
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wang-Tso Lee | + 1357 word(s) | 1357 | 2021-01-20 10:21:45 | | | |
| 2 | Catherine Yang | Meta information modification | 1357 | 2021-01-27 04:46:01 | | |
Video Upload Options
Limbic encephalitis (LE) is a rare cause of encephalitis presenting as an acute and subacute onset of neuropsychiatric manifestations, particularly with memory deficits and confusion as core features, along with seizure occurrence, movement disorders, or autonomic dysfunctions.
1. Introduction
Limbic encephalitis (LE) is an inflammatory encephalitis involving the limbic system, which encompasses the medial temporal lobe, hippocampus, and frontobasal and cingulate cortex [1]. LE is frequently associated with antibodies against the neuronal cell surface, synaptic vesicles, and intracellular proteins; therefore, it belongs to the autoimmune encephalitis (AE) category. Triggering factors include tumors, viral infections, or immune check point inhibitors (ICI’s) [2][3]. LE most often occurs in adults older than 45 years but can affect people of all ages. In addition, gender predominance varies with the type of antibody.
The clinical hallmark of LE includes acute and subacute onset of memory and cognitive deficits. Other symptoms include confusion, psychiatric symptoms (such as anxiety, depression, or psychosis), behavioral changes, seizures, movement disorders (such as ataxia, dystonia, or myoclonus), autonomic disturbances, and sleep disturbances [1][4]. The amygdala is a core region of the limbic system involved in the control of positive and negative affect, modulation of memory, and social and cognitive functions as well as behavioral adaptation to stress [5], which explains the core neuropsychiatric manifestations of LE. The basolateral complex of the amygdala is the main input site for sensory information from the thalamus and cortical regions, which plays a central role in seizure generation, particularly in the temporal lobe epilepsy [6].
2. Antibodies
Antibodies associated with LE are thought to be of peripheral origin that penetrates a leaky blood–brain barrier (BBB) or to be synthesized intrathecally. Most AE has higher serum than CSF antibody levels, implicating that antibodies are likely to be initiated by a peripheral immune response [7]. The autoantibodies are predominantly of IgG1 subclass. However, LGI1 and CASPR2 antibodies are mainly IgG4 subclass [8][9]. IgG4 antibody is hetero-bispecific (continuously undergoing half antibody exchange), hence less effective than IgG1 in crosslinking and internalizing the target antigen and does not fix complement [10]. In general, IgG1-associated AE (e.g., N-methyl-D-asparate receptor [NMDAR], γ-aminobutyric acid-B receptor [GABA-BR], and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor [AMPAR]) have more inflammatory signs than IgG4-associated AE (e.g., LGI1 and CASPR2) [11]. Nevertheless, complement-mediated neuronal loss was still observed in LGI1 and CASPR2-associated neurologic syndromes [12][13]. These patients had higher complement-fixing IgG1 subclass with more prolonged clinical course, more severe cognitive impairment, and frequent hippocampal atrophy in advanced stages of the disease [8][14].
Antibodies associated with LE target: (1) cell-surface receptors (GABA, AMPA, and glycine receptors); (2) ion channels (voltage-gated potassium channels [VGKC]); (3) neighboring proteins that stabilize channel complex into the membrane (LGI1 and CASPR2); (4) enzymes that catalyze the formation of neurotransmitters glutamic acid decarboxylase (GAD); and (5) intracellular proteins (Hu, Ma2, collapsin response-mediator protein-5 [CRMP5], and amphiphysin) [15]. These antibodies are classified as neuronal cell surface, synaptic vesicle, and intracellular antibodies according to the location of their targeting antigens. Antibodies against intracellular antigens are also known as onconeural antibodies because of their large association with tumors, and cause paraneoplastic syndromes (PNS) [16]. Pathologic effects include direct blockage of receptors and ion channels, indirect disruption with neighboring molecules interaction, and crosslinking and internalization of receptors to deplete them from the cell surface (Figure 1).
