| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Loredana Maiuolo | + 5201 word(s) | 5201 | 2021-01-19 07:42:25 | | | |
| 2 | Rita Xu | -1327 word(s) | 3874 | 2021-01-25 08:43:25 | | |
Video Upload Options
Bacterial nanocellulose (BNC) is a biomaterial with a growing interest in the field of nanocomposites and sustainable materials. It is produced through fermentative processes by several species of bacteria as extracellular secretion. BNC looks like a highly pure and flexible 3D porous network suitable for various applications including biodegradable materials, reinforcing agents, packaging films, transpiring membranes, and medical devices. Among the many applications, the use of BNC functionalized with organic and inorganic groups has found wide use as a catalyst in chemical transformations.
1. Introduction
Cellulose is the main component of lignocellulosic biomasses, with an average percentage of 40–50 of the total weight. It is the most abundant polysaccharide on Earth, with an estimated annual production of 7.5 × 1010 tons [1][2]. From a chemical point of view, it is composed of β-D-glucopyranoses (ca. 5000 and 10,000 units), linked by β-1,4-glycosidic bonds. β-Glycosidic bonds force the entire structure of the polysaccharide to a linear arrangement, due to the pyranose chair conformation and the equatorial disposition of the substituents within the subunits, that corresponds to a minimum of energy of the entire structure [3].
Due to the extensive potential applications of this inexhaustible material [4][5], nanocelluloses (NCs) became in brief time one of the main topics of research worldwide. In particular, NCs have shown excellent properties like strength, high Young’s modulus, biocompatibility, tunable self-assembly, and thixotropic behavior, becoming groundbreaking in plenty of fields like optoelectronics, antibacterial coatings, packaging, engineered polymer composites, medicine (tissue scaffolds, drug delivery, biosensors, etc.), energy storage, catalysis, and environmental remediation [6][7]. Very often the nanocellulose (NC) is subjected to functional modifications through chemical reactions. These modifications are frequently performed to improve the characteristics and performance of nanocellulose-based materials and more specifically to obtain nanocellulose derivatives with enhanced lipophilic characteristics through acetylation, benzoylation or alkylation and with increased hydrophilic features by sulfonation or oxidation reactions. At the same time, it is necessary to preserve the structural integrity of modified nanocellulose to ensure its stability during the use as heterogeneous catalyst in organic transformations. Moreover, the almost always superficial modifications ensure the excellent catalytic performance and the maintenance of its intimate structure for an easy recovery and a possible reuse of the catalyst.
Despite the always growing number of studies about the preparation and their applicative evaluation, NC materials can be classified into just three main nanoforms: bacterial nanocellulose (BNC), cellulose nanocrystals (CNCs), and cellulose nanofibers (CNFs). The parameters which define the classification of these materials are mainly related to the methods of preparation (biological, chemical, and mechanical), the morphological attributes, and the crystallinity degree of the material; for a deeper point of view on the physical properties of NCs, the reader can refer to [8].
In spite of a lot of works have been published about the preparation, the chemical transformation and the general employ of this nanomaterials, to our knowledge literature reports only two papers which examine the role of nanoparticles (NPs)-decorated NC-derivatives as inorganic catalysts [9][10]. On the other hand, the catalytic application of organic-functionalized NCs still have not been properly reviewed. In this context, the reader will find below a more thorough description of BNC and its catalytic applications.
2. Bacterial Nanocellulose
Bacterial nanocellulose (BNC), also defined bacterial cellulose (BC), is an exceptional natural polymer with a great variety of technical applications. BNC was firstly described in a scientific paper by A.J. Brown in 1886, as produced by Bacterium aceti through fermentative processes to produce nanoscale cellulose [11]. Cellulose nanofibers can be also produced, as extracellular secretion, by several species of bacteria, such as Aerobacter, Acetobacter, Agrobacterium, Azotobacter, Rhizobium, and Pseudomonas [12][13]. Acetobacter xylinus, also known as Gluconacetobacter or Komagataeibacter xylinus, a non-pathogenic Gram-negative aerobic bacteria, is considered the most efficient cellulose producer, and the most extensively studied [12]. BNC has the same molecular formula as plant cellulose but is characterized by a 3D porous network structure with unique features. First of all, it has high purity since it is hemicellulose- and lignin-free, with a high water content of 99% and hydrophilicity [13]. It possesses high crystallinity (up to 80%) with a resulting high thermal stability and a high degree of polymerization (up to 20,000). Finally, BNC exhibits high flexibility, with a value of 118 GPa for Young’s modulus of single nanofiber, which is comparable to steel [14]. BNC nanofibers have a high aspect ratio around 20–100 nm in width and 1–9 µm length, with a surface area superior to that of plant cellulose [12]. BNC is very versatile since it can be obtained in different shapes and thicknesses, such as pellicle, disk, or aggregate. Finally, BNC is more environmentally friendly than its vegetal counterpart, due to its high purity, which does not require additional purification procedures. Moreover, the identification of different waste biomass as a useful carbon source for a prospective industrial BNC production, together with its considerable environmental biodegradability and biocompatibility make it an ecofriendly material. The BNC features are influenced by the fermentation conditions such as the carbon and nitrogen sources, the temperature, the incubation time, and agitation. Furthermore, it is possible to alter the BNC structure and the morphology by adding chemical reagents in the culture medium or the growing fibers, to give nanocomposite properties [15][16]. In this way it is possible to obtain a versatile template material for various applications, from medical devices to innovative materials with electrical properties [16][17].
