
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Małgorzata Dmitryjuk | + 1150 word(s) | 1150 | 2021-01-20 13:15:27 | | | |
| 2 | Lily Guo | Meta information modification | 1150 | 2021-02-02 05:20:13 | | | | |
| 3 | Lily Guo | Meta information modification | 1150 | 2021-02-02 09:52:47 | | |
Video Upload Options
Borrelia miyamotoi is a Gram-negative bacteria included in the genus Borrelia from the family Spirochaetaceae, within the phylum Spirochaetes and the order Spirochaetales. B. miyamotoi is spirochete from the relapsing fever (RF) group of Borrelia. In RF-Borrelia complex only B. miyamotoi is transmitted by Ixodes ticks - a vector of B. burgdorferi s.l. complex. The biological feature that distinguishes B. miyamotoi from B. burgdorferi s.l. is transovarial transmission. B. miyamotoi was first isolated from questing I. persulcatus ticks and mouse Apodemus argentus in Japan in 1994. The main vector of B. miyamotoi is I. persulcatus (Asia), I. pacificus, I. scapularis (North America), and I. ricinus (Europe). Worldwide, B. miyamotoi prevalence in questing Ixodes ticks ranges from 0.2 to 10%. A phylogenetic analysis based on selected sequences of B. miyamotoi genome revealed genetic differences between isolates from Asia, North America, and Europe, which are clearly separated into three genotypes. Human symptomatic cases of Borrelia miyamotoi disease (BMD) were first reported in 2011 in Russia and then in North America, Europe, and Asia. BMD is usually manifested by several episodes of fever and flu-like symptoms (chills, headaches, muscle, and joint aches and general fatigue). However, serious symptoms such as meningoencephalitis can be observed.
1. Introduction
In Europe, tick-borne diseases transmitted by Ixodes ricinus are the most common zoonoses with significant medical and veterinary importance[1]. This hematophagous arthropod is a reservoir and vector of many pathogenic microorganisms, including the bacteria Borrelia burgdorferi sensu lato (s.l.) complex—the causative agent of Lyme borreliosis (LB), Rickettsia spp., and Anaplasma spp., as well as the flavivirus responsible for tick-borne encephalitis (TBE) and the etiological protozoan agents of babesiosis[2][3]. With advanced methods of molecular biology, new tick-borne microorganism species and their genetic variants with confirmed or potential pathogenicity for humans and animals are still being identified[4]. One of the emerging Ixodes-borne diseases in the northern temperate climate zones of the world, including Europe, is Borrelia miyamotoi disease (BMD), caused by spirochete from the relapsing fever (RF) group of Borrelia[5][6]. Since 1994, when B. miyamotoi was first isolated from questing I. presulcatus ticks and mouse Apodemus argentus in Japan[7], it was considered to be a non-pathogenic endosymbiont. However, since 2011 many symptomatic B. miyamotoi infections in humans have been noted in Asia, North America, and Europe [8][9][10][11][12][13][14].
2. Recent Studies
2.1. Taxonomic Position
B. miyamotoi is a Gram-negative bacteria included in the genus Borrelia from the family Spirochaetaceae, within the phylum Spirochaetes and the order Spirochaetales [15]. Borrelia species are obligate parasites, transmitted by arthropod vectors to vertebrate hosts. The biological feature that distinguishes B. miyamotoi and several other relapsing fever species from B. burgdorferi s.l. is transovarial transmission[16].
The Borrelia spirochete cells are 0.2–0.5 mm in diameter by 3–30 mm in length, with 15–20 periplasmic flagella (endoflagella) located in the periplasmic space between the outer membrane and the protoplasmic cylinder. These cells can move actively with frequent reversal of direction [15][17]. Due to limited B. miyamotoi biosynthetic potential, its in vitro culture is difficult (as other Borrelia species) and requires microaerophilic conditions and complex nutrition. However, it can be propagated in Kelly-Pettenkofer medium with fetal calf serum (MKP-F) [18].
