| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna K. Blakney | + 1729 word(s) | 1729 | 2021-02-12 08:47:10 | | | |
| 2 | Vicky Zhou | Meta information modification | 1729 | 2021-02-13 05:09:30 | | |
Video Upload Options
The core principle behind mRNA vaccines is to encode the antigen in the mRNA and then to deliver the transcript to the host cell cytoplasm using a non-viral delivery system, allowing antigen expression and induction of an antigen-specific immune response. Self-amplifying RNA (saRNA) is a type of mRNA that also encodes viral replicase, which enables the RNA to self-replicate upon delivery into the cell.
1. Introduction
In December 2019, the SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) virus emerged, causing a respiratory illness, coronavirus disease 2019 (COVID-19), in Hubei province, China [1][2]. The virus has spread globally, with the World Health Organization (WHO) declaring it a Public Health Emergency of International concern on 30 January 2020 and a pandemic officially on 7 March 2020 [3]. There is a strong consensus globally that a COVID-19 vaccine is likely the most effective approach to sustainably controlling the COVID-19 pandemic [4]. There has been an unprecedented research effort and global coordination which has resulted in the rapid development of vaccine candidates and initiation of human clinical trials. This has included conventional vaccine technologies such as viral vectors and adjuvanted subunits, but we have witnessed a renaissance in the field of RNA vaccines and a shift towards synthetic RNA platforms (Figure 1) [5][6]. In fact, one of the first vaccine to start clinical trials was a non-replicating mRNA vaccine from Moderna, mRNA-1273 [7][8][9]; the first patient was vaccinated on 16 March at the same time as a Chinese clinical trial was initiated with an adenovirus type-5 (Ad5) vector [10]. Furthermore, the BioNTech/Pfizer vaccine, BNT162b2, was the first COVID-19 vaccine to receive approval, first in the United Kingdom and then Canada, with an impressive 95% efficacy [11]. Since this time there have been several mRNA vaccine trials initiated, and publication of corresponding preclinical and clinical data, see Table 1.
