| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Keiji Hasumi | + 2131 word(s) | 2131 | 2021-01-28 03:06:15 | | | |
| 2 | Bruce Ren | -9 word(s) | 2122 | 2021-01-29 10:56:34 | | |
Video Upload Options
Stachybotrys microspora triprenyl phenol (SMTP) is a large family of small molecules derived from the fungus S. microspora. SMTP acts as a zymogen modulator (specifically, plasminogen modulator) that alters plasminogen conformation to enhance its binding to fibrin and subsequent fibrinolysis. Certain SMTP congeners exert anti-inflammatory effects by targeting soluble epoxide hydrolase. SMTP congeners with both plasminogen modulation activity and anti-inflammatory activity ameliorate various aspects of ischemic stroke in rodents and primates. A remarkable feature of SMTP efficacy is the suppression of hemorrhagic transformation, which is exacerbated by conventional thrombolytic treatments.
The hemostatic system is finely regulated to achieve continuous blood circulation and prevent blood loss based on the balance between blood coagulation and fibrinolysis (blood clot degradation) [1][2][3]. The zymogens in this system contribute to autonomous regulation through spatiotemporal activation in response to physiological demands or stimuli [4][5]. Defects in the hemostatic system lead to bleeding or thrombotic disorders [6][7][8][9]. To date, several drugs have been developed to treat diseases related to or resulting from such defects in the hemostatic system. Particularly, antithrombotics, which inhibit blood clot formation, and thrombolytics, which accelerate the degradation of blood clots, are widely used to treat, control, or prevent thrombotic complications such as ischemic diseases of the heart, brain, lungs, and kidneys [10][11][12][13][14]. Despite several efforts to elaborate the use of these drugs, a significant population of treated patients suffer from bleeding events, which are occasionally severe or fatal [15][16][17]. Thus, it is believed that antithrombotics and thrombolytics are associated with an inherent inevitable bleeding risk [18].
In 1993, we initiated an investigation to identify a bioactive compound that controls the hemostatic system, particularly a small molecule that promotes endogenous fibrinolysis [4]. Our theory was that a molecule that modulates the physiological process will achieve a therapeutic effect without excessive bleeding risk. Using several systems to screen random microbial metabolites, we discovered multiple compounds with a novel activity, zymogen modulation [4]. Stachybotrys microspora triprenyl phenol (SMTP) is a class of identified compounds that modulate the conformation of plasminogen to promote plasminogen binding to fibrin or the cell surface [9]. SMTP is a large family of metabolites from S. microspora, comprising more than 60 congeners (Table S1) [19][20]. In agreement with our hypothesis, SMTP promotes thrombolysis without causing excessive bleeding [21][22][23][24]. Subsequently, we observed an unexpected additional function of SMTP, the inhibition of soluble epoxide hydrolase (sEH), which is a key enzyme that controls inflammation [25]. Along with the radical-scavenging activity inherent in the SMTP structure, the combination of thrombolytic and anti-inflammatory actions of SMTP played a key role in the treatment of ischemic stroke in several models of rodents and monkeys [26][27][28][29][30].
1. Origin of SMTP
Background: Thrombotic and Thromboembolic Disorders and Treatment
Thrombotic and thromboembolic disorders are ischemic diseases that occur due to vascular occlusion by a blood clot formed in situ (thrombosis) or in an upstream vessel (embolism) that occludes a vessel at a downstream site [31][32]. The clot shuts off the supply of blood and oxygen, resulting in the death of the affected tissue. Thrombosis/thromboembolism can occur in both arteries and veins. Arterial thrombosis is the cause of most cases of heart attack (myocardial infarction) and ischemic stroke (brain infarction). Venous thrombosis/thromboembolism includes deep vein thrombosis, which accounts for most cases of pulmonary embolism (pulmonary infarction) [31]. These cardiovascular diseases constitute the most common causes of death in the developed world.
Atherosclerosis, or the rupture of an atherosclerotic plaque, is one of the most influential triggers for arterial thrombosis [33][34]. Atherosclerosis develops in the vessel wall through an accumulation of lipid deposits, mediated by macrophage foam cells that accumulate large amounts of cholesterol derived from lipoproteins such as low-density lipoprotein (LDL) that is oxidized in the vessel wall. Upon rupture of an atherosclerotic plaque, platelets rapidly aggregate to form a hemostatic plug through binding to collagen and von Willebrand factor. The aggregated platelets are activated to release several factors that promote the coagulation cascade and platelet aggregation/activation.