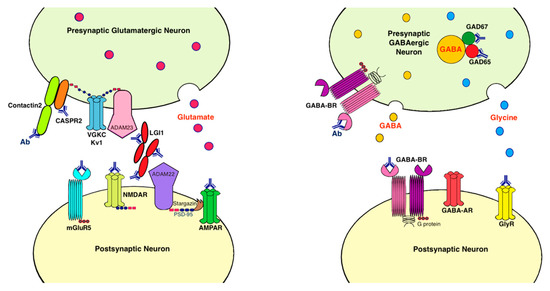
Figure 1. Antibodies and their pathogenic effects in limbic encephalitis. The pathologic effects of antibodies include blocking of receptors or ion channels, disruption of interaction with neighboring molecules, and crosslinking and internalization of receptors from cell surface. (1) CASPR2 and contactin2 antibodies inhibit the interaction between these proteins and reduce the clustering and surface expression of VGKC; (2) LGI1 antibody disrupts interaction between protein components such as LGI1 to ADAM22/23, downregulates VGKC and reduces AMPAR clustering and synaptic transmission; (3) AMPAR antibody causes cross-linking and receptor internalization; (4) NMDAR* antibody causes cross-linking and receptor internalization; (5) mGluR5 antibody causes decreased synaptic mGluR5 cluster density; (6) GAD antibodies target mostly the GAD65 subunits but also the GAD67 subunits, disrupting GABAergic signaling; (7) GABA-BR antibody prevents ligand binding to the receptor and alters receptor function; (8) GlyR antibody probably acts as antagonist to disrupt receptor function. Ab: antibody; ADAM: a disintegrin and metalloproteinase; AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor; CASPR2: contactin associated protein-like 2; GABA-AR: γ-aminobutyric acid-A receptor; GABA-BR: γ-aminobutyric acid-B receptor, GAD: glutamic acid decarboxylase; GlyR: glycine receptor; LGI1: leucine-rich, glioma inactivated 1; mGluR5: metabotropic glutamate receptor 5; NMDAR: N-methyl-D-asparate receptor; PSD-95: postsynaptic density protein 95; VGKC: voltage-gated potassium channels.
3. Immunopathology
LE with antibodies against cell-surface antigens act mainly through antibody and/or complement-mediated mechanisms. However, LE with antibodies against intracellular antigens is predominantly mediated by cluster of differentiation (CD) 8+ cytotoxic T cells with frequent neuronal loss [12][17]. T cells are the major immune cells in the CSF, which play a major immunosurveillance role in the central nervous system (CNS) [18]. In all AE cases, CD3+ lymphocytes comprised the majority of inflammatory cells within the brain. A significant difference in the proportion of parenchymal CD8+ T cells (CD8/CD3 ratio) between intracellular and surface antibodies (mean 75% vs. 43%, respectively) was noted. The percentage of CD8+ T cells in the GAD antibody-mediated disease is intermediate (54%) [12]. The CD8+ T cells cause impairment of neuronal excitability and integrity with neuronal degeneration via two independent pathways: (1) granule cytotoxicity by perforin and granzyme-B and (2) ligation of death receptors [19][20]. Perforin is a Ca2+-dependent protein, which can form transmembranous pores, alter electrical excitability and signaling, and cause neuronal necrosis with swelling and rupture of cell membranes. Perforin mediates the delivery of granzymes into the target cells to promote apoptosis [21]. Apposition of multiple granzyme-B lymphocytes to single neurons, which is consistent with a specific cytotoxic T-cell attack, was observed in several onconeural antibody-mediated diseases. Therefore, higher CD8/CD3 ratio and more frequent appositions of granzyme-B+ cytotoxic T cells to neurons were associated with greater neuronal loss [12]. Cytotoxic T cells can also liberate neurotoxic cytokines, such as interferon-γ (IFN- γ) and tumor necrosis factor-α as well as excitotoxic glutamate [22]. CD8+ T cells also destroy the myelin sheath or glial cells in both white and gray matter [19].