Although the Borrelia species share spirochetal morphology, they have different biological, clinical, and epidemiological features. Based on their arthropod vectors and genetic characteristics two major groups of Borrelia were distinguished. The first group contains 20 Borrelia species, including the B. burgdorferi s.l. complex, an agent of LB, and are transmitted by Ixodes hard ticks. The second group includes 25 Borrelia species associated with human RF and mostly found in soft ticks (Argasidae) but also in lice (B. recurensis) and hard ticks (B. miyamotoi, B. lonestari, B. theileri). In RF-Borrelia complex only B. miyamotoi is transmitted by Ixodes ticks—a vector of B. burgdorferi s.l. complex [15][19][20]. These two groups are genetically similar but form distinct, independent monophyletic clades and share a common ancestor. In 2014, Adeolu and Gupta[21] proposed splitting the spirochetes from the genus Borrelia into two separate genera: a novel genus, Borreliella gen. nov., containing the causative agents of Lyme disease and a revised genus Borrelia, with spirochetes causing RF, including B. miyamotoi. However, the proposed change in the name of this pathogenic bacteria species proved controversial and did not receive support among scientists, clinicians or public health authorities, who felt it would lead to confusion and pose a risk to patient safety [20][22][23].
2.2. Genome Organization and Genetic Diversity
The first information about the organization of the B. miyamotoi genome and its differences in relation to the known species from the LB- and RF-Borrelia groups was published in 1995[7]. Later, more advanced molecular analysis of Asian, American, and European B. miyamotoi isolates from Ixodes ticks and clinical samples revealed the complexity of the genome structure typical of Borrelia spirochetes[24][25][26][27][28][29]. However, the most information was obtained by sequencing the genome of B. miyamotoi Izh-4 isolate from a Russian patient[30]. The complete genome of a single B. miyamotoi cell consists of one linear chromosome (~900 kb) and 12 linear and two circular plasmids (from 6 to 73 kb). Two of the plasmids (lp70 and lp64) had not previously been found in other Borrelia species. A total of 1362 genes, including 1222 protein-coding genes, 103 pseudogenes, 31 genes for transfer RNA (tRNA), a cluster of three genes of ribosomal RNA (rRNA), and three genes of non-coding RNA (ncRNA) were identified. In B. miyamotoi virulence, a significant role is played by plasmid lp4, which includes genes of variable membrane proteins (VMPs), necessary to mask the bacteria from the host immune system and prolong the infection[30][31][32]. A comparison of different B. miyamotoi isolates revealed that the number and order of VMPs genes were unique for each of them[30].
Phylogenetic analysis based on genome sequences of B. miyamotoi showed genetic differences between isolates from Asia, North America and Europe which are clearly separated into three types (genotypes) and form a monophyletic clade inside the RF-Borrelia spirochetes[30]. However, the genetic differences between the B. miyamotoi isolates are probably not connected with geographic origin, but rather with pathogenicity, vector competence, and host range[24].
The B. miyamotoi genetic distance from other LB species and the relationship with the species from the RF group is evidenced by the carriage and expression of a glpQ gene, coding the immunoreactive protein glycerophosphodiester phosphodiesterase [33][34]. The glpQ gene and GlpQ protein are conserved among the members of the genus Borrelia, except LB spirochetes (Figure 1). Therefore, GlpQ is usually used as a marker in molecular and serological tests to detect RF spirochete infections and to distinguish cases of LB and other tick-borne infections (e.g., anaplasmosis, babesiosis) [8][35][36][37].
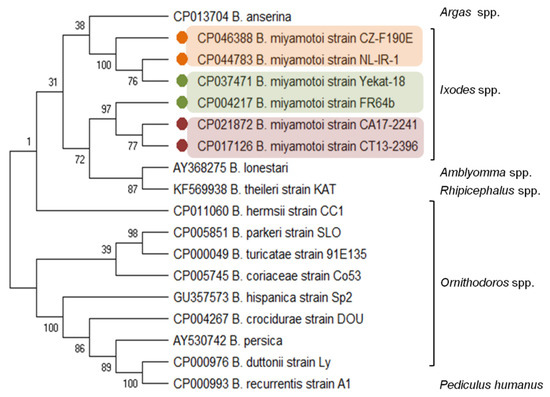
Figure 1. Molecular relationships between B. miyamotoi and other RF Borrelia species based on the sequences of the glpQ gene selected from GenBank. The consensus tree constructed using the neighbor-joining method and the maximum composite likelihood as the distance method; numbers at the tree nodes indicate bootstrap value from 1000 replicates; analyses were conducted in MEGA X[38]. Marks: orange—European type, green—Asian type, red—American type of B. miyamotoi. The genus names of the vectors were added.