The coagulation cascade primarily consists of a sequential process of protease zymogen activation that results in the formation of fibrin and thrombus. The exposure of coagulation factor VII to tissue factor, a transmembrane cell surface glycoprotein, is a pathophysiological trigger for the initiation of the coagulation cascade. The hemostatic thrombus can be removed via another cascade reaction, the fibrinolytic system [35]. In both systems, the activation of protease zymogens is a key feature that regulates the local propagation of each event. The regulatory mechanism involves the instant response of the zymogen conformation to pathophysiological stimuli, triggering coagulation and fibrinolysis; the changes in conformation affect the localization and proteolytic activation of zymogens.
Generally, drugs targeting platelets (which inhibit platelet aggregation, resulting in the inhibition of blood clot formation) are used to treat arterial thrombosis [36], and venous thrombosis is treated with drugs targeting coagulation cascade proteases [37]. However, agents targeting the coagulation system are increasingly used in arterial disease, as evidenced by the COMPASS trial, where patients with stable atherosclerotic vascular disease were treated with a combination of Xa inhibitor and aspirin [38][39]. Although these drugs treat or prevent arterial and venous thrombosis/thromboembolism, a significant inherent risk of bleeding limits their use. Another important class of drugs used to treat thrombotic/thromboembolic disorders is thrombolytics, such as tissue-type plasminogen activator (t-PA), which selectively cleaves plasminogen to form plasmin, a protease that degrades fibrin, the major component of blood clots [40]. However, the timing of thrombolytic intervention crucially affects the outcome: the earlier, the better. For example, t-PA therapy is beneficial only when used within 3 to 4.5 h of ischemic stroke onset, and the risk of intracranial hemorrhage increases significantly when used beyond this time window [41]. Thus, no drug that prevents blood coagulation or promotes thrombolysis without causing bleeding has been developed to date. Nevertheless, statins, a class of drugs that lower LDL cholesterol levels and suppress or retard atherosclerosis, are unique in that they reduce the risk of thromboembolic events such as heart attack and ischemic stroke without elevating the risk of bleeding [42]. However, statins do not directly treat thrombotic/thromboembolic diseases.
Search for a Bioactive Compound that Enhances Physiological Thrombolysis
One of the authors (K.H.) was involved in the identification of inhibitors of cholesterol biosynthesis and macrophage foam cell conversion led by Akira Endo, who discovered the first statin drug, ML-236B (compactin), and the second, monacolin K (lovastatin) [42]. Although we discovered several interesting molecules over more than 10 years of research, none of these compounds was developed further. By the early 1990s, several clinical trials had proven the clinical benefit of statin drugs for reducing LDL cholesterol, cardiovascular disease incidence, and mortality [43]. Meanwhile, we explored an approach to discover a new drug that directly controls thrombotic/thromboembolic disease through a hitherto unsought mechanism.
The theory behind our investigation was that a compound that enhances plasminogen binding to fibrin or the cell surface would promote physiological fibrinolysis, serving as an ideal approach to achieve regulated thrombus degradation. The basis of this theory was that (i) binding of plasminogen to fibrin or the cell surface is crucial for its activation to plasmin [44]; (ii) lipoprotein(a), a risk factor for cardiovascular diseases and atherosclerosis [45], competes with plasminogen for binding to fibrin and the cell surface [46]; and (iii) regulated fibrinolysis can occur without bleeding. Although this theory has no solid basis, especially regarding whether a small molecule could mediate protein (plasminogen)-to-protein (fibrin or receptor) binding, we started a pilot project to search for a molecule that enhances the binding of plasminogen to monocytoid cells (screening 1). Fortunately, a screening of random microbial cultures soon yielded several hits, including complestatin and its analog [47][48]. On the basis of these results, we expanded the project to screen for compounds that enhance plasminogen binding to fibrin (screening 2), cell-mediated fibrinolysis in plasma (screening 3), vascular endothelial cell surface generation of plasmin (screening 4), and reciprocal activation of plasminogen and single-chain urokinase-type plasminogen activator (scu-PA or prourokinase) (screening 5). These investigations led to the discovery of novel small molecules such as SMTP family, plactin family, and surfactin family compounds (see Table S1 and references therein). SMTP was discovered in screening 2. Notably, several compounds in these studies act through a unique mechanism, zymogen modulation.