Thanks to its numerous hydroxyl groups it could be chemically functionalized to give materials with enhanced chemical and physical properties. As an example, BNC oxidation enhances its biodegradability and solubility. Instead, esterification of the BNC could be a strategy for improving the capability of integration with other organic polymers, or for the delivery of active pharmaceutical ingredients [12].
Very attractive aerogels can be obtained from BNC, through freeze-drying processes. The resulting porous solid material can be used as a template for numerous catalysts in diverse applications [12][18][19]. It is worth noting that these BNC aerogels also are the starting material in high-temperature pyrolysis processes under an inert atmosphere, to obtain carbon nanofibers with a 3D nanostructure [20]. Although numerous papers reported the use of these advantageous catalysts, this topic is out of scope for the present review, and only pristine BNC, suitably modified without pyrolysis processes will be examined.
Readers can find a complete survey about the advancements in bacterial cellulose applications in previous, very exhaustive reviews [9][21][22][23][24][25][26]. The most interesting bacterial cellulose applications as a catalyst from 2016 until the present, are reported herein.
2.1. Inorganic Functionalization of Bacterial Nanocellulose and Catalytic Applications
Many inorganic compounds, like metal oxides, metal sulfides, silica, or metal nanoparticles, have been integrated into BNC, exploiting its isotropic 3D nanostructure to obtain versatile heterogeneous catalysts. With the aim to prepare nanocomposites with enhanced activity, it is possible to add these inorganic materials through different synthetic strategies. Among them, three strategies have been most used and reported in the literature: (i) the mixing under simple agitation or ultrasound-assisted conditions; (ii) the absorption through solvothermal processes; and (iii) in situ incorporation in the growth medium. The catalytic applications of these new materials are classified in the following sections.
2.1.1. Catalytic Transformation of Organic Compounds for Environmental Purposes
Many efforts have been made in the last years for the treatment of industrial wastewater, to eliminate or limit the pollutants. For example, the textile industries contribute a lot to aquatic pollution with azoic dyes. The reduction of these contaminants in non- or less-toxic compounds was investigated, using transition metal-based nanoparticles, which are more economical than noble metal nanoparticles. Thiruvengadam and Vitta in 2017, proposed the use of BNC as flexible and multifunctional Ni-based nanocomposite to reduce methyl orange (MO) used as a model dye pollutant [27]. The authors obtained Nickel-Bacterial Cellulose (NiBNC) nanocomposite at different nickel concentration using the “inverse chemical reduction” technique. It consisted of the immersion of a BNC hydrogel firstly in a sodium borohydride (NaBH4) solution and subsequently in a NiCl2 solution. Finally, the resulting black colored NiBNC was hot-pressed at 70 °C and 4 MPa, to obtain a dried NiBNC sheet with a final formation of highly interconnected metal nanoparticles in a BNC network, with magnetic and electrical properties. The reduction catalytic activity was also tested, and the NiBNC was able to reduce the UV-absorption of a methyl orange solution if compared with pristine BNC. The reusability of this catalyst was checked, and the authors highlighted that a high efficiency was maintained if the catalyst was not stored in an aqueous solution between the cycles. Xu et al., in 2018, developed a novel flexible membrane for the water pollution treatment, based on BNC loaded with graphene oxide (GO) and palladium nanoparticles (Pd-NPs) [28]. At first, GO flakes were introduced as a uniform dispersion in the growing BNC nanofibers, allowing intercalation in a “layer by layer” manner. The resulting material showed more robustness of the pristine BNC, a crucial property for water treatment materials. After the formation and cleaning in alkaline solution, the GO/BNC hydrogel was cut in the desired measures and freeze-dried to give GO/BNC aerogel with a large specific surface area. To add Pd-NPs to the GO/BNC structure, it was immersed in a PdCl2 solution, dried, and then washed with a NaBH4 solution leading to in situ formations of Pd-NPs (Figure 1). The newly formed Pd/GO/BNC membrane showed a large loading of Pd-NPs in the final membrane and was used dried for the catalytic test, in which methyl orange dye-contaminated water, in presence of NaBH4, was filtered through it. The rate of methyl orange degradation was measured with a simple Pd/BNC membrane that was lower compared to that of Pd/GO/BNC membrane. Its powerful activity was highlighted testing this sheet as a filter for a cocktail solution of contaminants with a concentration of 10 mg L−1: methylene blue 1 (MB), 4-nitrophenol 2 (4-NP), and rhodamine 6G 3 (R6G). In all cases, the colored solution became completely colorless after filtration through the Pd/GO/BNC membrane, probably due to the lamellar structure of the membrane that assures an excellent interaction with the Pd-NPs for the reduction activity. It is worth noting that the entire membrane preparation procedure is easily scalable. This feature together with the high spread catalytic activity and the stability showed in the stress test, make this new material interesting for industrial application.
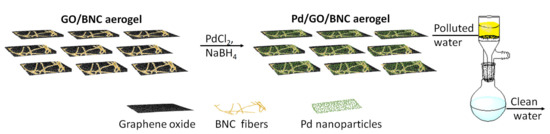
Figure 1. Schematic illustration of the steps involved in the fabrication of Pd/graphene oxide (GO)/bacterial nanocellulose (BNC) and its use.