References
- Mysterud, A.; Jore, S.; Østerås, O.; Viljugrein, H. Emergence of tick-borne diseases at northern latitudes in Europe: A comparative approach. Sci. Rep. 2017, 7, 1–12, doi:10.1038/s41598-017-15742-6.
- Dantas-Torres, F.; Chomel, B.; Otranto, D. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol. 2012, 28, 437–446, doi:10.1016/j.pt.2012.07.003.
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Špitalská, E.; et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: New hazards and relevance for public health. Front. Public Heal. 2014, 2, 1–26, doi:10.3389/fpubh.2014.00251.
- Azagi, T.; Hoornstra, D.; Kremer, K.; Hovius, J.W.R.; Sprong, H. Evaluation of disease causality of rare Ixodes ricinus-borne infections in Europe. Pathogens 2020, 9, 1–22, doi:10.3390/pathogens9020150.
- Telford, S.R.; Goethert, H.K.; Molloy, P.J.; Berardi, V.P.; Chowdri, H.R.; Gugliotta, J.L.; Lepore, T.J. Borrelia miyamotoi disease: neither Lyme disease nor relapsing fever. Clin. Lab. Med. 2015, 35, 867–882, doi:10.1016/j.cll.2015.08.002.
- Cutler, S.J.; Vayssier-Taussat, M.; Estrada-Peña, A.; Potkonjak, A.; Mihalca, A.D.; Zeller, H. A new Borrelia on the block: Borrelia miyamotoi - A human health risk? Eurosurveillance 2019, 24, 1800170, doi:10.2807/1560-7917.ES.2019.24.18.1800170.
- Fukunaga, M.; Takahashi, Y.; Tsuruta, Y.; Matsushita, O.; Ralph, D.; McClelland, M.; Nakao, M. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int. J. Syst. Bacteriol. 1995, 45, 804–810, doi:10.1099/00207713-45-4-804.
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V. V.; Fish, D.; Krause, P.J. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823, doi:10.3201/eid1710.101474.
- Sato, K.; Takano, A.; Konnai, S.; Nakao, M.; Ito, T.; Koyama, K.; Kaneko, M.; Ohnishi, M.; Kawabata, H. Human infections with Borrelia miyamotoi, Japan. Emerg. Infect. Dis. 2014, 20, 1391–1393, doi:10.3201/eid2008.131761.
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Rollend, L.; Fikrig, E.; Lepore, T.; Barbour, A.; Fish, D. Human Borrelia miyamotoi infection in the United States. N. Engl. J. Med. 2013, 368, 291–293, doi:10.1056/NEJMc1215469.
- Molloy, P.J.; Telford, S.R.; Chowdri, H.R.; Lepore, Timothy J Gugliotta, J.L.; Weeks, K.E.; Hewins, M.E.; Goethert, H.K.; Berardi, V.P. Borrelia miyamotoi disease in the Northeastern United States: A case series. Ann. Intern. Med. 2015, 163, 91–98, doi:10.7326/M15-0333.
- Hansford, K.M.; Fonville, M.; Jahfari, S.; Sprong, H.; Medlock, J.M. Borrelia miyamotoi in host-seeking Ixodes ricinus ticks in England. Epidemiol. Infect. 2015, 143, 1079–1087, doi:10.1017/S0950268814001691.
- Hovius, J.W.R.; De Wever, B.; Sohne, M.; Brouwer, M.C.; Coumou, J.; Wagemakers, A.; Oei, A.; Knol, H.; Narasimhan, S.; Hodiamont, C.J.; et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 2013, 382, 658, doi:10.1016/S0140-6736(13)61644-X.