Table 1. Published preclinical and clinical trial data with mRNA COVID-19 vaccines.
| Sponsor | Type of mRNA | Delivery System | Preclinical Data | Clinical Data |
|---|---|---|---|---|
| Moderna | bmRNA | LNP | [9][12] | [7][8] |
| BioNTech/Pfizer | bmRNA | LNP | [13] | [11][14][15][16][17] |
| ICL | saRNA | LNP | [18] | |
| Arcturus | saRNA | LNP | [19] | |
| CureVac | mRNA | LNP | [20] |
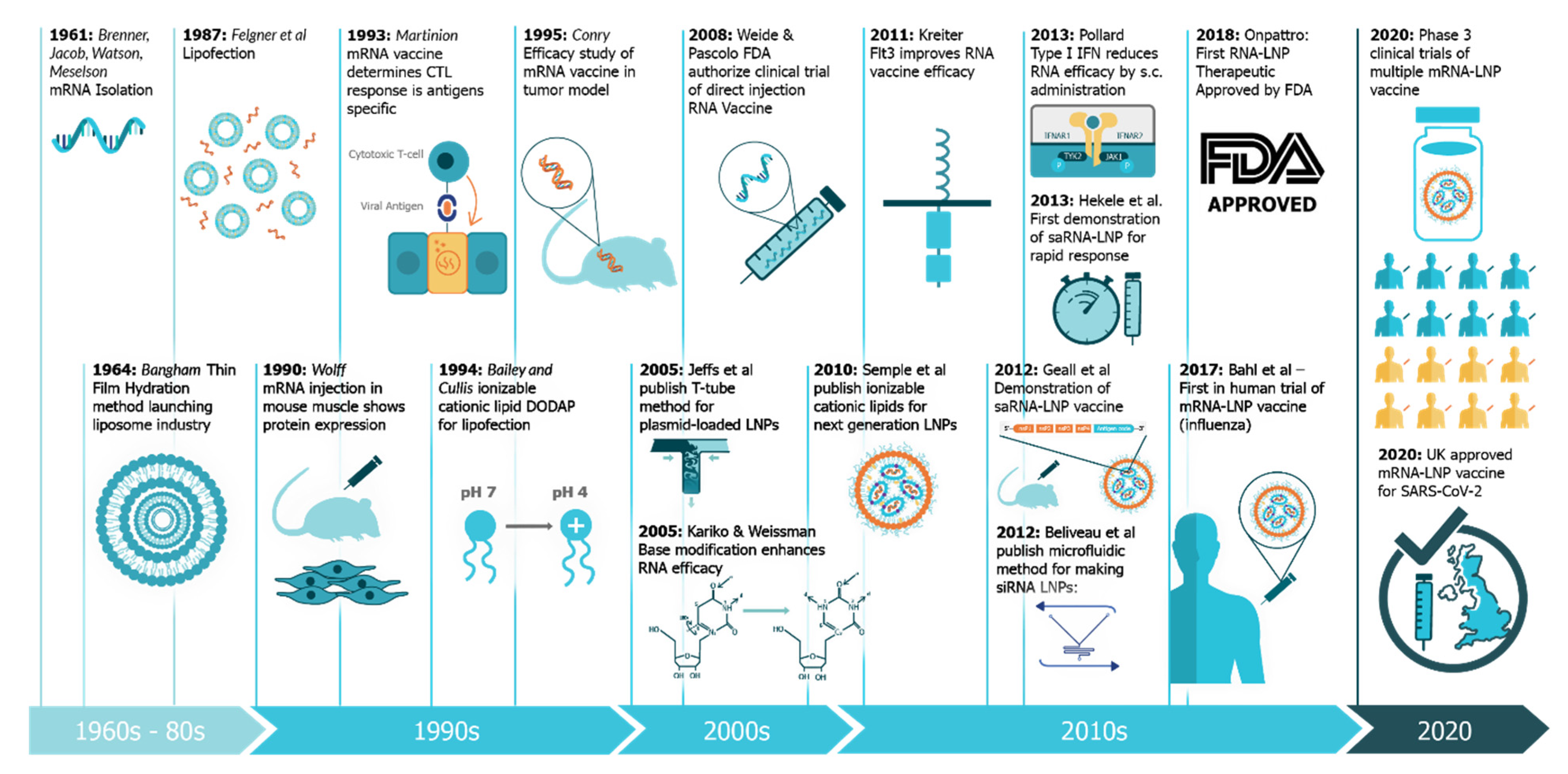
The use of mRNA vaccines for pandemic response has been well described previously in preclinical [12][21][22][23][24][25][26][27][28][29][30][31][32][33] and clinical settings [25], but this is the first time we have seen the platforms deployed in a real pandemic setting [34]. The core principle behind mRNA vaccines is to encode the antigen in the mRNA and then to deliver the transcript to the host cell cytoplasm using a non-viral delivery system, allowing antigen expression and induction of an antigen-specific immune response. This is especially advantageous as a vaccine platform as mRNA vaccines can be produced for any pathogen with a known protein target. mRNA is made using a cell-free enzymatic transcription reaction, which allows rapid and scalable manufacturing, as is evident from the swift pursuit of RNA vaccines in the current pandemic. Currently, there are three major types of RNA vaccines: conventional, non-amplifying mRNA molecules (mRNA), base-modified, non-amplifying mRNA molecules (bmRNA), which incorporate chemically modified nucleotides, and self-amplifying mRNA (saRNA or replicons) that maintain auto-replicative activity derived from an RNA virus vector. Self-amplifying RNA is beneficial compared to non-amplifying RNA as it maintains the advantages of mRNA vaccines, such as rapid development, modular design, and cell-free synthesis, but requires a lower dose of RNA due to the self-replicative properties. This reduces the burden of manufacturing for both the drug substance and product and is potentially advantageous in the context of pandemic response as it would enable a greater percentage of the population to be vaccinated in a shorter amount of time.
