
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ludmila Krizova | + 1476 word(s) | 1476 | 2021-01-20 08:02:51 | | | |
| 2 | Camila Xu | Meta information modification | 1476 | 2021-02-05 06:58:21 | | |
Video Upload Options
Mycotoxins are a structurally diverse group of mostly low-molecular-weight compounds. Their structures range from single heterocyclic rings to irregularly arranged rings of six to eight members and their molecular weights are usually less than 1000 Da. Therefore, they do not induce any response in the human immune system.
1. Introduction
Mycotoxins are produced mainly by the secondary metabolism of certain filamentous fungi, which grow under specific temperature and humidity and cause serious risks for human and animal health. As secondary metabolites, instead of playing a role in growth and normal metabolism of the fungus, many mycotoxins are involved in pathogenesis or in competing with other organisms [1][2].
Many of the toxigenic fungi are ubiquitous and, in some cases, have a conjunction with food and feed production. From these, the most common toxigenic species belong to four genera: Fusarium, Aspergillus, Penicillium, and Alternaria [3]. Fusarium and Alternaria usually produce mycotoxins before harvest or in freshly harvested products, whereas Aspergillus and Penicillium species represent a higher risk during drying and storage of food and feed products [1][3]. Fusarium genus includes over 90 described species and is responsible for the production of some of the most important classes of mycotoxins: trichothecenes, fumonisins, and zearalenones. Moreover, this genus produces less studied mycotoxins called minor or emerging mycotoxins: fusaproliferin, beauvericin (BEA), enniatins (ENs), and moniliformin. The toxicity of the toxins produced by Fusarium varies greatly depending on the toxin and the target organism [3][4]. The most important species that produce these toxic metabolites are Fusarium proliferatum, Fusarium subglutinans, Fusarium moniliforme, and Fusarium avenacum, involved in crop diseases, such as stalk and maize ear rot disease [5].
To date, more than 300 mycotoxins have been identified, and research is focused mainly on those that have been proven to have diverse health effects on humans and animals, like teratogenicity, carcinogenicity, and mutagenicity [6][7]. The exposure of humans to mycotoxins occurs either directly through the consumption of contaminated plant foods (e.g., cereals) or indirectly through the intake of animal-derived products (e.g., milk and eggs) that origin from animals fed with contaminated diets [6]. From the perspective of livestock breeding and nutrition, mycotoxins in feedstuffs are of dual concern. First one is connected with the safety of animal-derived food and is related to occurrence of mycotoxins in feed and their (partial) carry-over from feed to edible animal tissues such as milk, eggs, or meat. The occurrence of mycotoxins in food can be legislatively monitored (presence of aflatoxin M1 in milk [8]). The second one is connected with detrimental effects of mycotoxins on animal health and performance. Such detrimental mycotoxins include deoxynivalenol (DON), fumonisins, ochratoxin A, and zearalenone (ZEA). Most, but not all, of these mycotoxins are produced by the Fusarium species. Although these mycotoxins are significant contaminants when entering the food chain directly via food of plant origin, they are not considered relevant in food of animal origin because their carry-over from feed to animal-derived food products is negligible [9][10][11][12]. However, Fusarium species are also responsible for the production of minor mycotoxins, namely, enniatins and beauvericin, which are currently in the center of interest because of the wide range of their biological activities.
2. Beauvericin
Beauvericin (BEA) is a cyclic lactone trimer, which contains an alternate sequence of three N-methylphenylalanyl and three D-α-hydroxyisovaleryl residues (Figure 1). It was first isolated from the fungus Beauverina bassiana, an insect pathogen [13]. The first Fusarium species identified to produce BEA was Fusarium subglutinans [14]. Subsequently, other Fusarium species such as Fusarium bulbicola, Fusarium denticulatum, Fusarium lactis, Fusarium phyllophillum, Fusarium pseudocircinatum, and Fusarium succisae have been proven to produce BEA [15].
