52][53][54] and that 5–50% of EC have no GnRH-R [18][21][22][23], it is reasonable to speculate that the Zoptarelin Doxorubicin is more efficacious in patients with EC that express GnRH-R.
3. Conclusions and Perspectives
Treatment with conventional doses of GnRH-agonists that suppress pituitary gonadotropin secretion and ovarian estrogen production has become part of fertility preserving therapy either alone or in combination with LNG-IUD (52 mg) or aromatase inhibitors in young patients with AEH or early EC (EEC).
There is convincing evidence that EC express GnRH-I-R and GnRH-I as a negative autocrine system, limiting cell proliferation and likely apoptosis. It is unclear whether a functional GnRH-II-R exist in humans. The GnRH-I-R and, if existent, the putative GnRH-II-R can be targeted by analogs of GnRH-I and GnRH-II to inhibit proliferation and to induce apoptosis. Clinical trials to exploit these direct anti-tumor effects have, so far, been performed with conventional doses of GnRH agonists, resulting in marginal efficacy, but low toxicity. Trials using higher doses of GnRH-I analogs or the more potent GnRH-II analogs still have to be performed. The cytotoxic GnRH-analog Zoptarelin Doxorubicin has been shown to have meaningful activity in animal models and a phase II trial. A well-designed phase III trial with patients with advanced or recurrent EC expressing GnRH-R is warranted.
References
- International Agency for Research on Cancer. Cancer Today. Corpus Uteri. Available online: https://gco.iarc.fr/today/factsheets-cancers (accessed on 7 October 2020).
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108.
- Tempfer, C.B.; Hilal, Z.; Kern, P.; Juhasz-Boess, I.; Rezniczek, G.A. Menopausal hormone therapy and risk of endometrial cancer: A systematic review. Cancers 2020, 12, 2195.
- Ignatov, A.; Ortmann, O. Endocrine risk factors of endometrial cancer: Polycystic ovary syndrome, oral contraceptives, infertility, tamoxifen. Cancers 2020, 12, 1766.
- Emons, G.; Mustea, A.; Tempfer, C. Tamoxifen and endometrial cancer: A janus-headed drug. Cancers 2020, 12, 2535.
- Emons, G.; Schally, A.V. The use of luteinizing hormone releasing hormone agonists and antagonists in gynaecological cancers. Hum. Reprod. 1994, 9, 1364–1379.
- Peterson, C.M.; Jolles, C.J.; Carrell, D.T.; Straight, R.C.; Jones, K.P.; Poulson, A.M., Jr.; Hatasaka, H.H. GnRH agonist therapy in human ovarian epithelial carcinoma (OVCAR-3) heterotransplanted in the nude mouse is characterized by latency and transience. Gynecol. Oncol. 1994, 52, 26–30.
- Chatzaki, E.; Bax, C.M.; Eidne, K.A.; Anderson, L.; Grudzinskas, J.G.; Gallagher, C.J. The expression of gonadotropin-releasing hormone and its receptor in endometrial cancer, and its relevance as an autocrine growth factor. Cancer Res. 1996, 56, 2059–2065.
- Borri, P.; Coronnello, M.; Noci, I.; Pesciullesi, A.; Peri, A.; Caligiani, R.; Maggi, M.; Torricelli, F.; Scarselli, G.; Chieffi, O.; et al. Differential inhibitory effects on human endometrial carcinoma cell growth of luteinizing hormone-releasing hormone analogues. Gynecol. Oncol. 1998, 71, 396–403.
- Srkalovic, G.; Wittliff, J.L.; Schally, A.V. Detection and partial characterization of receptors for [D-Trp6]-luteinizing hormone-releasing hormone and epidermal growth factor in human endometrial carcinoma. Cancer Res. 1990, 50, 1841–1846.
- Emons, G.; Schroder, B.; Ortmann, O.; Westphalen, S.; Schulz, K.D.; Schally, A.V. High affinity binding and direct antiproliferative effects of luteinizing hormone-releasing hormone analogs in human endometrial cancer cell lines. J. Clin. Endocrinol. Metab. 1993, 77, 1458–1464.
- Imai, A.; Ohno, T.; Iida, K.; Fuseya, T.; Furui, T.; Tamaya, T. Presence of gonadotropin-releasing hormone receptor and its messenger ribonucleic acid in endometrial carcinoma and endometrium. Gynecol. Oncol. 1994, 55, 144–148.