2. Four Major Pillars of saRNA Vaccine Development
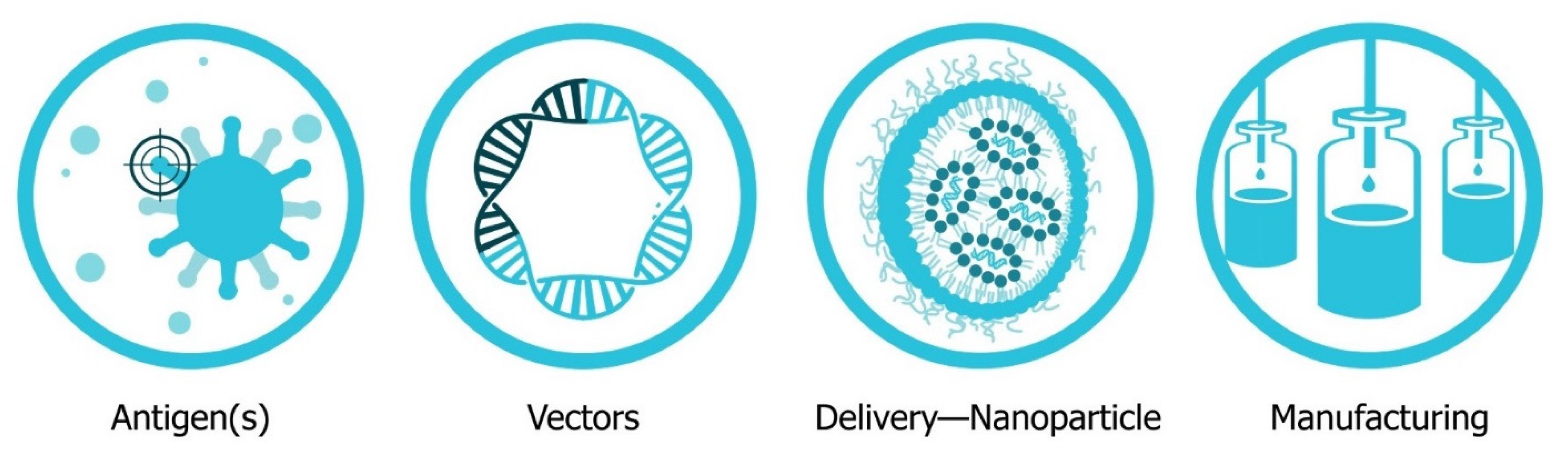
The four major pillars required for design and development of an saRNA vaccine are presented in Figure 2: Antigen design, vector design, non-viral delivery systems, and manufacturing (both saRNA and lipid nanoparticles (LNP)). In will report on the major innovations, preclinical and clinical data reported in the last five years and will discuss future prospects (Figure 3). Pertinent reviews on plasmid DNA and non-replicating messenger RNA vaccines can be found at the following references, which provide insight into the mechanism of immune response and effects of route of delivery [35][36][37][38].
2.1. Antigen Design
saRNA vaccines have been primarily investigated for active vaccination strategies for prevention of infectious diseases, wherein the host’s cells produce a pathogenic antigen encoded in saRNA to induce a humoral and cellular immune response. saRNA encoding viral glycoproteins are the most predominant application, although this has recently been expanded to include bacterial infections (Chlamydia trachomatis [57], Group A and B Streptococci [58]), parasites (Toxoplasma gondii [59][60]) and cancer (colon carcinoma, [61][62] melanoma [62]). A more novel approach to saRNA antigen design includes encoding monoclonal antibodies for passive vaccination [63]. While it is possible to incorporate relatively large (>4000 nt) or multiple antigens into an saRNA construct, the pDNA construct does have size limitations, so it may be advantageous to use separate saRNA constructs to encode multiple antigens if necessary [64].
2.2. Vector Design
There are three major forms of RNA vaccines based on the auto-replicative capacity of the mRNA and the inclusion of mammalian base-modifications. This section will focus on saRNA, or replicons, that maintain replicative activity derived from an RNA viral vector. Historically, positive-sense single-stranded RNA viruses, such as alphaviruses, flaviviruses, and picornaviruses have been used for replicons. The best-studied self-amplifying mRNA molecules are derived from alphavirus genomes, such as those of the Sindbis virus, which have been previously reviewed in references [5][24][30][65][66].