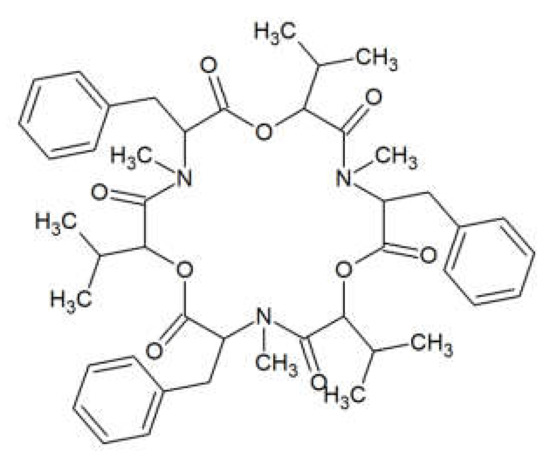
Figure 1. Beauvericin structure.
BEA possesses insecticidal and phytotoxic properties and is involved in the etiology of insect and plant diseases caused by the producer fungal strains [16]. The antimicrobial and antibiotic activities of BEA have been tested on human or mammalian intestinal bacteria (see Table 1). BEA also showed endocrine disrupting antagonistic effects at the androgen receptor [17]. It also acts on cellular level as an enzyme inhibitor [18], and as a compound inducing oxidative stress. BEA eases apoptosis, interferes with smooth muscle contraction, impedes with steatosis caused by the storage of cholesterol in liver cells, and according to various bioassays, it is believed to be toxic. Nevertheless, it was discovered that cytotoxicity of BEA depends on the dose, length, and also way of exposure [19][20], because it is able to penetrate to the body through the skin, although its permeation is relatively low [21].
Table 1. Antimicrobial effects of beauvericin and enniatins B and B1 on bacteria isolated from the human or mammalian intestinal tract.
| Beauvericin | Bacillus cereus, B. mycoides, B. pumilis, B. sphaericus, Bifidobacterium adolescentis, Clostridium perfringens, Escherichia coli, Enterococcus faecium, Eubacterium biforme, Listeria monocytogenes, Paenibacillus alvei, P. azotofixans, P. macerans, P. macquariensis, P. pabuli, P. productus, P. pulvifaciens, P. validus, Peptostreptococcus anaerobius, Pseudomonas aeruginosa, Salmonella enterica, Shigella dysenteriae, Yersinia enterocolitica, | [24][26] |
| Enniatin B | Escherichia coli, E. faecium, Clostridium perfringens, Listeria monocytogenes, Pseudomonas aeruginosa, Salmonella enterica, Shygella dysenteriae, Staphylococcus aureus, Yersinia enterocolitica | [36] |
| Enniatin B1 | Bifidobacterium adolescentis | [37] |
Furthermore, the effect of BEA on human and animal health might not be just negative, BEA was also proven to have several positive qualities such as antifungal [22], antiviral [23] or antibiotic effect. The antibiotic effects of BEA were tested on the following bacterial species including those from GI tract: Bacillus cereus, Bacillus mycoides, Bacillus pumilis, Bacillus sphaericus, Bifidobacterium adolescentis Clostridium perfringens, Escherichia coli, Enterococcus faecium, Eubacterium biforme, Listeria monocytogenes, Paenibacillus alvei, Paenibacillus azotofixans, Paenibacillus macerans, Paenibacillus macquariensis, Paenibacillus pabuli, Paenibacillus productus, Paenibacillus pulvifaciens, Paenibacillus Validus, Peptostreptococcus anaerobius, Pseudomonas aeruginosa, Salmonella enterica, Shigella dysenteriae, Yersinia enterocolitica, and two strains of Staphylococcus aureus, using microbial bioassay techniques [24][25][26]. The highest activity was observed for C. perfringens with a minimum inhibitory concentration (MIC) of 1 ng per disc, followed by S. enterica (MIC = 10 ng per disc) and B. pumilus together with L. monocytogenes (MIC = 100 ng per disc). Generally, Gram-positive bacteria were more inhibited than Gram-negative ones. Furthermore, BEA, which acts as an inhibitor of activated T cells, is a possible drug candidate for the colon inflammation treatment [27].