Discovery of SMTP
Screening 2 identified several hits. Of these, an extract of a culture of S. microspora IFO 30018 (current repository code NCBI 30018) showed activity to enhance plasminogen-fibrin binding; however, isolation of the active principle was challenging, as the activity was distributed over a wide range of retention times with a turtle shell-like UV absorption peak, according to HPLC analysis. Initially, we believed that the mobile phase conditions were insufficient to achieve clear resolution; however, we subsequently realized that the broad turtle shell-like peak was due to several overlapping peaks with similar UV spectra. Repeated preparative HPLC fractionations corresponding to a relatively sharp convex shape in the turtle shell-like area yielded the first SMTP congener, staplabin (Table S1) [49]. The name staplabin was derived from Stachybotrys plasminogen-binding stimulator. Following the isolation of staplabin, we isolated two minor analogs, SMTP-1 and SMTP-2 (Table S1) [50]. Additionally, we developed a method to isolate multiple congeners by changing the composition of the culture medium to alter the turtle shell-shaped pattern to a truly peaked pattern. To clearly define each anticipated congener that would follow staplabin, we used a new designation, consisting of SMTP and a number.
SMTP Congeners
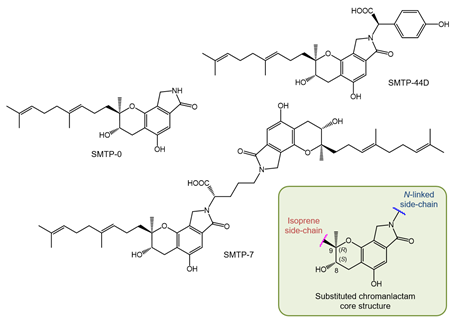
The structure of the SMTP congeners consists of a chromanlactam moiety, an isoprene side chain, and a side chain linked to the nitrogen atom of the chromanlactam moiety (N-linked side chain) (Figure 1). Using the newly devised culture conditions mentioned above, we discovered six additional SMTP congeners, SMTP-3 to SMTP-8 (Table S1) [51][52]. These differed with respect to the N-linked side chains, all of which constituted known a-amino acids. Therefore, we hypothesized that the N-linked side chains originated from amino acids present inside the cell or in medium. To test this possibility, we used a poor medium in which amine compounds were restricted but which contained a specific amine to be incorporated as the N-linked side chain [53]. This precursor amine-feeding method enabled us to produce large amounts (up to 10 g L−1 of culture) of a specific SMTP congener quite selectively. This is a huge achievement, considering that the yield of staplabin was only 24 mg L−1 . Furthermore, selective incorporation of the fed amine into the N-linked side chain was confirmed by the robust incorporation of rare amines such as D-amino acids following feeding [54]. Several SMTP congeners were isolated using a fermentation method that was fed with the precursor amine [55][56][57][58][59] (see Table S1 for details). Furthermore, some analogs differing in the isoprene side chain structure have been identified by microbial conversions of SMTP-0, which has a hydrogen atom as the N-linked side chain (Figure 1) [60].
Figure 1. Structures of key Stachybotrys microspora triprenyl phenol (SMTP) congeners and substituted chromanlactam core unit.
Structure of SMTP
The structures of the SMTP congeners were elucidated using a combination of spectroscopic methods, including NMR and MS. The initial absolute stereochemistry was proposed utilizing NMR techniques using the simplest congener, SMTP-0 (Figure 1), and its derivatives. The results obtained were consistent with the 8S, 9S configuration. However, a recent investigation that utilized a combination of NMR and crystallographic techniques proved an 8S, 9R configuration for an analog of SMTP, stachybotrin C (Table S1) [61][62]. Considering that stachybotrin C can be produced by feeding S. microspora with the precursor amine and that SMTP is produced from a common precursor, pre-SMTP (see Section 2.6), we conclude that all SMTP congeners exhibit an 8S, 9R configuration, and herein revise their stereochemistry (Figure 1).