Very recently, the research group of Kamal et al. proposed different examples in which BNC was used as a high surface area support for transition metal nanoparticles stabilized with carboxymethyl cellulose (CMC) like cobalt (CMC-Co-BNC) [29], copper (CMC-Cu-BNC) [30], and nickel (CMC-Ni-BNC) [31]. In these works, metal chloride and CMC (1 wt%) solutions were mixed, then hydrazine hydrate and ascorbic acid were slowly added. Microwave heating of the resulting solution led to the carboxymethyl cellulose-metal (CMC-M) nanoparticles. Finally, the CMC-M-BNC dip-catalyst was obtained by simple dropping and spread of the CMC-M suspension on the BNC sheets previously obtained by a Gluconacetobacter xylinum culture (Scheme 1).
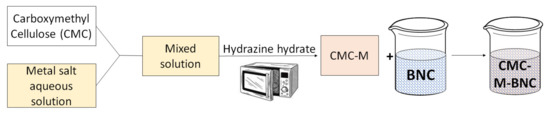
Scheme 1. Preparation of the colloidal carboxymethyl cellulose (CMC)-M nanoparticles and carboxymethyl cellulose (CMC)-M-BNC dip-catalyst.
All these catalysts were tested on the reduction of methylene blue 1 (MB) and different nitrophenols: 4-nitrophenol 2 (2-NP), 2,6-dinitrophenol 4 (2,6-DNP), and 2-nitrophenol 5 (2-NP) with CMC-Co-BNC, CMC-Cu-BNC, and CMC-Ni-BNC, respectively. In all cases, the CMC-M-BNC catalyst was able to reduce the dye pollutants, both alone and in combination, even if the colloidal CMC-M was found to catalyze the reaction faster than the heterogeneous CMC-M-BNC. The reusability was also tested with good conversion results after four cycles. The easier separation procedure of the heterogeneous catalyst, compared to the colloidal one that requires long centrifugation times for its recovery, suggests that the supported CMC-M-BNC could be more useful for real applications.
The hypothesized reaction mechanism could explain the different reaction rates measured for the CMC-M and the CMC-M-BNC. Both the reducible molecules and the sodium borohydride move on the nanoparticle surface. The NaBH4 transfers a hydride on the reducible molecule, while an electron was transferred from it to the catalyst. Then, the reduced molecules leave the catalyst surface and spread in the solution. By then, another reducible molecule could be adsorbed. The authors hypothesized that the anionic surface of the CMC favored a strong interaction with the reducible molecules. Consequently, a fast catalytic reaction occurs (Scheme 2).
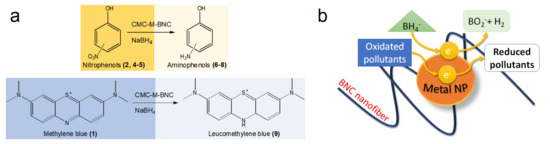
Scheme 2. (a) Chemical reduction of nitrophenols (2, 4, 5) and methylene blue (1) by NaBH4 using CMC-M or CMC-M-BNC; (b) reaction mechanism.
After simple oxidation of the pristine BNC, it is possible to highly improve the metal absorption capacity maintaining, at the same time the crystallinity and crystal size of the nanofibers [15]. In addition, BNC TEMPO-mediated oxidation has been combined, also with defibrillation to allow a higher metal nanoparticles inclusion capability. In 2017, Chen et al. exploited this option using the 2,2,6,6-tetramethylpiperidine-1-oxyradical (TEMPO)-mediated oxidation reaction to obtain a BNC with carboxyl groups on the glucose moieties (TOBNC) as support for Au nanoparticles (Au-TOBNC) [32]. Before the oxidation reaction, it was necessary to fibrillate BNC into a slurry, then the reaction proceeded by adding TEMPO, NaBr, and NaClO under mild aqueous conditions. The TOBNC as obtained was then mixed with a HAuCl4 solution in presence of sodium borohydride to give the Au-TOBNC catalyst (Scheme 3).
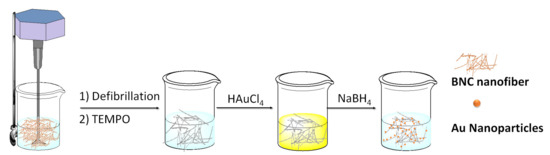
Scheme 3. Synthetic procedure of AuNPs supported by TOBNCs.
The average particle size for AuNP-TOBNC was lower than the unsupported AuNPs, and it was probably due to the carboxyl groups, which in the dissociate form could immobilize tighter the AuNP, allowing a more uniform distribution of the particle size. The influence of the pH on the amount of AuNPs loaded was studied. When pH was 3.2–11.3 it was observed a changing color of the suspension reflecting the increase of the amount of AuNPs. It was probably due to the associate or dissociated form of the carboxyl groups. Then, the Au-TOBNC catalyst was tested for the reduction of 4-nitrophenol (4-NP) 2 by NaBH4 as a reaction model. The reduction of 4-nitrophenol (4-NP) 2 to 4-aminophenol 6 (4-AP) proceed almost 20-fold faster than the AuNP without TOBNC support. Probably, because the TOBNC allowed a better Au exposure to the reagent when adsorbed on the single nanofiber, it was well dispersed. Finally, the influence of the temperature on the reduction reaction was evaluated, and the rate of substrate activation and the rate of product desorption achieved a balance almost at 65 °C. This work was very important because of the higher catalytic efficiency than the values reported before for analog catalyst [33].