3. Enniatins
Enniatins (ENs) were discovered in the cultures of Fusarium orthoceras, later renamed Fusarium oxysporum [28]. ENs represent a large group of related mycotoxins with the structure of cyclic hexadepsipeptides, comprised of D-α-hydroxy-isovaleryl-(2-hydroxy-3-methylbutanoic acid) and N-methylamino acid residues linked with peptide bonds and intra-molecular ester (lactone) bonds (see Figure 2). ENs of type A and B contain N-methyl-valine or N-methyl-isoleucine or the mixtures of these two amino acids [29]. Currently, 29 naturally occurring enniatin analogues are known [30] and seven of them (ENs A, A1, B, B1, B2, B3, and B4) have been found in cereals. ENs A, A1, B, and B1 are most frequently reported in foods and feeds [15]. ENs are produced by strains of some species of Fusarium, Alternaria, Halosarpheia, and Verticillium genera [31].
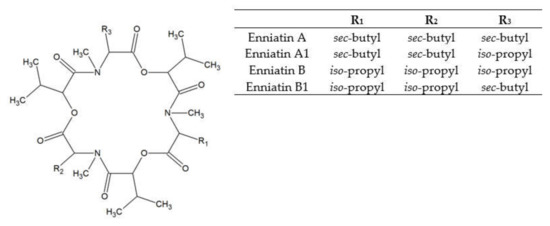
Figure 2. Enniatin structure.
ENs are phytotoxic [32], antifungal (towards Aspergillus flavus, A. parasiticus, A. fumigatus, A. ochraceus, Beauveria bassiana, Fusarium verticilloides, F. sporotrichioides, F. tricinctum, F. poae, F. oxysporum, F. proliferatum, Penicillium expansum, and Trichoderma harzianum) [24], antiyeast (towards Candida albicans, Trichosporum cutaneum, and Cryptococcus neoformans) [33] and antibacterial (towards Bacillus subtilis, Mycobacterium spp., Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, and lactic acid bacteria) [34] and insecticidal agents [35]. The antimicrobial activities of ENs tested on human or mammalian intestinal bacteria are shown in Table 1.
ENs have cytotoxic activities that have been tested on several mammalian and cancer cell types such us Hep-G2 [36][38], Caco-2, HT-29 [36], MRC-5 [38] or CHO-K1 cells [39]. These studies gave proof of the potential cytotoxicity of ENs in mammal cell lines at quite low micromolar concentrations. Furthermore, synergistic effect of the combination of several individual ENs was observed [39]. It has been proved that ENs might even have an effect of genotoxicity. When eaten in larger doses, symptoms that are frequently occurring in transition cows include reduced rumen fermentation [40]. ENs are able to penetrate to the body through the skin and their permeation is higher than that of BEA with the highest permeation found in enniatin B (k (p, v) = 9.44 × 10–6 cm/h) [21].
ENs are also known as ionophores [41][42], antibiotics [24], and antimicrobial compounds [43][44] against human, animal, and plant pathogenic bacteria with no selectivity between Gram-positive and Gram-negative bacteria. Undeniable benefit of ENs is also an anti-helminthic effect [45]. Their biological activities may be explained by their ability to selectively increase the flux of alkali metal ions through biological membranes. Using the patch clamp technique in the inside-out mode, enniatin was shown to incorporate into the cell membrane, where it forms pores selective for cations [46]. Recent study suggested that EN B1 can destabilize the lysosome-associated membrane proteins 2 which results in the alkalinization of lysosomes and partial lysosomal membrane permeabilization [47]. In addition to their effect on cells, ENs exerts a hypolipidemic effect partly by inhibiting enzymes such as acyl-CoA: cholesterol acyl transferase (ACAT) and partly by reducing triglyceride synthesis and diminishing the free fatty acid pool in the cells. Furthermore, ENs inhibit 30,50-cyclo-nucleotide phosphodiesterase and can attach to calmodulin. Even though, ENs are currently used only to the local treatment of respiratory infections [46].
References
- Pitt, J.I. Toxigenic fungi and mycotoxins. Br. Med. Bull. 2000, 56, 184–192, doi:10.1258/0007142001902888.
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural functions of mycotoxins and control of their biosynthe-sis in fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911, doi:10.1007/s00253-010-2657-5.