Biosynthesis of SMTP
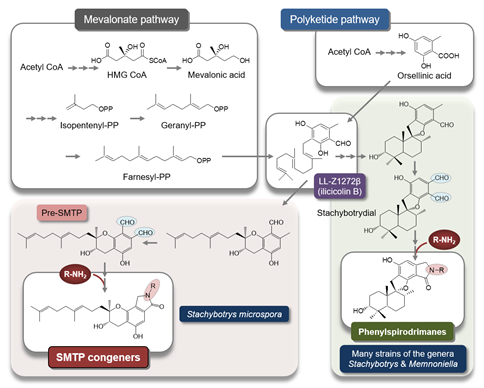
To elucidate the mechanism underlying the generation of a wide variety of SMTP congeners, we searched for a biosynthesis precursor of SMTP. We hypothesized that such a precursor might accumulate and disappear before and after amine feeding, respectively. We isolated two candidates: LL-Z1272b (ilicicolin B) and a novel compound. The latter, designated pre-SMTP, has no lactam but two aldehydes in the chroman moiety (Figure 2) [59]. Pre-SMTP spontaneously reacts with primary amines to yield an SMTP congener with the amine as an N-linked side chain. Thus, various SMTP congeners can be nonenzymatically derived from pre-SMTP. Incorporating relevant information from other studies [63][64][65], we propose an overall pathway for SMTP biosynthesis, as presented in Figure 2.
Figure 2. Putative pathway for SMTP biosynthesis. The synthetic pathway for SMTP-type and phenylspirodrimane-type triprenyl phenols may diverge, through differential cyclization mechanisms, from LL-Z1272b (ilicicolin B), a key intermediate synthesized from farnesyl diphosphate and orsellinic acid. Pre-SMTP forms various SMTPs via a nonenzymatic reaction with an amine. Stachybotrydial [66] may yield a wide variety of phenylspirodrimanes via a similar mechanism, because aromatic o-dialdehydes (shaded in light blue) are highly reactive with an amine [67][68]. The precursor amine feeding method can be applied to selectively synthesize a phenylspirodrimane of interest [69] using Stachybotrys sp. F462, which forms stachybotramide, a phenylspirodrimane-type triprenyl phenol [70].
References
- Versteeg, H.H.; Heemskerk, J.W.M.; Levi, M.; Reitsma, P.H. New Fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358, doi:10.1152/physrev.00016.2011.
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336, doi:10.3109/10409238.2015.1050550.
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24, doi:10.1016/j.blre.2014.09.003.
- Hasumi, K.; Yamamichi, S.; Harada, T. Small-molecule modulators of zymogen activation in the fibrinolytic and coagulation systems: Review Article. FEBS J. 2010, 277, doi:10.1111/j.1742-4658.2010.07783.x.
- Urano, T.; Castellino, F.J.; Suzuki, Y. Regulation of plasminogen activation on cell surfaces and fibrin. J. Thromb. Haemost. 2018, 16, 1487–1497, doi:10.1111/jth.14157.
- Peyvandi, F.; Garagiola, I.; Biguzzi, E. Advances in the treatment of bleeding disorders. J. Thromb. Haemost. 2016, 14, 2095–2106, doi:10.1111/jth.13491
- Kolev, K.; Longstaff, C. Bleeding related to disturbed fibrinolysis. Br. J. Haematol. 2016, 175, 12–23, doi:10.1111/bjh.14255.
- Chang, Y.; Dabiri, G.; Damstetter, E.; Baiyee Ebot, E.; Powers, J.G.; Phillips, T. Coagulation disorders and their cutaneous presentations: Pathophysiology. J. Am. Acad. Dermatol. 2016, 74, 783–792, doi:10.1016/j.jaad.2015.08.072.
- Hoffman, M.; Pawlinski, R. Hemostasis: Old system, new players, new directions. Thromb. Res. 2014, 133, doi:10.1016/j.thromres.2014.03.001.
- Mega, J.L.; Simon, T. Pharmacology of antithrombotic drugs: An assessment of oral antiplatelet and anticoagulant treatments. Lancet 2015, 386, 281–291, doi:10.1016/S0140-6736(15)60243-4.
- McFadyen, J.D.; Peter, K. Novel Antithrombotic Drugs on the Horizon. Circ. Res. 2017, 121, 1133–1135, doi:10.1161/circresaha.117.312012.