- Shibata, S.; Sato, H.; Ota, H.; Karube, A.; Takahashi, O.; Tanaka, T. Involvement of annexin V in antiproliferative effects of gonadotropin-releasing hormone agonists on human endometrial cancer cell line. Gynecol. Oncol. 1997, 66, 217–221.
- Ohta, H.; Sakamoto, H.; Satoh, K. In vitro effects of gonadotropin-releasing hormone (GnRH) analogue on cancer cell sensitivity to cis-platinum. Cancer Lett. 1998, 134, 111–118.
- Kim, J.W.; Lee, Y.S.; Kim, B.K.; Park, D.C.; Lee, J.M.; Kim, I.K.; Namkoong, S.E. Cell cycle arrest in endometrial carcinoma cells exposed to gonadotropin-releasing hormone analog. Gynecol. Oncol. 1999, 73, 368–371.
- Noci, I.; Coronnello, M.; Borri, P.; Borrani, E.; Giachi, M.; Chieffi, O.; Marchionni, M.; Paglierani, M.; Buccoliero, A.M.; Cherubini, A.; et al. Inhibitory effect of luteinising hormone-releasing hormone analogues on human endometrial cancer in vitro. Cancer Lett. 2000, 150, 71–78.
- Volker, P.; Grundker, C.; Schmidt, O.; Schulz, K.D.; Emons, G. Expression of receptors for luteinizing hormone-releasing hormone in human ovarian and endometrial cancers: Frequency, autoregulation, and correlation with direct antiproliferative activity of luteinizing hormone-releasing hormone analogues. Am. J. Obs. Gynecol. 2002, 186, 171–179.
- Nagai, N.; Oshita, T.; Mukai, K.; Shiroyama, Y.; Shigemasa, K.; Ohama, K. GnRH agonist inhibits human telomerase reverse transcriptase mRNA expression in endometrial cancer cells. Int. J. Mol. Med. 2002, 10, 593–597.
- Yang, W.H.; Wieczorck, M.; Allen, M.C.; Nett, T.M. Cytotoxic activity of gonadotropin-releasing hormone (GnRH)-pokeweed antiviral protein conjugates in cell lines expressing GnRH receptors. Endocrinology 2003, 144, 1456–1463.
- Engel, J.B.; Keller, G.; Schally, A.V.; Nagy, A.; Chism, D.D.; Halmos, G. Effective treatment of experimental human endometrial cancers with targeted cytotoxic luteinizing hormone-releasing hormone analogues AN-152 and AN-207. Fertil. Steril. 2005, 83 (Suppl S1), 1125–1133.
- Jeon, Y.T.; Kim, Y.B.; Park, S.Y.; Kim, J.W.; Park, N.H.; Kang, S.B.; Song, Y.S. Gonadotropin-releasing hormone receptor expression in endometrial cancer. Int. J. Gynecol. Pathol. 2009, 28, 19–22.
- Jankowska, A.G.; Andrusiewicz, M.; Fischer, N.; Warchol, P.J. Expression of hCG and GnRHs and their receptors in endometrial carcinoma and hyperplasia. Int. J. Gynecol. Cancer 2010, 20, 92–101.
- Emons, G.; Gorchev, G.; Harter, P.; Wimberger, P.; Stahle, A.; Hanker, L.; Hilpert, F.; Beckmann, M.W.; Dall, P.; Grundker, C.; et al. Efficacy and safety of AEZS-108 (LHRH agonist linked to doxorubicin) in women with advanced or recurrent endometrial cancer expressing LHRH receptors: A multicenter phase 2 trial (AGO-GYN5). Int. J. Gynecol. Cancer 2014, 24, 260–265.
- Wu, H.M.; Cheng, J.C.; Wang, H.S.; Huang, H.Y.; MacCalman, C.D.; Leung, P.C. Gonadotropin-releasing hormone type II induces apoptosis of human endometrial cancer cells by activating GADD45alpha. Cancer Res. 2009, 69, 4202–4208.
- Wu, H.M.; Wang, H.S.; Huang, H.Y.; Lai, C.H.; Lee, C.L.; Soong, Y.K.; Leung, P.C. Gonadotropin-releasing hormone type II (GnRH-II) agonist regulates the invasiveness of endometrial cancer cells through the GnRH-I receptor and mitogen-activated protein kinase (MAPK)-dependent activation of matrix metalloproteinase (MMP)-2. BMC Cancer 2013, 13, 300.
- Hao, D.; Sun, L.; Hu, X.; Hao, X. (99m)Tc-LHRH in tumor receptor imaging. Oncol. Lett. 2017, 14, 569–578.