2.1.2. Photocatalytic Applications of BNC-Inorganic Composites for Environmental Remediation
The advanced oxidation processes (AOPs) are another way for the water removal of dyes and pollutants, which were under investigation through heterogeneous catalysis in the last year. Thanks to its unique features, the BNC can be considered an excellent material substrate for the design of a potential Fenton-type catalyst. In 2018, Wibowo et al. synthesized a Fenton-BNC catalyst, by immersing a BNC hydrogel in FeCl2 and FeCl3 solutions at different concentrations, with a 1:2 molar ratio between Fe(II) and Fe(III) [34]. After one night, the hydrogel color changed from white to yellow and was immersed in a NaOH (4 M) solution to oxidize the metal, with a final black color for the hydrogel. Finally, the Fenton-BNC hydrogel was freeze-dried to give a Fenton-BNC aerogel that was used for the catalytic degradation studies of methylene blue (MB) 1, used as dye pollutant model. The authors demonstrated that the heterogeneous Fenton catalyst supported by BNC possessed better catalytic activity than the bare Fenton catalyst prepared without BNC. As a further advantage, Fenton-BNC could be easily recovered by using an external magnetic field thanks to its magnetic features.
Very recently, Hu and co-workers proposed a new efficient and recyclable photocatalyst based on TiO2, non-woven polypropylene (NWP), and BNC [35]. In particular, TiO2 nanoparticles were loaded onto NWP, and this sample was placed in the culture medium of Gluconacetobacter x., in order to fix the nanoparticles in the growing cellulose (Figure 2). The TiO2-NWPBNC was used for the MB degradation catalytic tests, confirming that TiO2 was efficiently embedded into the composite film, maintaining a good degradation performance with an MB removal rate over 92% after 2 h of reaction. Furthermore, after 5 cycles less than 10% of removal rate reduction was measured, showing also good reusability.
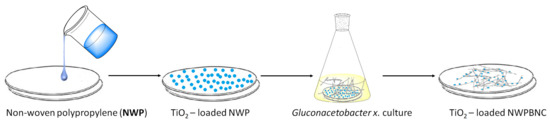
Figure 2. Preparation process of TiO2-loaded NWPBNC composite film.
2.1.3. Electro-Catalytic Applications of Inorganic-Functionalized Bacterial Nanocellulose
As previously reported [9], BNC can be converted into a material with electrical properties by inorganic functionalization with suitable transition metals. In this work, the authors measured an increase of the room temperature electrical conductance for a flexible BNC sheet functionalized with a 20 vol% of Ni nanoparticles. It is worth noting that the NiBNC membrane could be useful both for biodegradable and bendable electronic devices thanks to its unique properties to conduct both in the hydrogel or dried state, respectively.
Zhou and co-workers in 2019, developed a CuO/Cu nanocomposite electrode supported on BNC as a catalyst for efficient CO2 electrochemical reduction to CO [36]. This electrode was obtained by applying an in situ chemical reduction process, in which BNC sheets were immersed in a Cu(II) salt solution of ethanol and water. NaBH4 was added as a reducing agent, together with an NaOH solution (0.1 M), and the mixture was heated at 30 °C for 90 min. Different CuO/Cu-BNC membranes were obtained as a function of the NaBH4 concentration after washing with deionized water. Electrochemical activity and performance tests were carried out and in particular, the CuO/Cu4:3-BNC prepared with a 0.3 M concentration in NaBH4 (with a 4:3 molar ratio between CuSO4·5H2O and NaBH4) was evaluated as an efficient catalyst for electrochemical CO2 reduction. The CuO/Cu4:3-BNC catalyst showed high faradaic efficiency of 53% for CO formation at a low overpotential of 490 mV, for over 40 h. Furthermore, it was compared with a conventional CuO/Cu composite catalyst supported on carbon paper and it was highlighted that an enhanced electro-catalytic performance was obtained due to its unique structural advantages, such as high surface area, optimal CuO/Cu composition that allowed a facilitating charge, and mass transport and ability to promote the conversion of CO2.