- Bivard, A.; Lin, L.; Parsonsb, M.W. Review of Stroke Thrombolytics. J. Stroke 2013, 15, 90, doi:10.5853/jos.2013.15.2.90.
- Martin, C.; Sobolewski, K.; Bridgeman, P.; Boutsikaris, D. Systemic thrombolysis for pulmonary embolism: A review. P T 2016, 41, 770–775.
- Ibrahim, H.; Rondina, M.; Welt, F.G.P. Antithrombotic drugs in cardiovascular medicine: A year in review. Curr. Opin. Cardiol. 2018, 33, 369–374, doi:10.1097/HCO.0000000000000530.
- Goldstein, J.N.; Marrero, M.; Masrur, S.; Pervez, M.; Barrocas, A.M.; Abdullah, A.; Oleinik, A.; Rosand, J.; Smith, E.E.; Dzik, W.H.; et al. Management of thrombolysis-associated symptomatic intracerebral hemorrhage. Arch. Neurol. 2010, 67, 965–969, doi:10.1001/archneurol.2010.175.
- Piran, S.; Schulman, S. Treatment of bleeding complications in patients on anticoagulant therapy. Blood 2019, 133, 425–435, doi:10.1182/blood-2018-06-820746.
- De Andrade, N.K.; Motta, R.H.L.; Bergamaschi, C.D.C.; Oliveira, L.B.; Guimarães, C.C.; Araújo, J.D.O.; Lopes, L.C. Bleeding risk in patients using oral anticoagulants undergoing surgical procedures in dentistry: A systematic review and meta-analysis. Front. Pharmacol. 2019, 10, doi:10.3389/fphar.2019.00866.
- Macman, N. Triggers, targets and treatments for thrombosis. Nature 2008, 451, 914–918, doi:10.1038/nature06797.
- Hu, W.; Narasaki, R.; Nishimura, N.; Hasumi, K. SMTP (Stachybotrys microspora triprenyl phenol) enhances clot clearance in a pulmonary embolism model in rats. Thromb. J. 2012, 10, doi:10.1186/1477-9560-10-2.
- Matsumoto, N.; Suzuki, E.; Tsujihara, K.; Nishimura, Y.; Hasumi, K. Structure-activity relationships of the plasminogen modulator SMTP with respect to the inhibition of soluble epoxide hydrolase. J. Antibiot. (Tokyo). 2015, 68, 685–690, doi:10.1038/ja.2015.58.
- Shibata, K.; Hashimoto, T.; Nobe, K.; Hasumi, K.; Honda, K. A novel finding of a low-molecular-weight compound, SMTP-7, having thrombolytic and anti-inflammatory effects in cerebral infarction of mice. Naunyn. Schmiedebergs. Arch. Pharmacol. 2010, 382, doi:10.1007/s00210-010-0542-5.
- Sawada, H.; Nishimura, N.; Suzuki, E.; Zhuang, J.; Hasegawa, K.; Takamatsu, H.; Honda, K.; Hasumi, K. SMTP-7, a novel small-molecule thrombolytic for ischemic stroke: A study in rodents and primates. J. Cereb. Blood Flow Metab. 2014, 34, doi:10.1038/jcbfm.2013.191.
- Ito, A.; Niizuma, K.; Shimizu, H.; Fujimura, M.; Hasumi, K.; Tominaga, T. SMTP-7, a new thrombolytic agent, decreases hemorrhagic transformation after transient middle cerebral artery occlusion under warfarin anticoagulation in mice. Brain Res. 2014, 1578, doi:10.1016/j.brainres.2014.07.004.
- Huang, Y.; Ohta, Y.; Shang, J.; Li, X.; Liu, X.; Shi, X.; Feng, T.; Yamashita, T.; Sato, K.; Takemoto, M.; et al. Reduction of Ischemia Reperfusion-Related Brain Hemorrhage by Stachybotrys Microspora Triprenyl Phenol-7 in Mice With Antioxidant Effects. J. Stroke Cerebrovasc. Dis. 2018, 27, doi:10.1016/j.jstrokecerebrovasdis.2018.08.018.
- Matsumoto, N.; Suzuki, E.; Ishikawa, M.; Shirafuji, T.; Hasumi, K. Soluble epoxide hydrolase as an anti-inflammatory target of the thrombolytic stroke drug SMTP-7. J. Biol. Chem. 2014, 289, 35826–35838, doi:10.1074/jbc.M114.588087.