In addition to antibodies and lymphocytes, microglia and astrocytes also play roles in mediating neuronal damages in the hippocampus and other brain regions. Microglia and astrocytes are required for synaptic integrity structurally and synaptic transmission functionally [23]. Activated microglia have dual functions, either anti-inflammatory or pro-inflammatory [24]. Previous studies showed that activated microglia in hippocampus may be associated with chronic epilepsy and was also found in VGKC-associated LE [25][26][27]. Immunotherapy may decrease the activation of microglia leading to improvement in seizure. Astrocytes and their autoimmune glial fibrillary acidic protein (GFAP) have also been shown to be associated with AE including NMDAR encephalitis and other encephalitis mimicking LE [28][29], and play important roles in neuroinflammation. The activation of astrocytes may trigger an astrocyte-neuron signaling cascade leading to persistent functional change in hippocampal excitatory synapses [30], which may be associated with cognitive impairment.
4. Inflammatory Mediators
Conventional CSF inflammatory markers such as white blood cells or protein are neither sensitive nor specific for LE, although the detection of oligoclonal band may increase diagnostic sensitivity [31]. A lower CD4/CD8+ T-cell ratio is detected in the CSF of all patients with LE [32].
The serum and CSF levels of C-X-C motif chemokine ligand 13 (CXCL13) were significantly higher in patients with LGI1 encephalitis [33]. The serum levels of CXCL10 were elevated in CASPR2 encephalitis. CXCL10 is a cytokine that recruits C-X-C motif chemokine receptor 3 cells such as activated T cells [34]. CASPR2 encephalitis seems to elicit a higher immune response than LGI1 encephalitis, with more intrathecal IgG and higher cytokine levels, indicated by higher CXCL13 and soluble intercellular adhesion molecule-1 (sICAM1) in the CSF. CXCL13 points to a B-cell mediated, whereas sICAM1 to a T-cell mediated neuroinflammation [35]. Patients with PNS also had high levels of chemokine CXCL10 in the CSF with the presence of IFN-γ receptors on their T cells [34].
CSF mediators are different in infectious and immune-mediated encephalitis [36]. CSF cytokines IL-21 and IFN-γ-induced protein 10 kDa (IP10) are promising in differentiating between viral encephalitis and AE [37]. IL-21 causes autoantibody production, which downregulates regulatory T cells leading to enhanced autoimmunity and increased CD8+ T cells and NK cells in AE [38], whereas IP10/CXCL10 is secreted in response to IFN-γ, which is produced as part of the Th1 in response to viral infection [39].
References
- Graus, F.; Titulaer, M.J.; Balu, R.; Benseler, S.; Bien, C.G.; Cellucci, T.; Cortese, I.; Dale, R.C.; Gelfand, J.M.; Geschwind, M.; et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016, 15, 391–404.
- Linnoila, J.J.; Binnicker, M.J.; Majed, M.; Klein, C.J.; McKeon, A. CSF herpes virus and autoantibody profiles in the evaluation of encephalitis. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e245.
- Graus, F.; Dalmau, J. Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2019, 16, 535–548.
- Budhram, A.; Leung, A.; Nicolle, M.W.; Burneo, J.G. Diagnosing autoimmune limbic encephalitis. CMAJ 2019, 191, E529–E534.
- Pape, H.C.; Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 2010, 90, 419–463.
- Aroniadou-Anderjaska, V.; Fritsch, B.; Qashu, F.; Braga, M.F. Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res. 2008, 78, 102–116.
- Giannoccaro, M.P.; Menassa, D.A.; Jacobson, L.; Coutinho, E.; Prota, G.; Lang, B.; Leite, M.I.; Cerundolo, V.; Liguori, R.; Vincent, A. Behaviour and neuropathology in mice injected with human contactin-associated protein 2 antibodies. Brain 2019, 142, 2000–2012.
- Thompson, J.; Bi, M.; Murchison, A.G.; Makuch, M.; Bien, C.G.; Chu, K.; Farooque, P.; Gelfand, J.M.; Geschwind, M.D.; Hirsch, L.J.; et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 2018, 141, 348–356.
- Joubert, B.; Saint-Martin, M.; Noraz, N.; Picard, G.; Rogemond, V.; Ducray, F.; Desestret, V.; Psimaras, D.; Delattre, J.Y.; Antoine, J.C.; et al. Characterization of a Subtype of Autoimmune Encephalitis with Anti-Contactin-Associated Protein-like 2 Antibodies in the Cerebrospinal Fluid, Prominent Limbic Symptoms, and Seizures. JAMA Neurol. 2016, 73, 1115–1124.