2.1.4. Synthetic Applications of BNCs Grafted with Inorganic Catalysts
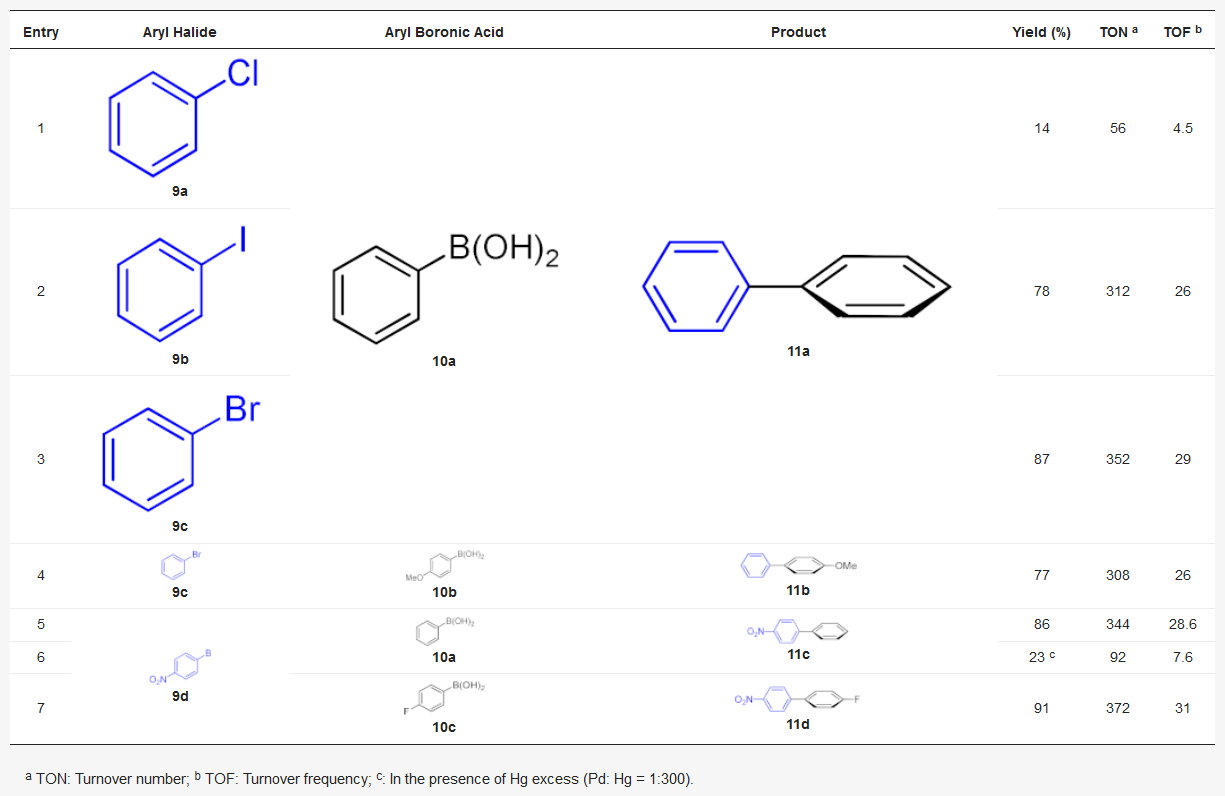
The use of a heterogeneous catalyst is in great demand, also in organic synthesis, and BNC responds with its peculiar features. In 2019, Jeremic et al. carried out an extensive study on the influence of the BNC production parameters and its use in transition metal nanocomposites for the catalysis of cross-coupling reactions [37]. Firstly, the authors studied the influence of the single parameters, such as pH, glucose concentration, and day of incubation, for the optimization of BNC production. In addition, for the first time, they successfully used a sugar mixture, a product of grass biomass treatment [38], as a carbon source for BNC production, obtaining the valorization of non-food renewable biomass as starting material. Then, the authors evaluated BNC application as solid supports for transition metals for the synthesis of heterogeneous catalysts. They loaded onto BNC, Pd(II) or Cu(II) salts in a sodium borohydride solution in water at 140 and 70 °C, respectively. Differences were observed in the metal catalyst’s distribution, showing that Cu formed a spherical structure on the surface, while Pd nanoparticles were incorporated inside the BNC network. Furthermore, quadrupole inductively coupled plasma mass spectrometry (ICP-QMS) analysis showed that the amount of Cu on Cu/BNC was 10-fold lower than Pd on Pd/BNC, indicating more efficient incorporation for the Pd catalyst than the Cu catalyst. For this reason, the Pd/BNC catalyst was chosen for the catalytic tests. The results of the Suzuki–Miyaura reaction performed with the Pd/BNC catalyst are reported in Table 1.
Table 1. Pd/BNC catalyzed Suzuki–Miyaura reaction of aryl halogenides with arylboronic acid in water.
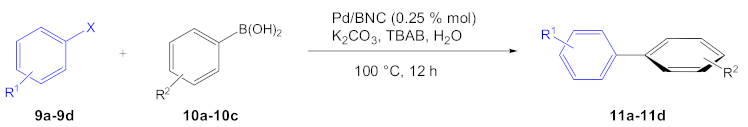
As expected, the coupling reaction gave higher yields using bromobenzene 9a with different arylboronic acids 10a–10c, using water as an ecofriendly solvent, and highlighting the greenness of the whole process. Subsequently, the same recovered catalyst was used to catalyze the reduction of the nitro- to amino group of 11c and 11d with H2, with 99% yield for both compounds (Scheme 4).
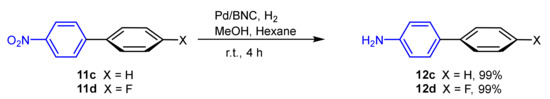
Scheme 4. Reduction of nitro-group catalyzed by Pd/BNC.
Finally, Cu/BNC catalyst was also tested for the Chan–Lam coupling reaction between benzylamine 12 and phenylboronic acid 10a (Scheme 5). The authors supposed that the moderate observed yield was due to the low Cu incorporation on BNC. Anyway, the easy recovery and reusability, together with the negligible leaching of the metals confirmed these materials as good heterogeneous catalysts.
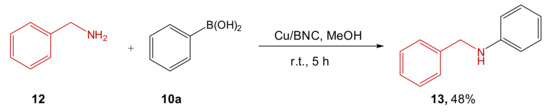
Scheme 5. Chan–Lam coupling reaction catalyzed by Cu/BNC.