- Hashimoto, T.; Shibata, K.; Nobe, K.; Hasumi, K.; Honda, K. A novel embolic model of cerebral infarction and evaluation of Stachybotrys microspora triprenyl phenol-7 (SMTP-7), a novel fungal triprenyl phenol metabolite. J. Pharmacol. Sci. 2010, 114, doi:10.1254/jphs.10131FP.
- Akamatsu, Y.; Saito, A.; Fujimura, M.; Shimizu, H.; Mekawy, M.; Hasumi, K.; Tominaga, T. Stachybotrys microspora triprenyl phenol-7, a novel fibrinolytic agent, suppresses superoxide production, matrix metalloproteinase-9 expression, and thereby attenuates ischemia/reperfusion injury in rat brain. Neurosci. Lett. 2011, 503, doi:10.1016/j.neulet.2011.08.018.
- Suzuki, E.; Nishimura, N.; Yoshikawa, T.; Kunikiyo, Y.; Hasegawa, K.; Hasumi, K. Efficacy of SMTP-7, a small-molecule anti-inflammatory thrombolytic, in embolic stroke in monkeys. Pharmacol. Res. Perspect. 2018, 6, doi:10.1002/prp2.448.
- Shibata, K.; Hashimoto, T.; Hasumi, K.; Honda, K.; Nobe, K. Evaluation of the effects of a new series of SMTPs in the acetic acid-induced embolic cerebral infarct mouse model. Eur. J. Pharmacol. 2018, 818, doi:10.1016/j.ejphar.2017.10.055.
- Shi, X.; Ohta, Y.; Shang, J.; Morihara, R.; Nakano, Y.; Fukui, Y.; Liu, X.; Feng, T.; Huang, Y.; Sato, K.; et al. Neuroprotective effects of SMTP-44D in mice stroke model in relation to neurovascular unit and trophic coupling. J. Neurosci. Res. 2018, 96, doi:10.1002/jnr.24326.
- Cushman, M. Epidemiology and Risk Factors for Venous Thrombosis. Semin. Hematol. 2007, 44, 62–69, doi:10.1053/j.seminhematol.2007.02.004.
- Previtali, E.; Bucciarelli, P.; Passamonti, S.M.; Martinelli, I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011, 9, 120–138, doi:10.2450/2010.0066-10.
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632, doi:10.1111/joim.12296.
- Otsuka, F.; Yasuda, S.; Noguchi, T.; Ishibashi-Ueda, H. Pathology of coronary atherosclerosis and thrombosis. Cardiovasc. Diagn. Ther. 2016, 6, 396–408, doi:10.21037/cdt.2016.06.01.
- Rijken, D.C.; Lijnen, H.R. New insights into the molecular mechanisms of the fibrinolytic system. J. Thromb. Haemost. 2009, 7, 4–13, doi:10.1111/j.1538-7836.2008.03220.x.
- Majithia, A.; Bhatt, D.L. Novel Antiplatelet Therapies for Atherothrombotic Diseases. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 546–557, doi:10.1161/ATVBAHA.118.310955.
- Nakamura, M.; Yamada, N.; Ito, M. Novel Anticoagulant Therapy of Venous Thromboembolism: Current Status and Future Directions. Ann. Vasc. Dis. 2017, 10, 92–98, doi:10.3400/avd.ra.17-00015.
- 38. Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N. Engl. J. Med. 2017, 377, 1319–1330.
- 39. Steffel, J.; Eikelboom, J.W.; Anand, S.S.; Shestakovska, O.; Yusuf, S.; Fox, K.A.A. The COMPASS Trial. Circulation 2020, 142, 40–48, doi:10.1161/circulationaha.120.046048.
- Miller, D.J.; Simpson, J.R.; Silver, B.; Silver, B. Safety of Thrombolysis in Acute Ischemic Stroke: A Review of Complications, Risk Factors, and Newer Technologies. The Neurohospitalist 2011, 1, 138–147, doi:10.1177/1941875211408731.
- Demaerschalk, B.M.; Cheng, N.T.; Kim, A.S. Intravenous Thrombolysis for Acute Ischemic Stroke Within 3 Hours Versus Between 3 and 4.5 Hours of Symptom Onset. The Neurohospitalist 2015, 5, 101–109, doi:10.1177/1941874415583116.