- Jahfari, S.; Hofhuis, A.; Fonville, M.; van der Giessen, J.; van Pelt, W.; Sprong, H. Molecular detection of tick-borne pathogens in humans with tick bites and erythema migrans, in the Netherlands. PLoS Negl. Trop. Dis. 2016, 10, 1–15, doi:10.1371/journal.pntd.0005042.
- Wang, G.; Schwartz, I. Genus Borrelia. In Bergey’s Manual of Systematic Bacteriology Vol. 4: The Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes; Krieg, N., Staley, J., Brown, D., Hedlund, B., Paster, B., Ward, N., Ludwig, W., Whitman, W., Eds.; Springer: New York, 2011; pp. 484–531 ISBN 978-0-387-68572-4.
- Krause, P.J.; Fish, D.; Narasimhan, S.; Barbour, A.G. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect. 2015, 21, 631–639, doi:10.1016/j.cmi.2015.02.006.
- Wang, G. Borrelia burgdorferi and other Borrelia species. In Molecular Medical Microbiology (Second Edition); Yi-Wei, T., Max, S., Dongyou, L., Ian, P., Joseph, S., Eds.; Academic Press, 2015; pp. 1867–1909 ISBN 9780123971692.
- Wagemakers, A.; Oei, A.; Fikrig, M.M.; Miellet, W.R.; Hovius, J.W. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasites and Vectors 2014, 7, 4–9, doi:10.1186/1756-3305-7-418.
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex-clinical significance of genomic species? Clin. Microbiol. Infect. 2011, 17, 487–493, doi:10.1111/j.1469-0691.2011.03492.x.
- Margos, G.; Marosevic, D.; Cutler, S.; Derdakova, M.; Diuk-Wasser, M.; Emler, S.; Fish, D.; Gray, J.; Hunfeldt, K.P.; Jaulhac, B.; et al. There is inadequate evidence to support the division of the genus Borrelia. Int. J. Syst. Evol. Microbiol. 2017, 67, 1081–1084, doi:10.1099/ijsem.0.001717.
- Adeolu, M.; Gupta, R.S. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: The emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members o. Antonie van Leeuwenhoek, Int. J. Gen. Mol. Microbiol. 2014, 105, 1049–1072, doi:10.1007/s10482-014-0164-x.
- Margos, G.; Fingerle, V.; Oskam, C.; Stevenson, B.; Gofton, A. Comment on: Gupta, 2019, Distinction between Borrelia and Borreliella is more robustly supported by molecular and phenotypic characteristics than all other neighbouring prokaryotic genera: Response to Margos’ et al. “The genus Borrelia reloaded” PLoS One. Ticks Tick. Borne. Dis. 2020, 11, doi:10.1016/j.ttbdis.2019.101320.
- Stevenson, B.; Fingerle, V.; Wormser, G.P.; Margos, G. Public health and patient safety concerns merit retention of Lyme borreliosis-associated spirochetes within the genus Borrelia, and rejection of the genus novum Borreliella. Ticks Tick. Borne. Dis. 2019, 10, 1–4, doi:10.1016/j.ttbdis.2018.08.010.
- Barbour, A.G. Phylogeny of a relapsing fever Borrelia species transmitted by the hard tick Ixodes scapularis. Infect. Genet. Evol. 2014, 27, 551–558, doi:10.1016/j.meegid.2014.04.022.
- Hue, F.; Langeroudi, A.G.; Barbour, A.G. Borne agent of human infection. Genome Announc. 2013, 1, 5–6, doi:10.1371/journal.pone.0069802.10.
- Kuleshov, K. V; Koetsveld, J.; Goptar, I.A.; Markelov, M.L.; Kolyasnikova, N.M. Whole-genome sequencing of six Borrelia miyamotoi clinical strains isolated in Russia. Genome Announc. 2018, 6, 1–2, doi:10.1128/genomeA.01424-17.