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520.
- Zuliani, L.; Nosadini, M.; Gastaldi, M.; Spatola, M.; Iorio, R.; Zoccarato, M.; Mariotto, S.; De Gaspari, P.; Perini, F.; Ferrari, S.; et al. Management of antibody-mediated autoimmune encephalitis in adults and children: Literature review and consensus-based practical recommendations. Neurol. Sci. 2019, 40, 2017–2030.
- Bien, C.G.; Vincent, A.; Barnett, M.H.; Becker, A.J.; Blumcke, I.; Graus, F.; Jellinger, K.A.; Reuss, D.E.; Ribalta, T.; Schlegel, J.; et al. Immunopathology of autoantibody-associated encephalitides: Clues for pathogenesis. Brain 2012, 135, 1622–1638.
- Bauer, J.; Bien, C.G. Neuropathology of autoimmune encephalitides. Handb. Clin. Neurol. 2016, 133, 107–120.
- van Sonderen, A.; Petit-Pedrol, M.; Dalmau, J.; Titulaer, M.J. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat. Rev. Neurol. 2017, 13, 290–301.
- Alexopoulos, H.; Dalakas, M.C. The immunobiology of autoimmune encephalitides. J. Autoimmun. 2019, 104, 102339.
- Lancaster, E.; Dalmau, J. Neuronal autoantigens—Pathogenesis, associated disorders and antibody testing. Nat. Rev. Neurol. 2012, 8, 380–390.
- Dalmau, J.; Geis, C.; Graus, F. Autoantibodies to Synaptic Receptors and Neuronal Cell Surface Proteins in Autoimmune Diseases of the Central Nervous System. Physiol. Rev. 2017, 97, 839–887.
- Pilli, D.; Zou, A.; Tea, F.; Dale, R.C.; Brilot, F. Expanding Role of T Cells in Human Autoimmune Diseases of the Central Nervous System. Front. Immunol. 2017, 8, 652.
- Melzer, N.; Meuth, S.G.; Wiendl, H. CD8+ T cells and neuronal damage: Direct and collateral mechanisms of cytotoxicity and impaired electrical excitability. FASEB J. 2009, 23, 3659–3673.
- Ehling, P.; Melzer, N.; Budde, T.; Meuth, S.G. CD8(+) T Cell-Mediated Neuronal Dysfunction and Degeneration in Limbic Encephalitis. Front. Neurol. 2015, 6, 163.
- Waterhouse, N.J.; Sutton, V.R.; Sedelies, K.A.; Ciccone, A.; Jenkins, M.; Turner, S.J.; Bird, P.I.; Trapani, J.A. Cytotoxic T lymphocyte-induced killing in the absence of granzymes A and B is unique and distinct from both apoptosis and perforin-dependent lysis. J. Cell Biol. 2006, 173, 133–144.
- Kreutzfeldt, M.; Bergthaler, A.; Fernandez, M.; Bruck, W.; Steinbach, K.; Vorm, M.; Coras, R.; Blumcke, I.; Bonilla, W.V.; Fleige, A.; et al. Neuroprotective intervention by interferon-gamma blockade prevents CD8+ T cell-mediated dendrite and synapse loss. J. Exp. Med. 2013, 210, 2087–2103.
- Chung, W.S.; Welsh, C.A.; Barres, B.A.; Stevens, B. Do glia drive synaptic and cognitive impairment in disease? Nat. Neurosci. 2015, 18, 1539–1545.
- Wesselingh, R.; Butzkueven, H.; Buzzard, K.; Tarlinton, D.; O’Brien, T.J.; Monif, M. Innate Immunity in the Central Nervous System: A Missing Piece of the Autoimmune Encephalitis Puzzle? Front. Immunol. 2019, 10, 2066.
- Khan, N.L.; Jeffree, M.A.; Good, C.; Macleod, W.; Al-Sarraj, S. Histopathology of VGKC antibody-associated limbic encephalitis. Neurology 2009, 72, 1703–1705.