- Escrivá, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the Presence of Mycotoxins in Biological Samples: An Overview. Toxins 2017, 9, 251, doi:10.3390/toxins9080251.
- Guerre, P. Fusariotoxins in Avian Species: Toxicokinetics, Metabolism and Persistence in Tissues. Toxins 2015, 7, 2289–2305, doi:10.3390/toxins7062289.
- Monti, S.M.; Fogliano, V.; Logrieco, A.; Ferracane, R.; Ritieni, A. Simultaneous Determination of Beauvericin, Enniatins, and Fusaproliferin by High Performance Liquid Chromatography. J. Agric. Food Chem. 2000, 48, 3317–3320, doi:10.1021/jf990373n.
- Capriotti, A.L.; Caruso, G.; Cavaliere, C.; Foglia, P.; Samperi, R.; Laganà, A. Multiclass mycotoxin analysis in food, envi-ronmental and biological matrices with chromatography/mass spectrometry. Mass Spectrom. Rev. 2012, 31, 466–503, doi:10.1002/mas.20351.
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158, doi:10.1016/j.anifeedsci.2011.12.014.
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] re-lated to Aflatoxin B1 as undesirable substance in animal feed. EFSA J. 2004, 2, 39, doi:10.2903/j.efsa.2004.39.
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] re-lated to Deoxynivalenol (DON) as undesirable substance in animal feed. EFSA J. 2004, 2, 73, doi:10.2903/j.efsa.2004.73.
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on contaminants in the food chain [CON-TAM]related to Zearalenone as undesirable substance in animal feed. EFSA J. 2004, 2, 89, doi:10.2903/j.efsa.2004.89.
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] re-lated to ochratoxin A (OTA) as undesirable substance in animal feed. EFSA J. 2004, 2, 101, doi:10.2903/j.efsa.2004.101.
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] re-lated to fumonisins as undesirable substances in animal feed. EFSA J. 2005, 3, 235, doi:10.2903/j.efsa.2005.235.
- Hamill, R.L.; Higgens, C.E.; Boaz, H.E.; Gorman, M. Structure of Beauvericin, a New Depsipeptide Antibiotic Toxic to Arte-mia Salina. Tetrahedron Lett. 1969, 49, 4255–4258.
- Gupta, S.; Krasnoff, S.B.; Underwood, N.L.; Renwick, J.A.A.; Roberts, D.W. Isolation of beauvericin as an insect toxin from Fusarium semitectum and Fusarium moniliforme var. subglutinans. Mycopathologia 1991, 115, 185–189, doi:10.1007/BF00462223.
- Santini, A.; Meca, G.; Uhlig, S.; Ritieni, A. Fusaproliferin, beauvericin and enniatins: Occurrence in food—A review. World Mycotoxin J. 2012, 5, 71–81, doi:10.3920/WMJ2011.1331.
- Paciolla, C.; Dipierro, N.; Mulè, G.; Logrieco, A.; Dipierro, S. The mycotoxins beauvericin and T-2 induce cell death and alteration to the ascorbate metabolism in tomato protoplasts. Physiol. Mol. Plant Pathol. 2004, 65, 49–56, doi:10.1016/j.pmpp.2004.07.006.
- García-Herranz, V.; Valdehita, A.; Navas, J.M.; Fernández-Cruz, M.L. Cytotoxicity against Fish and Mammalian Cell Lines and Endocrine Activity of the Mycotoxins Beauvericin, Deoxynivalenol and Ochratoxin-A. Food Chem. Toxicol. 2019, 127, 288–297, doi:10.1016/j.fct.2019.01.036.
- Juan, C.; Manyes, L.; Font, G.; Juan-García, A. Evaluation of immunologic effect of Enniatin A and quantitative determina-tion in feces, urine and serum on treated Wistar rats. Toxicon 2014, 87, 45–53, doi:10.1016/j.toxicon.2014.05.005.
- Calo’, L.; Fornelli, F.; Nenna, S.; Tursi, A.; Caiaffa, M.F.; Macchia, L. Beauvericin cytotoxicity to the invertebrate cell line SF-9. J. Appl. Genet. 2003, 44, 515–520.