- Endo, A. A historical perspective on the discovery of statins. Proc. Japan Acad. Ser. B Phys. Biol. Sci. 2010, 86, 484–493, doi:10.2183/pjab.86.484.
- Hajar, R. Statins: Past and Present. Hear. Views 2011, 12, 121, doi:10.4103/1995-705x.95070.
- Anglés-Cano, E. Overview on fibrinolysis: Plasminogen activation pathways on fibrin and cell surfaces. Chem. Phys. Lipids 1994, 67–68, 353–362, doi:10.1016/0009-3084(94)90157-0.
- McLean, J.W.; Tomlinson, J.E.; Kuang, W.J.; Eaton, D.L.; Chen, E.Y.; Fless, G.M.; Scanu, A.M.; Lawn, R.M. cDNA sequence of human apolipoprotein(a) is homologous to plasminogen. Nature 1987, 330, 132–137, doi:10.1038/330132a0.
- Hajjar, K.A.; Gavishi, D.; Breslow, J.L.; Nachman, R.L. Lipoprotein(a) modulation of endothelial cell surface fibrinolysis and its potential role in atherosclerosis. Nature 1989, 339, 303–305, doi:10.1038/339303a0.
- Tachikawa, K.; Hasumi, K.; Endo, A. Enhancement of plasminogen binding to U937 cells and fibrin by complestatin. Thromb. Haemost. 1997, 77.
- Tachikawa, K.; Hasumi, K.; Endo, A. Enhancement of plasminogen binding and fibrinolysis by chloropeptin I. Thromb. Res. 1997, 87, doi:10.1016/S0049-3848(97)00186-2.
- Shinohara, C.; Hasumi, K.; Hatsumi, W.; Endo, A. Staplabin, a novel fungal triprenyl phenol which stimulates the binding of plasminogen to fibrin and U937 cells. J. Antibiot. (Tokyo). 1996, 49, doi:10.7164/antibiotics.49.961.
- Kohyama, T.; Hasumi, K.; Hamanaka, A.; Endo, A. SMTP-1 and -2, novel analogs of staplabin produced by Stachybotrys microspora IFO30018. J. Antibiot. (Tokyo). 1997, 50, doi:10.7164/antibiotics.50.172.
- Hasumi, K.; Ohyama, S.; Kohyama, T.; Ohsaki, Y.; Takayasu, R.; Endo, A. Isolation of SMTP-3, 4, 5 and -6, novel analogs of staplabin, and their effects on plasminogen activation and fibrinolysis. J. Antibiot. (Tokyo). 1998, 51, doi:10.7164/antibiotics.51.1059.
- Hu, W.; Ohyama, S.; Hasumi, K. Activation of fibrinolysis by SMTP-7 and -8, novel staplabin analogs with a pseudosymmetric structure. J. Antibiot. (Tokyo). 2000, 53, doi:10.7164/antibiotics.53.241.
- Hu, W.; Narasaki, R.; Ohyama, S.; Hasumi, K. Selective production of staplabin and SMTPs in cultures of Stachybotrys microspora Fed with precursor amines. J. Antibiot. (Tokyo). 2001, 54, doi:10.7164/antibiotics.54.962.
- Hu, W.; Kitano, Y.; Hasumi, K. SMTP-4D, -5D, -7D and -8D, a new series of the non-lysine-analog plasminogen modulators with a D-amino acid moiety. J. Antibiot. (Tokyo). 2003, 56, doi:10.7164/antibiotics.56.832.
- Hasumi, K.; Hasegawa, K.; Kitano, Y. Isolation and absolute configuration of SMTP-0, a simplest congener of the SMTP family nonlysine-analog plasminogen modulators. J. Antibiot. (Tokyo). 2007, 60, doi:10.1038/ja.2007.60.
- Hasegawa, K.; Koide, H.; Hu, W.; Nishimura, N.; Narasaki, R.; Kitano, Y.; Hasumi, K. Structure-activity relationships of 11 new congeners of the SMTP plasminogen modulator. J. Antibiot. (Tokyo). 2010, 63, doi:10.1038/ja.2010.101.