2.3. Delivery Systems
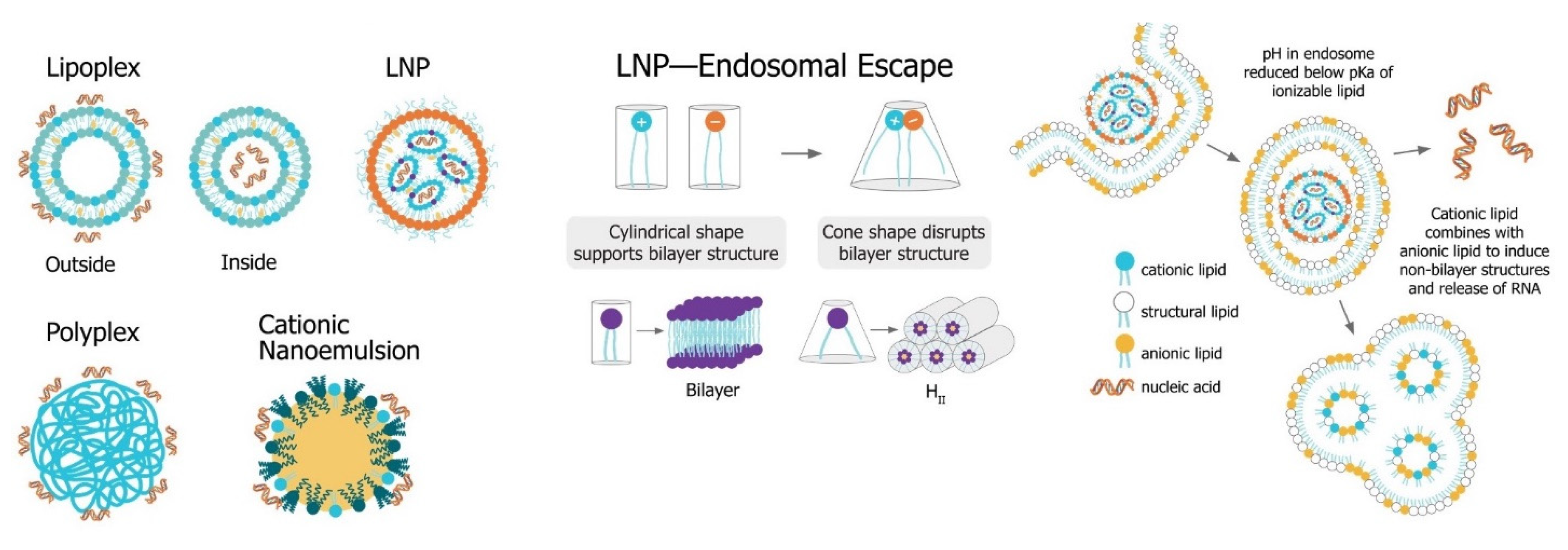
The main challenge for saRNA vaccines is achieving sufficient delivery of saRNA to the target cells or tissue. saRNA constructs are relatively large (9000 to 15,000 nt), anionic molecules, which precludes efficient cellular uptake of unformulated saRNA. Despite the use of “naked” saRNA in some studies, three predominant delivery platforms have emerged: Polymeric nanoparticles, lipid nanoparticles, and nanoemulsions. These delivery strategies share a central dogma wherein the anionic saRNA is condensed by a cationic (or ionizable cationic) carrier to a nanoparticle of ~100 nm in size, that protects the saRNA from degradation and encourages uptake into target cells (Figure 4).
Figure 4. Non-viral saRNA delivery systems. Lipid-, polymer-, and emulsion-based delivery systems all use cationic groups to mediate condensation of the anionic RNA as well as delivery across the cell membrane. LNP systems, which have been found to be the most potent vaccine formulatinos, utilize a pH-sensitive ionizable cationic lipids and are taken up in cells through receptor-mediated endocytosis. In the endosome, the lower pH environment ionizes the cationic lipids, which then interacts electrostatically with anionic lipids in the endosomal membrane. These ion pairs cause a phase transition into a porous hexagonal phase (HII) that disrupts the endosome and facilitates release of the RNA into the cytoplasm.
2.4. Manufacturing Considerations for Formulated mRNA Drug Product
While the manufacturing and production process for the formulated mRNA drug product can differ considerably depending on the type of formulation, a clinically relevant manufacturing process can be generalized into four steps: (1) Formulation, which involves one or more mixing steps, (2) downstream processing and purification, (3) sterile filtration through a 0.2 µm filter, (4) fill and finish. Presently, little information has been published regarding the specific manufacturing processes utilized for saRNA vaccine candidates currently in clinical trials. Hence, the preclinical processes for each formulation type will be generalized from methods published in the literature for lipid nanoparticles and nanoemulsions. To focus on potential clinical production, scalable continuous flow process steps are favored over fixed-volume processes.