2.2. Surface Chemical Functionalization of Bacterial Nanocellulose and Catalytic Applications
The introduction of sulfuric acid as functional group on the BNC surface can modify the surface polarity leading to a heterogeneous acid catalyst, with a high crystallinity and surface area. In 2018, Nikoofar and co-workers exploited this chemical modification to catalyze a multicomponent reaction in order to obtain pyrimidine fused heterocycles [39]. Multicomponent reactions (MCR) are very interesting and green tools to obtain useful scaffolds for medicinal chemistry [40]. Many green protocols have been developed in the last years [41][42], and the use of an heterogeneous catalyst can accelerate the reaction times and increases the reaction yields [43]. In this work, biodegradable and stable heterogeneous catalysts were tested in a four MCR (4-MCR). BNC sulfuric acid (s-BNC) together with nanofibrillated cellulose sulfuric acid (s-NFC) were obtained by the authors in mild condition by adding chlorosulfonic acid in n-hexane at 0 °C for 20 min. The mixture was stirred at room temperature to remove the HCl. Then, the catalysts were washed and dried to give s-BNC and s-NFC ready for the MCR reaction to give dihydropyrimido [4,5-b]quinolinetriones (Scheme 6).
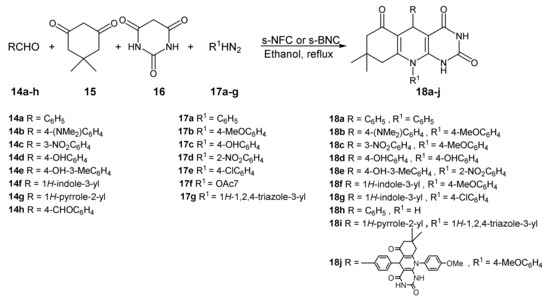
Scheme 6. Multicomponent reaction catalyzed by s-BNC and s-NFC.
The reaction conditions were tested using s-NFC, which showed good results both in water and ethanol. The optimized reaction conditions were applied for both the catalysts, and the results are reported in Table 2.
Both the catalysts allowed the formation of the products with a range yield of 83-95% in short reaction times. In particular, s-BNC showed a short reaction time in all cases, despite its porosity reduction, if compared with the s-NFC porosity. Finally, the heterogeneous catalysts were successfully recovered and reused for the synthesis of the compound 18a for three times.
Table 2. Multicomponent reactions (MCR) for the synthesis of dihydropyrimido[4,5-b]quinolinetriones 18a-18j catalyzed by s-NFC or s-BNC in refluxing ethanol.
|
Entry |
Aldehyde |
Amine |
Product |
s-NFC |
s-BNC |
||
|
Time (Min) |
Yield (%) |
Time (Min) |
Yield (%) |
||||
|
1 |
14a |
15a |
18a |
45 |
92,90,90 a |
40 |
94 |
|
2 |
14b |
15b |
18b |
90 |
90 |
70 |
91 |
|
3 |
14c |
15b |
18c |
60 |
85 |
50 |
83 |
|
4 |
14d |
15c |
18d |
105 |
95 |
90 |
94 |
|
5 |
14e |
15d |
18e |
90 |
93 |
80 |
90 |
|
6 |
14f |
15b |
18f |
165 |
93 |
150 |
91 |
|
7 |
14f |
15e |
18g |
105 |
95 |
95 |
90 |
|
8 |
14a |
15f |
18h |
75 |
90 |
60 |
88 |
|
9 |
14g |
15g |
18i |
75 |
90 |
65 |
88 |
|
10 b |
14h |
15b |
18j |
90 |
55 |
80 |
54 |
a: Results for recovery and reusability of catalyst; b: 14h:15:16:17b at molar ratio of 1:2:2:2 with the double amount of each catalyst.
Very recently, Said et al. developed an heterogeneous fluoride complex between BNC and tetrabutylammonium fluoride (TBAF) that allowed to be react as highly stable and selective fluoride source for fluorination reactions [44]. Although the use of a complex could be considered as out of scope for this review, this work is very interesting since the BNC was used both in batch and in flow condition with good to excellent results, demonstrating a high stability and versatility that could justify this concise regression. In brief, they tested different polysaccharides, such as BNC, vegetable cellulose, pectine, and starch, as support for (TBAF), in order to find a stable and non-hygroscopic heterogeneous complex for the SN2 type fluorination reactions performed both in batch and flow conditions (Scheme 7).
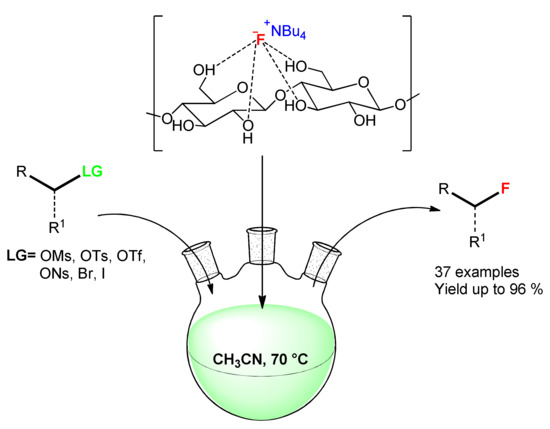
Scheme 7. Hydrogen bonded fluoride complexes for SN2 fluorination reaction.
References
- French, A.D.; Pérez, S.; Bulone, V.; Rosenau, T.; Gray, D. Cellulose; John Wiley & Sons: New York, NY, USA, 2018; Volume 1838; ISBN 0471440264.
- Kirk, O. Kirk-Othmer Encyclopedia of Chemical Technology, 5th ed.; John Wiley & Sons: New York, NY, USA, 2004.