- Koide, H.; Hasegawa, K.; Nishimura, N.; Narasaki, R.; Hasumi, K. A new series of the SMTP plasminogen modulators with a phenylamine-based side chain. J. Antibiot. (Tokyo). 2012, 65, doi:10.1038/ja.2012.29.
- Koide, H.; Narasaki, R.; Hasegawa, K.; Nishimura, N.; Hasumi, K. A new series of the SMTP plasminogen modulator with a phenylglycine-based side chain. J. Antibiot. (Tokyo). 2012, 65, doi:10.1038/ja.2011.108.
- Nishimura, Y.; Suzuki, E.; Hasegawa, K.; Nishimura, N.; Kitano, Y.; Hasumi, K. Pre-SMTP, a key precursor for the biosynthesis of the SMTP plasminogen modulators. J. Antibiot. (Tokyo). 2012, 65, doi:10.1038/ja.2012.47.
- Otake, S.; Ogawa, N.; Kitano, Y.; Hasumi, K.; Suzuki, E. Isoprene side-chain of SMTP is essential for soluble epoxide hydrolase inhibition and cellular localization. Nat. Prod. Commun. 2016, 11.
- Jacolot, M.; Jean, M.; Tumma, N.; Bondon, A.; Chandrasekhar, S.; Van De Weghe, P. Synthesis of stachybotrin C and all of its stereoisomers: Structure revision. J. Org. Chem. 2013, 78, 7169–7175, doi:10.1021/jo401116r.
- Kuroda, Y.; Hasegawa, K.; Noguchi, K.; Chiba, K.; Hasumi, K.; Kitano, Y. Confirmation of the absolute configuration of Stachybotrin C using single-crystal X-ray diffraction analysis of its 4-bromobenzyl ether derivative. J. Antibiot. (Tokyo). 2018, 71, doi:10.1038/s41429-018-0042-2.
- Li, C.; Matsuda, Y.; Gao, H.; Hu, D.; Yao, X.S.; Abe, I. Biosynthesis of LL-Z1272β: Discovery of a New Member of NRPS-like Enzymes for Aryl-Aldehyde Formation. ChemBioChem 2016, 17, 904–907, doi:10.1002/cbic.201600087.
- Semeiks, J.; Borek, D.; Otwinowski, Z.; Grishin, N. V. Comparative genome sequencing reveals chemotype-specific gene clusters in the toxigenic black mold Stachybotrys. BMC Genomics 2014, 15, doi:10.1186/1471-2164-15-590.
- Yin, Y.; Fu, Q.; Wu, W.; Cai, M.; Zhou, X.; Zhang, Y. Producing novel fibrinolytic isoindolinone derivatives in marine fungus Stachybotrys longispora FG216 by the rational supply of amino compounds according to its biosynthesis pathway. Mar. Drugs 2017, 15, doi:10.3390/md15070214.
- Ayer, W.A.; Miao, S. Secondary metabolites of the aspen fungus Stachybotrys cylindrospora. Can. J. Chem. 1993, 71, 487–493, doi:10.1139/v93-069.
- Alajarín, M.; Sánchez-Andrada, P.; López-Leonardo, C.; Álvarez, Á. On the mechanism of phthalimidine formation via o-phthalaldehyde monoimines. New [1,5]-H sigmatropic rearrangements in molecules with the 5-aza-2,4-pentadienal skeleton. J. Org. Chem. 2005, 70, 7617–7623, doi:10.1021/jo0508494.
- Gyimesi-Forrás, K.; Leitner, A.; Akasaka, K.; Lindner, W. Comparative study on the use of ortho-phthalaldehyde, naphthalene-2,3- dicarboxaldehyde and anthracene-2,3-dicarboxaldehyde reagents for α-amino acids followed by the enantiomer separation of the formed isoindolin-1-one derivatives using quinine-type chir. J. Chromatogr. A 2005, 1083, 80–88, doi:10.1016/j.chroma.2005.06.012.
- Kawano, Y. Development of a novel PAI-1 inhibitor using beta-SMTP, Master’s thesis, Tokyo University of Agriculture and Technology, Tokyo, Japan, March 2010.
- Takahashi, F.; Hasumi, K.; Endo, A. Modulation of the plasma cholesteryl ester transfer by stachybotramide. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1995, 1258, 70–74, doi:10.1016/0005-2760(95)00102-I.