- Macchia, L.; Caiffa, M.F.; Fornelli, F.; Calo, L.; Nenna, S.; Moretti, A.; Logrieco, A.; Tursi, A. Apoptosis induced by the Fusarium mycotoxin beauvericin in mammalian cells. Appl Genet 2002, 43, 363–371.
- Taevernier, L.; Veryser, L.; Roche, N.; Peremans, K.; Burvenich, C.; Delesalle, C.; De Spiegeleer, B. Human skin permeation of emerging mycotoxins (beauvericin and enniatins). J. Expo. Sci. Environ. Epidemiol. 2016, 26, 277–287, doi:10.1038/jes.2015.10.
- Olleik, H.; Nicoletti, C.; Lafond, M.; Courvoisier-Dezord, E.; Xue, P.; Hijazi, A.; Baydoun, E.; Perrier, J.; Maresca, M. Com-parative Structure–Activity Analysis of the Antimicrobial Activity, Cytotoxicity, and Mechanism of Action of the Fungal Cyclohexadepsipeptides Enniatins and Beauvericin. Toxins 2019, 11, 514, doi:10.3390/toxins11090514.
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206, doi:10.1016/j.fct.2015.02.005.
- Meca, G.; Sospedra, I.; Soriano, J.M.; Ritieni, A.; Moretti, A.; Mañes, J. Antibacterial effect of the bioactive compound beau-vericin produced by Fusarium proliferatum on solid medium of wheat. Toxicon 2010, 56, 349–354, doi:10.1016/j.toxicon.2010.03.022.
- Madhyastha, M.S.; Marquardt, R.R.; Frohlich, A.A.; Borsa, J. Optimization of Yeast Bioassay for Trichothecene Mycotoxins. J. Food Prot. 1994, 57, 490–495, doi:10.4315/0362-028X-57.6.490.
- Castlebury, L.A.; Sutherland, J.B.; Tanner, L.A.; Henderson, A.L.; Cerniglia, C.E. Short Communication: Use of a bioassay to evaluate the toxicity of beauvericin to bacteria. World J. Microbiol. Biotechnol. 1999, 15, 131–133.
- Wu, X.-F.; Xu, R.; Ouyang, Z.-J.; Qian, C.; Shen, Y.; Wu, X.-D.; Gu, Y.-H.; Xu, Q.; Sun, Y. Beauvericin Ameliorates Experi-mental Colitis by Inhibiting Activated T Cells via Downregulation of the PI3K/Akt Signaling Pathway. PLoS ONE 2013, 8, doi:10.1371/journal.pone.0083013.
- Plattner, P.A.; Nager, U. Über die Chemie des Enniatins. Experientia 1947, 3, 325–326, doi:10.1007/BF02164246.
- Blais, L.A.; Simon, J.W.A.; Blackwell, B.A.; Greenhalgh, R.; Miller, J.D. Isolation and characterization of enniatins from Fusarium avenaceum DAOM 196490. Can. J. Chem. 1992, 70, 1281–1287.
- Sy-Cordero, A.A.; Pearce, C.J.; Oberlies, N.H. Revisiting the enniatins: A review of their isolation, biosynthesis, structure determination and biological activities. J. Antibiot. 2012, 65, 541–549, doi:10.1038/ja.2012.71.
- Supothina, S.; Isaka, M.; Kirtikara, K.; Tanticharoen, M.; Thebtaranonth, Y. Enniatin Production by the Entomopathogenic Fungus Verticillium hemipterigenum BCC 1449. J. Antibiot. 2004, 57, 732–738.
- Morrison, E.; Kosiak, B.; Ritieni, A.; Aastveit, A.H.; Uhlig, S.; Bernhoft, A. Mycotoxin Production by Fusarium avenaceum Strains Isolated from Norwegian Grain and the Cytotoxicity of Rice Culture Extracts to Porcine Kidney Epithelial Cells. J. Agric. Food Chem. 2002, 50, 3070–3075, doi:10.1021/jf011532h.
- Firakova, S.; Šturdíková; M; Liptaj, T.; Prónayová; N; Bezáková; L; Proksa, B. Enniatins produced by Fusarium dimerum, an endophytic fungal strain. Pharmazie 2008, 63, 539–541, doi:10.1691/ph.2008.7831.