3. Future Outlook
While historical (pre-2015) preclinical studies of saRNA vaccines were predominantly focused on viral replicon particles and cancer applications, the field has more recently shifted to applications in viral infectious diseases, although a few studies have also explored prevention of parasitic and bacterial infections. The investigation of saRNA for passive immunization by encoding a monoclonal antibody is also a highly promising application that warrants further development. The clinical trials for rabies and SARS-CoV-2 are an exciting opportunity for the field of saRNA vaccines, and will no doubt be informative as to the characteristics of the immune response, required dose, duration of immunity, and required regimen. The field is also starting to consider methods to modulate the innate response to saRNA, which will no doubt be imperative to the clinical success of these vaccines, so that the lesson of DNA vaccine clinical trials are not forgotten [67]. One strategy that may facilitate efficacious saRNA vaccines is utilizing evaluation models, such as skin explants that have human immune cells and innate sensing, in order to optimize molecular and delivery components. While the SARS-CoV-2 global pandemic has been detrimental to economies and health, it’s provided a valuable opportunity to test saRNA vaccines in the clinical that otherwise might have taken decades. Given the short timespan required to design and test new saRNA vaccines (reportedly as little as 14 days in the case of Imperial College London) [68], it is clear that this platform is particularly well-suited to outbreaks, and also possibly seasonal vaccines, such as influenza. The rapid and easy manufacture of saRNA vaccines may also pave the way for a distributed manufacturing model where vaccines are produced locally in order to minimize logistical and cold-chain issues that could hinder widespread distribution of a vaccine. While immense progress was made in RNA vaccine technology in 2020 [34], the main limitations are now the stability, which requires storage at <80 °C for most RNA formulations [69], and minimizing the required dose in order to reduce associated side effects [70]. Overall, saRNA vaccines have made monumental strides in the past five years, and the next five years will be telling as to the clinical utility and success of this promising vaccine platform.
References
- Oberfeld, B.; Achanta, A.; Carpenter, K.; Chen, P.; Gilette, N.M.; Langat, P.; Said, J.T.; Schiff, A.E.; Zhou, A.S.; Barczak, A.K.; et al. SnapShot: COVID-19. Cell 2020, 181, 954.e1.
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76.
- Koirala, A.; Joo, Y.J.; Khatami, A.; Chiu, C.; Britton, P.N. Vaccines for COVID-19: The current state of play. Paediatr. Respir. Rev. 2020, 35, 43–49.
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2020.
- Funk, C.D.; Laferrière, C.; Ardakani, A. A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic. Front. Pharmacol. 2020, 11, 937.
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438.
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020.
- Corbett, K.S.; Edwards, D.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA Vaccine Development Enabled by Prototype Pathogen Preparedness. bioRxiv 2020.
- Cohen, J. Vaccine designers take first shots at COVID-19. Science 2020, 368, 14.
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615.
- Pardi, N.; Parkhouse, K.; Kirkpatrick, E.; McMahon, M.; Zost, S.J.; Mui, B.L.; Tam, Y.K.; Karikó, K.; Barbosa, C.J.; Madden, T.D.; et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat. Commun. 2018, 9, 3361.
- Laczkó, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castaño, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H., 3rd; et al. A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 2020, 53, 724–732.e7.
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593.
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. Concurrent human antibody and Th1 type T-cell responses elicited by a COVID-19 RNA vaccine. medRxiv 2020.
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.P.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase 1/2 Study to Describe the Safety and Immunogenicity of a COVID-19 RNA Vaccine Candidate (BNT162b1) in Adults 18 to 55 Years of Age: Interim Report. medRxiv 2020.
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450.
- McKay, P.F.; Hu, K.; Blakney, A.K.; Samnuan, K.; Brown, J.C.; Penn, R.; Zhou, J.; Bouton, C.R.; Rogers, P.; Polra, K.; et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020, 11, 3523.
- De Alwis, R.; Gan, E.S.; Chen, S.; Leong, Y.S.; Tan, H.C.; Zhang, S.L.; Yau, C.; Matsuda, D.; Allen, E.; Hartman, P.; et al. A Single Dose of Self-Transcribing and Replicating RNA Based SARS-CoV-2 Vaccine Produces Protective Adaptive Immunity In Mice. bioRxiv 2020.
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. mRNA based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus neutralizing antibodies and mediates protection in rodents. bioRxiv 2020.
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555.
- Hekele, A.; Bertholet, S.; Archer, J.; Gibson, D.G.; Palladino, G.; Brito, L.A.; Otten, G.R.; Brazzoli, M.; Buccato, S.; Bonci, A.; et al. Rapidly produced SAM® vaccine against H7N9 influenza is immunogenic in mice. Emerg. Microbes Infect. 2013, 2, 1–7.