- Amhaoul, H.; Hamaide, J.; Bertoglio, D.; Reichel, S.N.; Verhaeghe, J.; Geerts, E.; Van Dam, D.; De Deyn, P.P.; Kumar-Singh, S.; Katsifis, A.; et al. Brain inflammation in a chronic epilepsy model: Evolving pattern of the translocator protein during epileptogenesis. Neurobiol. Dis. 2015, 82, 526–539.
- Yang, M.T.; Lin, Y.C.; Ho, W.H.; Liu, C.L.; Lee, W.T. Everolimus is better than rapamycin in attenuating neuroinflammation in kainic acid-induced seizures. J. Neuroinflamm. 2017, 14, 15.
- Tomczak, A.; Su, E.; Tugizova, M.; Carlson, A.M.; Kipp, L.B.; Feng, H.; Han, M.H. A case of GFAP-astroglial autoimmunity presenting with reversible parkinsonism. Mult. Scler. Relat. Disord. 2019, 39, 101900.
- Ismail, F.S.; Faustmann, P.M. Astrocytes and their potential role in anti-NMDA receptor encephalitis. Med. Hypotheses 2020, 139, 109612.
- Habbas, S.; Santello, M.; Becker, D.; Stubbe, H.; Zappia, G.; Liaudet, N.; Klaus, F.R.; Kollias, G.; Fontana, A.; Pryce, C.R.; et al. Neuroinflammatory TNFalpha Impairs Memory via Astrocyte Signaling. Cell 2015, 163, 1730–1741.
- Hebert, J.; Gros, P.; Lapointe, S.; Amtashar, F.S.; Steriade, C.; Maurice, C.; Wennberg, R.A.; Day, G.S.; Tang-Wai, D.F. Searching for autoimmune encephalitis: Beware of normal CSF. J. Neuroimmunol. 2020, 345, 577285.
- Hansen, N.; Schwing, K.; Onder, D.; Widman, G.; Leelaarporn, P.; Prusseit, I.; Surges, R.; Melzer, N.; Gross, C.; Becker, A.J.; et al. Low CSF CD4/CD8+ T-cell proportions are associated with blood-CSF barrier dysfunction in limbic encephalitis. Epilepsy Behav. 2020, 102, 106682.
- Lin, Y.T.; Yang, X.; Lv, J.W.; Liu, X.W.; Wang, S.J. CXCL13 Is A Biomarker of Anti-Leucine-Rich Glioma-Inactivated Protein 1 Encephalitis Patients. Neuropsychiatr. Dis. Treat. 2019, 15, 2909–2915.
- Roberts, W.K.; Blachere, N.E.; Frank, M.O.; Dousmanis, A.; Ransohoff, R.M.; Darnell, R.B. A destructive feedback loop mediated by CXCL10 in central nervous system inflammatory disease. Ann. Neurol. 2015, 78, 619–629.
- Kortvelyessy, P.; Goihl, A.; Guttek, K.; Schraven, B.; Pruss, H.; Reinhold, D. Serum and CSF cytokine levels mirror different neuroimmunological mechanisms in patients with LGI1 and Caspr2 encephalitis. Cytokine 2020, 135, 155226.
- Michael, B.D.; Griffiths, M.J.; Granerod, J.; Brown, D.; Davies, N.W.; Borrow, R.; Solomon, T. Characteristic Cytokine and Chemokine Profiles in Encephalitis of Infectious, Immune-Mediated, and Unknown Aetiology. PLoS ONE 2016, 11, e0146288.
- Jiang, J.X.; Fewings, N.; Dervish, S.; Fois, A.F.; Duma, S.R.; Silsby, M.; Bandodkar, S.; Ramanathan, S.; Bleasel, A.; John, B.; et al. Novel Surrogate Markers of CNS Inflammation in CSF in the Diagnosis of Autoimmune Encephalitis. Front. Neurol. 2019, 10, 1390.
- Spolski, R.; Leonard, W.J. Interleukin-21: A double-edged sword with therapeutic potential. Nat. Rev. Drug Discov. 2014, 13, 379–395.
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011, 22, 121–130.