- Kingry, L.C.; Replogle, A.; Dolan, M.; Sexton, C.; Padgett, K.A.; Schriefer, M.E. Chromosome and large linear plasmid sequences of a Borrelia miyamotoi strain isolated from Ixodes pacificus ticks from California. Genome Announc. 2017, 5, 13–14, doi:10.1128/genomeA.00960-17.
- Kingry, L.C.; Replogle, A.; Batra, D.; Rowe, L.A.; Sexton, C.; Dolan, M.; Connally, N.; Petersen, J.M.; Schriefer, M.E. Toward a complete north American Borrelia miyamotoi genome. Genome Announc. 2017, 5, 7–8, doi:10.1128/genomeA.01557-16.
- Kuleshov, K. V.; Hoornstra, D.; Sprong, H.; Platonov, A.E.; Hovius, J.W. Draft whole-genome sequences of two western European Borrelia miyamotoi isolates. Microbiol. Resour. Announc. 2019, 8, e01314-19, doi:10.1128/MRA.01314-19.
- Kuleshov, K. V.; Margos, G.; Fingerle, V.; Koetsveld, J.; Goptar, I.A.; Markelov, M.L.; Kolyasnikova, N.M.; Sarksyan, D.S.; Kirdyashkina, N.P.; Shipulin, G.A.; et al. Whole genome sequencing of Borrelia miyamotoi isolate Izh-4: Reference for a complex bacterial genome. BMC Genomics 2020, 21, 1–18, doi:10.1186/s12864-019-6388-4.
- Barbour, A.G. Multiple and diverse vsp and vlp sequences in Borrelia miyamotoi, a hard tick-borne zoonotic pathogen. PLoS One 2016, 11, e0146283, doi:10.1371/journal.pone.0146283.
- Bergström, S.; Normark, J. Microbiological features distinguishing Lyme disease and relapsing fever spirochetes. Wien. Klin. Wochenschr. 2018, 130, 484–490, doi:10.1007/s00508-018-1368-2.
- Schwan, T.G.; Battisti, J.M.; Porcella, S.F.; Raffel, S.J.; Schrumpf, M.E.; Fischer, E.R.; Carroll, J.A.; Stewart, P.E.; Rosa, P.; Somerville, G.A. Glycerol-3-phosphate acquisition in spirochetes: distribution and biological activity of glycerophosphodiester phosphodiesterase (GlpQ) among Borrelia species. J. Bacteriol. 2003, 185, 1346–1356, doi:10.1128/JB.185.4.1346-1356.2003.
- Bacon, R.M.; Pilgard, M.A.; Johnson, B.J.B.; Raffel, S.J.; Schwan, T.G. Glycerophosphodiester phosphodiesterase gene (glpQ) of Borrelia lonestari identified as a target for differentiating Borrelia species associated with hard ticks (Acari:Ixodidae). J. Clin. Microbiol. 2004, 42, 2326–2328, doi:10.1128/JCM.42.5.2326-2328.2004.
- Krause, P.J.; Carroll, M.; Fedorova, N.; Brancato, J.; Dumouchel, C.; Akosa, F.; Narasimhan, S.; Fikrig, E.; Lane, R.S. Human Borrelia miyamotoi infection in California: Serodiagnosis is complicated by multiple endemic Borrelia species. PLoS One 2018, 13, e0191725, doi:10.1371/journal.pone.0191725.
- Wagemakers, A.; Jahfari, S.; de Wever, B.; Spanjaard, L.; Starink, M. V.; de Vries, H.J.C.; Sprong, H.; Hovius, J.W. Borrelia miyamotoi in vectors and hosts in The Netherlands. Ticks Tick. Borne. Dis. 2017, 8, 370–374, doi:10.1016/j.ttbdis.2016.12.012.
- Hoornstra, D.; Koetsveld, J.; Sprong, H.; Platonov, A.E.; Hovius, J.W. Borrelia miyamotoi disease in an immunocompetent patient, Western Europe. Emerg. Infect. Dis. 2018, 24, 1770–1772, doi:10.7326/l15-5188.
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549, doi:10.1093/molbev/msy096.