- Gründker, C.; Völker, P.; Emons, G. Antiproliferative signaling of luteinizing hormone-releasing hormone in human endometrial and ovarian cancer cells through G protein alpha(I)-mediated activation of phosphotyrosine phosphatase. Endocrinology 2001, 142, 2369–2380.
- Irmer, G.; Burger, C.; Ortmann, O.; Schulz, K.D.; Emons, G. Expression of luteinizing hormone releasing hormone and its mRNA in human endometrial cancer cell lines. J. Clin. Endocrinol. Metab. 1994, 79, 916–919.
- Furui, T.; Imai, A.; Tamaya, T. Intratumoral level of gonadotropin-releasing hormone in ovarian and endometrial cancers. Oncol. Rep. 2002, 9, 349–352.
- Zhao, L.J.; Liu, N.; Li, X.P.; Wang, J.L.; Wei, L.H. Phosphatase and tensin homolog gene inhibits the effect induced by gonadotropin-releasing hormone subtypes in human endometrial carcinoma cells. Chin. Med. J. 2010, 123, 1170–1175.
- Park, D.W.; Choi, K.C.; MacCalman, C.D.; Leung, P.C. Gonadotropin-releasing hormone (GnRH)-I and GnRH-II induce cell growth inhibition in human endometrial cancer cells: Involvement of integrin beta3 and focal adhesion kinase. Reprod. Biol. Endocrinol. 2009, 7, 81.
- Kleinman, D.; Roberts, C.T., Jr.; LeRoith, D.; Schally, A.V.; Levy, J.; Sharoni, Y. Regulation of endometrial cancer cell growth by insulin-like growth factors and the luteinizing hormone-releasing hormone antagonist SB-75. Regul. Pept. 1993, 48, 91–98.
- Emons, G.; Muller, V.; Ortmann, O.; Grossmann, G.; Trautner, U.; Stuckrad, B.; Schulz, K.; Schally, A. Luteinizing hormone-releasing hormone agonist triptorelin antagonizes signal transduction and mitogenic activity of epidermal growth factor in human ovarian and endometrial cancer cell lines. Int. J. Oncol. 1996, 9, 1129–1137.
- Sica, G.; Schinzari, G.; Angelucci, C.; Lama, G.; Iacopino, F. Direct effects of GnRH agonists in human hormone-sensitive endometrial cells. Mol. Cell. Endocrinol. 2001, 176, 121–128.
- Emons, G.; Weiss, S.; Ortmann, O.; Grundker, C.; Schulz, K.D. LHRH might act as a negative autocrine regulator of proliferation of human ovarian cancer. Eur. J. Endocrinol. 2000, 142, 665–670.
- Gründker, C.; Völker, P.; Schulz, K.D.; Emons, G. Luteinizing hormone-releasing hormone agonist triptorelin and antagonist cetrorelix inhibit EGF-induced c-fos expression in human gynecological cancers. Gynecol. Oncol. 2000, 78, 194–202.
- Takagi, H.; Imai, A.; Horibe, S.; Fuseya, T.; Tamaya, T. GTP-binding protein and its associated event in membranes from endometrial carcinoma. Oncol. Rep. 1996, 3, 161–163.
- Imai, A.; Horibe, S.; Takagi, A.; Tamaya, T. Gi protein activation of gonadotropin-releasing hormone-mediated protein dephosphorylation in human endometrial carcinoma. Am. J. Obs. Gynecol. 1997, 176, 371–376.
- Imai, A.; Takagi, A.; Horibe, S.; Takagi, H.; Tamaya, T. Fas and Fas ligand system may mediate antiproliferative activity of gonadotropin-releasing hormone receptor in endometrial cancer cells. Int. J. Oncol. 1998, 13, 97–100.
- Grundker, C.; Emons, G. The role of gonadotropin-releasing hormone in cancer cell proliferation and metastasis. Front. Endocrinol. 2017, 8, 187.
- Grundker, C.; Gunthert, A.R.; Hellriegel, M.; Emons, G. Gonadotropin-releasing hormone (GnRH) agonist triptorelin inhibits estradiol-induced serum response element (SRE) activation and c-fos expression in human endometrial, ovarian and breast cancer cells. Eur. J. Endocrinol. 2004, 151, 619–628.
- Grundker, C.; Schlotawa, L.; Viereck, V.; Emons, G. Protein kinase C-independent stimulation of activator protein-1 and c-Jun N-terminal kinase activity in human endometrial cancer cells by the LHRH agonist triptorelin. Eur. J. Endocrinol. 2001, 145, 651–658.