- Bellesia, G.; Asztalos, A.; Shen, T.; Langan, P.; Redondo, A.; Gnanakaran, S. In silico studies of crystalline cellulose and its degradation by enzymes. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 1184–1188, doi:10.1107/S0907444910029483.
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Chem. 2015, 6, 4497–4559.
- Romeo, I.; Olivito, F.; Tursi, A.; Algieri, V.; Beneduci, A.; Chidichimo, G.; Maiuolo, L.; Sicilia, E.; De Nino, A. Totally green cellulose conversion into bio-oil and cellulose citrate using molten citric acid in an open system: Synthesis, characterization and computational investigation of reaction mechanisms. RSC Adv. 2020, 10, 34738–34751, doi:10.1039/d0ra06542k.
- Thomas, B.; Raj, M.C.; Athira, B.K.; Rubiyah, H.M.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Rev. 2018, 118, 11575–11625, doi:10.1021/acs.chemrev.7b00627.
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Today 2018, 21, 720–748, doi:10.1016/j.mattod.2018.02.001.
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. Polym. Sci. Part B Polym. Phys. 2014, 52, 791–806, doi:10.1002/polb.23490.
- Zhang, Q.; Zhang, L.; Wu, W.; Xiao, H. Methods and applications of nanocellulose loaded with inorganic nanomaterials: A review. Polym. 2020, 229, 115454, doi:10.1016/j.carbpol.2019.115454.
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637, doi:10.1039/c5gc02500a.
- Brown, A.J. On an acetic ferment which forms cellulose. Chem. Soc. Trans. 1886, 49, 432–439.
- Foresti, M.L.; Vázquez, A.; Boury, B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: A review of recent advances. Polym. 2017, 157, 447–467, doi:10.1016/j.carbpol.2016.09.008.
- Qiu, K.; Netravali, A.N. A review of fabrication and applications of bacterial cellulose based nanocomposites. Rev. 2014, 54, 598–626, doi:10.1080/15583724.2014.896018.
- Soriano, M.L.; Dueñas-Mas, M.J. Promising Sensing Platforms Based on Nanocellulose. In Carbon-Based Nanosensor Technology; Springer Series on Chemical Sensors and Biosensors (Methods and Applications); Kranz, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 273–301.
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized bacterial cellulose derivatives and nanocomposites. Polym. 2014, 101, 1043–1060, doi:10.1016/j.carbpol.2013.09.102.
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial cellulose nanocomposites: An all-nano type of material. Sci. Eng. C 2019, 98, 1277–1293, doi:10.1016/j.msec.2019.01.064.
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766, doi:10.1007/s10570-020-03273-9.
- Long, L.Y.; Weng, Y.X.; Wang, Y.Z. Cellulose aerogels: Synthesis, applications, and prospects. Polymers (Basel). 2018, 8, 1–28, doi:10.3390/polym10060623.
- Bonaccorsi, L.; Calandra, P.; Amenitsch, H.; Proverbio, E.; Lombardo, D. Growth of fractal aggregates during template directed SAPO-34 zeolite formation. Microporous Mesoporous Mater. 2013, 167, 3–9, doi:10.1016/j.micromeso.2012.10.024.
- Wu, Z.Y.; Liang, H.W.; Chen, L.F.; Hu, B.C.; Yu, S.H. Bacterial Cellulose: A Robust Platform for Design of Three Dimensional Carbon-Based Functional Nanomaterials. Chem. Res. 2016, 49, 96–105, doi:10.1021/acs.accounts.5b00380.
- Mohamed, M.A.; Abd Mutalib, M.; Mohd Hir, Z.A.; Zain, M.F.M.; Mohamad, A.B.; Jeffery Minggu, L.; Awang, N.A.; Salleh, W.N.W. An overview on cellulose-based material in tailoring bio-hybrid nanostructured photocatalysts for water treatment and renewable energy applications. J. Biol. Macromol. 2017, 103, 1232–1256, doi:10.1016/j.ijbiomac.2017.05.181.
- Li, Y.Y.; Wang, B.; Ma, M.G.; Wang, B. Review of Recent Development on Preparation, Properties, and Applications of Cellulose-Based Functional Materials. J. Polym. Sci. 2018, 2018, doi:10.1155/2018/8973643.
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial nanocellulose—a biobased polymer for active and intelligent food packaging applications: Recent advances and developments. Polymers (Basel) 2020, 12, 1–23, doi:10.3390/polym12102209.
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Polym. 2021, 251, 116986, doi:10.1016/j.carbpol.2020.116986.
- Jiang, Y.; Lawan, I.; Zhou, W.; Zhang, M.; Fernando, G.F.; Wang, L.; Yuan, Z. Synthesis, properties and photocatalytic activity of a semiconductor/cellulose composite for dye degradation-a review. Cellulose 2020, 27, 595–609, doi:10.1007/s10570-019-02851-w.
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Sci. Eng. C 2019, 104, 109963, doi:10.1016/j.msec.2019.109963.
- Thiruvengadam, V.; Vitta, S. Bacterial cellulose based flexible multifunctional nanocomposite sheets. Cellulose 2017, 24, 3341–3351, doi:10.1007/s10570-017-1350-6.
- Xu, T.; Jiang, Q.; Ghim, D.; Liu, K.K.; Sun, H.; Derami, H.G.; Wang, Z.; Tadepalli, S.; Jun, Y.S.; Zhang, Q.; et al. Catalytically Active Bacterial Nanocellulose-Based Ultrafiltration Membrane. Small 2018, 14, 1–8, doi:10.1002/smll.201704006.