- Kis, Z.; Shattock, R.; Shah, N.; Kontoravdi, C. Emerging Technologies for Low-Cost, Rapid Vaccine Manufacture. Biotechnol. J. 2019, 14, 1800376.
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. 2019, 27, 757–772.
- Ulmer, J.B.; Mansoura, M.K.; Geall, A.J. Vaccines ‘on demand’: Science fiction or a future reality. Expert Opin Drug Discov. 2015, 10, 101–106.
- Feldman, R.A.; Fuhr, R.; Smolenov, I.; Mick Ribeiro, A.; Panther, L.; Watson, M.; Senn, J.J.; Smith, M.; Almarsson, Ö.; Pujar, H.S.; et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 2019, 37, 3326–3334.
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, Ö.; Thompson, J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017, 25, 1316–1327.
- Petsch, B.; Schnee, M.; Vogel, A.B.; Lange, E.; Hoffmann, B.; Voss, D.; Schlake, T.; Thess, A.; Kallen, K.-J.; Stitz, L.; et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012, 30, 1210–1216.
- Scorza, F.B.; Pardi, N. New Kids on the Block: RNA-Based Influenza Virus Vaccines. Vaccines 2018, 6, 20.
- Lundstrom, K. Self-Amplifying RNA Viruses as RNA Vaccines. Int. J. Mol. Sci. 2020, 21, 5130.
- DeFrancesco, L. The ‘anti-hype’ vaccine. Nat. Biotechnol. 2017, 35, 193–197.
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. npj Vaccines 2020, 5, 11.
- Lutz, J.; Lazzaro, S.; Habbeddine, M.; Schmidt, K.E.; Baumhof, P.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Heidenreich, R.; et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2017, 2, 29.
- Dolgin, E. How COVID unlocked the power of RNA vaccines. Nature 2021, 589, 189–191.
- Lee, J.; Arun Kumar, S.; Jhan, Y.Y.; Bishop, C.J. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018, 80, 31–47.
- Suschak, J.J.; Williams, J.A.; Schmaljohn, C.S. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum. Vaccines Immunother. 2017, 13, 2837–2848.
- Liu, M.A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines 2019, 7, 37.
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279.
- Alnylam Pharmaceuticals Press. Alnylam Announces First-Ever FDA Approval of an RNAi Therapeutic, ONPATTRO (Patisiran) for the Treatment of the Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis in Adults; Alnylam Pharmaceuticals Press: Cambridge, MA, USA, 2018.
- Center for Leading Innovation. Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis SARS CoV-2 Infection (COVID-19); Center for Leading Innovation: Rochester, NY, USA, 2020.
- Brenner, S.; Jacob, F.; Meselson, M. An Unstable Intermediate Carrying Information from Genes to Ribosomes for Protein Synthesis. Nature 1961, 190, 576–581.
- Pfizer. Pfizer and Biontech Achieve First Authorization in the World for a Vaccine to Combat Covid-19. 2020. Available online: https://www.businesswire.com/news/home/20201201006304/en/ (accessed on 26 January 2021).
- Pollard, C.; Rejman, J.; De Haes, W.; Verrier, B.; Van Gulck, E.; Naessens, T.; De Smedt, S.; Bogaert, P.; Grooten, J.; Vanham, G.; et al. Type I IFN Counteracts the Induction of Antigen-Specific Immune Responses by Lipid-Based Delivery of mRNA Vaccines. Mol. Ther. 2013, 21, 251–259.
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465.
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.-G.; Lévy, J.-P.; Meulien, P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur. J. Immunol. 1993, 23, 1719–1722.
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660-IN610.
- Conry, R.M.; LoBuglio, A.F.; Wright, M.; Sumerel, L.; Pike, M.J.; Johanning, F.; Benjamin, R.; Lu, D.; Curiel, D.T. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 1995, 55, 1397–1400.
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417.
- Weide, B.; Pascolo, S.; Scheel, B.; Derhovanessian, E.; Pflugfelder, A.; Eigentler, T.K.; Pawelec, G.; Hoerr, I.; Rammensee, H.G.; Garbe, C. Direct injection of protamine-protected mRNA: Results of a phase 1/2 vaccination trial in metastatic melanoma patients. J. Immunother. 2009, 32, 498–507.
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175.