- Fister, S.; Schlotawa, L.; Gunthert, A.R.; Emons, G.; Grundker, C. Increase of doxorubicin-induced apoptosis after knock-down of gonadotropin-releasing hormone receptor expression in human endometrial, ovarian and breast cancer cells. Gynecol. Endocrinol. 2008, 24, 24–29.
- Ozturk, H.B.; Vural, B.; Caliskan, E.; Solakoglu, S. Effect of GnRH analogues and octreotide treatment on apoptosis and the cell proliferation of endometrium adenocarcinoma cell lines. J. Turk. Ger. Gynecol. Assoc. 2010, 11, 131–136.
- Gründker, C.; Günthert, A.R.; Millar, R.P.; Emons, G. Expression of gonadotropin-releasing hormone II (GnRH-II) receptor in human endometrial and ovarian cancer cells and effects of GnRH-II on tumor cell proliferation. J. Clin. Endocrinol. Metab. 2002, 87, 1427–1430.
- Gründker, C.; Schlotawa, L.; Viereck, V.; Eicke, N.; Horst, A.; Kairies, B.; Emons, G. Antiproliferative effects of the GnRH antagonist cetrorelix and of GnRH-II on human endometrial and ovarian cancer cells are not mediated through the GnRH type I receptor. Eur. J. Endocrinol. 2004, 151, 141–149.
- Eicke, N.; Gunthert, A.R.; Emons, G.; Grundker, C. GnRH-II agonist [D-Lys6]GnRH-II inhibits the EGF-induced mitogenic signal transduction in human endometrial and ovarian cancer cells. Int. J. Oncol. 2006, 29, 1223–1229.
- Fister, S.; Gunthert, A.R.; Emons, G.; Grundker, C. Gonadotropin-releasing hormone type II antagonists induce apoptotic cell death in human endometrial and ovarian cancer cells in vitro and in vivo. Cancer Res. 2007, 67, 1750–1756.
- Eicke, N.; Günthert, A.R.; Viereck, V.; Siebold, D.; Béhé, M.; Becker, T.; Emons, G.; Gründker, C. GnRH-II receptor-like antigenicity in human placenta and in cancers of the human reproductive organs. Eur. J. Endocrinol. 2005, 153, 605–612.
- Fister, S.; Gunthert, A.R.; Aicher, B.; Paulini, K.W.; Emons, G.; Grundker, C. GnRH-II antagonists induce apoptosis in human endometrial, ovarian, and breast cancer cells via activation of stress-induced MAPKs p38 and JNK and proapoptotic protein Bax. Cancer Res. 2009, 69, 6473–6481.
- Pashov, A.I.; Tskhay, V.B.; Ionouchene, S.V. The combined GnRH-agonist and intrauterine levonorgestrel-releasing system treatment of complicated atypical hyperplasia and endometrial cancer: A pilot study. Gynecol. Endocrinol. 2012, 28, 559–561.
- Grundker, C.; Volker, P.; Griesinger, F.; Ramaswamy, A.; Nagy, A.; Schally, A.V.; Emons, G. Antitumor effects of the cytotoxic luteinizing hormone-releasing hormone analog AN-152 on human endometrial and ovarian cancers xenografted into nude mice. Am. J. Obs. Gynecol. 2002, 187, 528–537.
- Schally, A.V.; Nagy, A. Cancer chemotherapy based on targeting of cytotoxic peptide conjugates to their receptors on tumors. Eur. J. Endocrinol. 1999, 141, 1–14.
- Westphalen, S.; Kotulla, G.; Kaiser, F.; Krauss, W.; Werning, G.; Elsasser, H.P.; Nagy, A.; Schulz, K.D.; Grundker, C.; Schally, A.V.; et al. Receptor mediated antiproliferative effects of the cytotoxic LHRH agonist AN-152 in human ovarian and endometrial cancer cell lines. Int. J. Oncol. 2000, 17, 1063–1069.
- Günthert, A.R.; Gründker, C.; Bongertz, T.; Schlott, T.; Nagy, A.; Schally, A.V.; Emons, G. Internalization of cytotoxic analog AN-152 of luteinizing hormone-releasing hormone induces apoptosis in human endometrial and ovarian cancer cell lines independent of multidrug resistance-1 (MDR-1) system. Am. J. Obs. Gynecol. 2004, 191, 1164–1172.