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to Prevent Mycotoxin Contamination of Food and Animal Feed: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619, doi:10.1080/10408390500436185.
- Grove, J.F.; Pople, M. The insecticidal activity of beauvericin and the enniatin complex. Mycopathologia 1980, 70, 103–105, doi:10.1007/BF00443075.
- Meca, G.; Sospedra, I.; Valero, M.A.; Mañes, J.; Font, G.; Ruiz, M.J. Antibacterial Activity of the Enniatin B, Produced by Fusarium Tricinctum in Liquid Culture, and Cytotoxic Effects on Caco-2 Cells. Toxicol. Mech. Methods 2011, 21, 503–512, doi:10.3109/15376516.2011.556202.
- Roig, M.; Meca, G.; Marín, R.; Ferrer, E.; Mañes, J. Antibacterial Activity of the Emerging Fusarium Mycotoxins Enniatins A, A1, A2, B, B1, and B4 on Probiotic Microorganisms. Toxicon 2014, 85, 1–4, doi:10.1016/j.toxicon.2014.04.007.
- Ivanova, L.; Skjerve, E.; Eriksen, G.S.; Uhlig, S. Cytotoxicity of enniatins A, A1, B, B1, B2 and B3 from Fusarium avenaceum. Toxicon 2006, 47, 868–876, doi:10.1016/j.toxicon.2006.02.012.
- Lu, H.; Fernández-Franzón, M.; Font, G.; Ruiz, M.J. Toxicity evaluation of individual and mixed enniatins using an in vitro method with CHO-K1 cells. Toxicol. In Vitro 2013, 27, 672–680, doi:10.1016/j.tiv.2012.11.009.
- Sotnichenko, A.; Pantsov, E.; Shinkarev, D.; Okhanov, V. Hydrophobized Reversed-Phase Adsorbent for Protection of Dairy Cattle against Lipophilic Toxins from Diet. Efficiensy In Vitro and In Vivo. Toxins 2019, 11, 256; doi:10.3390/toxins11050256..
- Kouri, K.; Lemmens, M.; Lemmens-Gruber, R. Beauvericin-induced channels in ventricular myocytes and liposomes. Bio-chim. Biophys. Acta BBA Biomembr. 2003, 1609, 203–210, doi:10.1016/S0005-2736(02)00689-2.
- Uhlig, S.; Ivanova, L.; Petersen, D.; Kristensen, R. Structural studies on minor enniatins from Fusarium sp. VI 03441: Novel N-methyl-threonine containing enniatins. Toxicon 2009, 53, 734–742, doi:10.1016/j.toxicon.2009.02.014.
- Kouri, K.; Duchen, M.R.; Lemmens-Gruber, R. Effects of Beauvericin on the Metabolic State and Ionic Homeostasis of Ven-tricular Myocytes of the Guinea Pig. Chem. Res. Toxicol. 2005, 18, 1661–1668, doi:10.1021/tx050096g.
- Uhlig, S.; Jestoi, M.; Parikka, P. Fusarium avenaceum—The North European situation. Int. J. Food Microbiol. 2007, 119, 17–24, doi:10.1016/j.ijfoodmicro.2007.07.021.
- Behm, C.; Degen, G.H.; Föllmann, W. The Fusarium toxin enniatin B exerts no genotoxic activity, but pronounced cytotoxi-city in vitro. Mol. Nutr. Food Res. 2009, 53, 423–430, doi:10.1002/mnfr.200800183.
- Kamyar, M.; Rawnduzi, P.; Studenik, C.R.; Kouri, K.; Lemmens-Gruber, R. Investigation of the electrophysiological proper-ties of enniatins. Arch. Biochem. Biophys. 2004, 429, 215–223, doi:10.1016/j.abb.2004.06.013.
- Oliveira, C.A.F.; Ivanova, L.; Solhaug, A.; Fæste, C.K. Enniatin B1-Induced Lysosomal Membrane Permeabilization in Mouse Embryonic Fibroblasts. Mycotoxin Res. 2020, 36, 23–30, doi:10.1007/s12550-019-00366-8.