- Jeffs, L.B.; Palmer, L.R.; Ambegia, E.G.; Giesbrecht, C.; Ewanick, S.; MacLachlan, I. A Scalable, Extrusion-Free Method for Efficient Liposomal Encapsulation of Plasmid DNA. Pharm. Res. 2005, 22, 362–372.
- Geall, A.J.; Verma, A.; Otten, G.R.; Shaw, C.A.; Hekele, A.; Banerjee, K.; Cu, Y.; Beard, C.W.; Brito, L.A.; Krucker, T.; et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl. Acad. Sci. USA 2012, 109, 14604–14609.
- Belliveau, N.M.; Huft, J.; Lin, P.J.; Chen, S.; Leung, A.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K.; et al. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Mol. Ther. Nucleic Acids 2012, 1, e37.
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176.
- Kreiter, S.; Diken, M.; Selmi, A.; Diekmann, J.; Attig, S.; Hüsemann, Y.; Koslowski, M.; Huber, C.; Türeci, Ö.; Sahin, U. FLT3 Ligand Enhances the Cancer Therapeutic Potency of Naked RNA Vaccines. Cancer Res. 2011, 71, 6132.
- Bailey, A.L.; Cullis, P.R. Modulation of Membrane Fusion by Asymmetric Transbilayer Distributions of Amino Lipids. Biochemistry 1994, 33, 12573–12580.
- Blakney, A.K.; McKay, P.F.; Christensen, D.; Yus, B.I.; Aldon, Y.; Follmann, F.; Shattock, R.J. Effects of cationic adjuvant formulation particle type, fluidity and immunomodulators on delivery and immunogenicity of saRNA. J. Control. Release 2019, 304, 65–74.
- Maruggi, G.; Chiarot, E.; Giovani, C.; Buccato, S.; Bonacci, S.; Frigimelica, E.; Margarit, I.; Geall, A.; Bensi, G.; Maione, D. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine 2017, 35, 361–368.
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142.
- Luo, F.; Zheng, L.; Hu, Y.; Liu, S.; Wang, Y.; Xiong, Z.; Hu, X.; Tan, F. Induction of Protective Immunity against Toxoplasma gondii in Mice by Nucleoside Triphosphate Hydrolase-II (NTPase-II) Self-amplifying RNA Vaccine Encapsulated in Lipid Nanoparticle (LNP). Front. Microbiol. 2017, 8, 605.
- Li, Y.; Su, Z.; Zhao, W.; Zhang, X.; Momin, N.; Zhang, C.; Wittrup, K.D.; Dong, Y.; Irvine, D.J.; Weiss, R. Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Cancer 2020, 1, 882–893.
- Li, Y.; Teague, B.; Zhang, Y.; Su, Z.; Porter, E.; Dobosh, B.; Wagner, T.; Irvine, D.J.; Weiss, R. In vitro evolution of enhanced RNA replicons for immunotherapy. Sci. Rep. 2019, 9, 6932.
- Erasmus, J.H.; Archer, J.; Fuerte-Stone, J.; Khandhar, A.P.; Voigt, E.; Granger, B.; Bombardi, R.G.; Govero, J.; Tan, Q.; Durnell, L.A.; et al. Intramuscular Delivery of Replicon RNA Encoding ZIKV-117 Human Monoclonal Antibody Protects against Zika Virus Infection. Mol. Ther. Methods Clin. Dev. 2020, 18, 402–414.
- Blakney, A.K.; McKay, P.F.; Bouton, C.R.; Hu, K.; Samnuan, K.; Shattock, R.J. Innate Inhibiting Protiens Enhance Expression and Immunogenicity of Self-Amplifying RNA. Mol. Ther. 2020.
- Tews, B.A.; Meyers, G. Self-Replicating RNA. Methods Mol. Biol. 2017, 1499, 15–35.
- Ljungberg, K.; Liljeström, P. Self-replicating alphavirus RNA vaccines. Expert Rev. Vaccines 2015, 14, 177–194.
- Liu, M.A.; Ulmer, J.B. Human clinical trials of plasmid DNA vaccines. Adv. Genet. 2005, 55, 25–40.
- Scheuber, A. Imperial Social Enterprise to Accelerate Low-Cost COVID-19 Vaccine. Available online: https://www.imperial.ac.uk/news/198053/imperial-social-enterprise-accelerate-lowcost-covid19/ (accessed on 26 January 2021).
- Kaiser, J. Temperature concerns could slow the rollout of new coronavirus vaccines. Science 2020.
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520.