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Bacterial cellulose as support for biopolymer stabilized catalytic cobalt nanoparticles. J. Biol. Macromol. 2019, 135, 1162–1170, doi:10.1016/j.ijbiomac.2019.05.057.
- Kamal, T.; Ahmad, I.; Khan, S.B.; Ul-Islam, M.; Asiri, A.M. Microwave Assisted Synthesis and Carboxymethyl Cellulose Stabilized Copper Nanoparticles on Bacterial Cellulose Nanofibers Support for Pollutants Degradation. Polym. Environ. 2019, 27, 2867–2877, doi:10.1007/s10924-019-01565-1.
- Kamal, T.; Ahmad, I.; Khan, S.B.; Asiri, A.M. Anionic polysaccharide stabilized nickel nanoparticles-coated bacterial cellulose as a highly efficient dip-catalyst for pollutants reduction. Funct. Polym. 2019, 145, 104395, doi:10.1016/j.reactfunctpolym.2019.104395.
- Chen, Y.; Chen, S.; Wang, B.; Yao, J.; Wang, H. TEMPO-oxidized bacterial cellulose nanofibers-supported gold nanoparticles with superior catalytic properties. Polym. 2017, 160, 34–42, doi:10.1016/j.carbpol.2016.12.020.
- Sen, I.K.; Maity, K.; Islam, S.S. Green synthesis of gold nanoparticles using a glucan of an edible mushroom and study of catalytic activity. Polym. 2013, 91, 518–528, doi:10.1016/j.carbpol.2012.08.058.
- Wibowo, A.; Indrawan, R.F.; Triadhi, U.; Aimon, H.A.; Iskandar, F.; Ardy, H. Simple preparation of Fenton catalyst@bacterial cellulose for waste water treatment. Res. Express 2018, 5, 024005.
- Hu, J.; Wu, D.; Feng, Q.; Wei, A.; Song, B. Soft High-Loading TiO2 Composite Biomaterial Film as an Efficient and Recyclable Catalyst for Removing Methylene Blue. Fibers Polym. 2020, 21, 1760–1766, doi:10.1007/s12221-020-9543-2.
- Zhou, Y.; Guo, X.; Li, X.; Fu, J.; Liu, J.; Hong, F.; Qiao, J. In-situ growth of CuO/Cu nanocomposite electrode for efficient CO2 electroreduction to CO with bacterial cellulose as support. CO2 Util. 2020, 37, 188–194, doi:10.1016/j.jcou.2019.12.009.
- Jeremic, S.; Djokic, L.; Ajdačić, V.; Božinović, N.; Pavlovic, V.; Manojlović, D.D.; Babu, R.; Senthamaraikannan, R.; Rojas, O.; Opsenica, I.; et al. Production of bacterial nanocellulose (BNC) and its application as a solid support in transition metal catalysed cross-coupling reactions. J. Biol. Macromol. 2019, 129, 351–360, doi:10.1016/j.ijbiomac.2019.01.154.
- Davis, R.; Kataria, R.; Cerrone, F.; Woods, T.; Kenny, S.; O’Donovan, A.; Guzik, M.; Shaikh, H.; Duane, G.; Gupta, V.K.; et al. Conversion of grass biomass into fermentable sugars and its utilization for medium chain length polyhydroxyalkanoate (mcl-PHA) production by Pseudomonas strains. Technol. 2013, 150, 202–209, doi:10.1016/j.biortech.2013.10.001.
- Nikoofar, K.; Heidari, H.; Shahedi, Y. Investigation the catalytic activity of nanofibrillated and nanobacterial cellulose sulfuric acid in synthesis of dihydropyrimidoquinolinetriones. Chem. Intermed. 2018, 44, 4533–4546, doi:10.1007/s11164-018-3402-4.
- Costanzo, P.; Nardi, M.; Oliverio, M. Similarity and Competition between Biginelli and Hantzsch Reactions: An Opportunity for Modern Medicinal Chemistry. European J. Org. Chem. 2020, 2020, 3954–3964, doi:10.1002/ejoc.201901923.
- Oliverio, M.; Costanzo, P.; Nardi, M.; Rivalta, I.; Procopio, A. Facile ecofriendly synthesis of monastrol and its structural isomers via biginelli reaction. ACS Sustain. Chem. Eng. 2014, 2, 1228–1233, doi:10.1021/sc5000682.
- Costanzo, P.; Calandruccio, C.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. First multicomponent reaction exploiting glycerol carbonate synthesis. Clean. Prod. 2018, 202, 504–509, doi:10.1016/j.jclepro.2018.08.120.
- Choudhury, P.; Ghosh, P.; Basu, B. Amine-functionalized graphene oxide nanosheets (AFGONs): An efficient bifunctional catalyst for selective formation of 1,4-dihydropyridines, acridinediones and polyhydroquinolines. Divers. 2020, 24, 283–294, doi:10.1007/s11030-019-09949-0.
- Said, M.S.; Khonde, N.S.; Thorat, M.N.; Atapalkar, R.S.; Kulkarni, A.A.; Gajbhiye, J.; Dastager, S.G. A New TBAF Complex, Highly Stable, Facile and Selective Source for Nucleophilic Fluorination: Applications in Batch and Flow Chemistry. Asian J. Org. Chem. 2020, 9, 1022–1026, doi:10.1002/ajoc.202000235.