- Nechushtan, A.; Yarkoni, S.; Marianovsky, I.; Lorberboum-Galski, H. Adenocarcinoma cells are targeted by the new GnRH-PE66 chimeric toxin through specific gonadotropin-releasing hormone binding sites. J. Biol Chem. 1997, 272, 11597–11603.
- Perez-Medina, T.; Bajo, J.; Folgueira, G.; Haya, J.; Ortega, P. Atypical endometrial hyperplasia treatment with progestogens and gonadotropin-releasing hormone analogues: Long-term follow-up. Gynecol. Oncol. 1999, 73, 299–304.
- Minig, L.; Franchi, D.; Boveri, S.; Casadio, C.; Bocciolone, L.; Sideri, M. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann. Oncol. 2011, 22, 643–649.
- Pronin, S.M.; Novikova, O.V.; Andreeva, J.Y.; Novikova, E.G. Fertility-sparing treatment of early endometrial cancer and complex atypical hyperplasia in young women of childbearing potential. Int J. Gynecol. Cancer 2015, 25, 1010–1014.
- Zhou, H.; Cao, D.; Yang, J.; Shen, K.; Lang, J. Gonadotropin-releasing hormone agonist combined with a levonorgestrel-releasing intrauterine system or letrozole for fertility-preserving treatment of endometrial carcinoma and complex atypical hyperplasia in young women. Int. J. Gynecol. Cancer 2017, 27, 1178–1182.
- Yin, J.; Ma, S.; Shan, Y.; Wang, Y.; Li, Y.; Jin, Y.; Pan, L. Risk factors for recurrence in patients with atypical endometrial hyperplasia and endometrioid adenocarcinoma after fertility-sparing treatments. Cancer Prev. Res. 2020, 13, 403–410.
- Zhang, Z.; Huang, H.; Feng, F.; Wang, J.; Cheng, N. A pilot study of gonadotropin-releasing hormone agonist combined with aromatase inhibitor as fertility-sparing treatment in obese patients with endometrial cancer. J. Gynecol. Oncol. 2019, 30, e61.
- Tock, S.; Jadoul, P.; Squifflet, J.L.; Marbaix, E.; Baurain, J.F.; Luyckx, M. fertility sparing treatment in patients with early stage endometrial cancer, using a combination of surgery and GnRH agonist: A monocentric retrospective study and review of the literature. Front. Med. 2018, 5, 240.
- Gallagher, C.J.; Oliver, R.T.; Oram, D.H.; Fowler, C.G.; Blake, P.R.; Mantell, B.S.; Slevin, M.L.; Hope-Stone, H.F. A new treatment for endometrial cancer with gonadotrophin releasing-hormone analogue. Br. J. Obs. Gynaecol. 1991, 98, 1037–1041.
- Jeyarajah, A.R.; Gallagher, C.J.; Blake, P.R.; Oram, D.H.; Dowsett, M.; Fisher, C.; Oliver, R.T. Long-term follow-up of gonadotrophin-releasing hormone analog treatment for recurrent endometrial cancer. Gynecol. Oncol. 1996, 63, 47–52.
- Covens, A.; Thomas, G.; Shaw, P.; Ackerman, I.; Osborne, R.; Lukka, H.; Carey, M.; Franssen, E.; Roche, K. A phase II study of leuprolide in advanced/recurrent endometrial cancer. Gynecol. Oncol. 1997, 64, 126–129.
- Lhomme, C.; Vennin, P.; Callet, N.; Lesimple, T.; Achard, J.L.; Chauvergne, J.; Luporsi, E.; Chinet-Charrot, P.; Coudert, B.; Couette, J.E.; et al. A multicenter phase II study with triptorelin (sustained-release LHRH agonist) in advanced or recurrent endometrial carcinoma: A French anticancer federation study. Gynecol. Oncol. 1999, 75, 187–193.
- Noci, I.; Borri, P.; Bonfirraro, G.; Chieffi, O.; Arcangeli, A.; Cherubini, A.; Dabizzi, S.; Buccoliero, A.M.; Paglierani, M.; Taddei, G.L. Longstanding survival without cancer progression in a patient affected by endometrial carcinoma treated primarily with leuprolide. Br. J. Cancer 2001, 85, 333–336.
- Asbury, R.F.; Brunetto, V.L.; Lee, R.B.; Reid, G.; Rocereto, T.F.; Gynecologic Oncology, G. Goserelin acetate as treatment for recurrent endometrial carcinoma: A gynecologic oncology group study. Am. J. Clin. Oncol. 2002, 25, 557–560.




