
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anastasios Zouboulis | + 5059 word(s) | 5059 | 2021-01-21 06:13:37 | | | |
| 2 | Nora Tang | Meta information modification | 5059 | 2021-01-21 08:58:59 | | | | |
| 3 | Rui Liu | Meta information modification | 5059 | 2021-01-27 09:06:38 | | | | |
| 4 | Vicky Zhou | -4561 word(s) | 498 | 2022-04-13 11:41:45 | | |
Video Upload Options
Catalytic membrane ozonation is a hybrid process that combines membrane filtration and catalytic ozonation. The membrane deposited with an appropriate solid material acts as catalyst. As a consequence, the catalytic membrane contactor can act simultaneously as contactor (i.e., improving the transfer/dissolution of gaseous ozone into the liquid phase), as well as reactor (i.e., oxidizing the organic compounds). It can be used in water and wastewater treatment limiting the disadvantages of membrane filtration (i.e., lower removal rates of emerging contaminants or fouling occurrence) and ozonation (i.e., selective oxidation, low mineralization rates, or bromate (BrO3−) formation). The catalytic membrane ozonation process can enhance the removal of micropollutants and bacteria, inhibit or decrease the BrO3− formation and additionally, restrict the membrane fouling (i.e., the major/common problem of membranes’ use). Nevertheless, the higher operational cost is the main drawback of these processes.
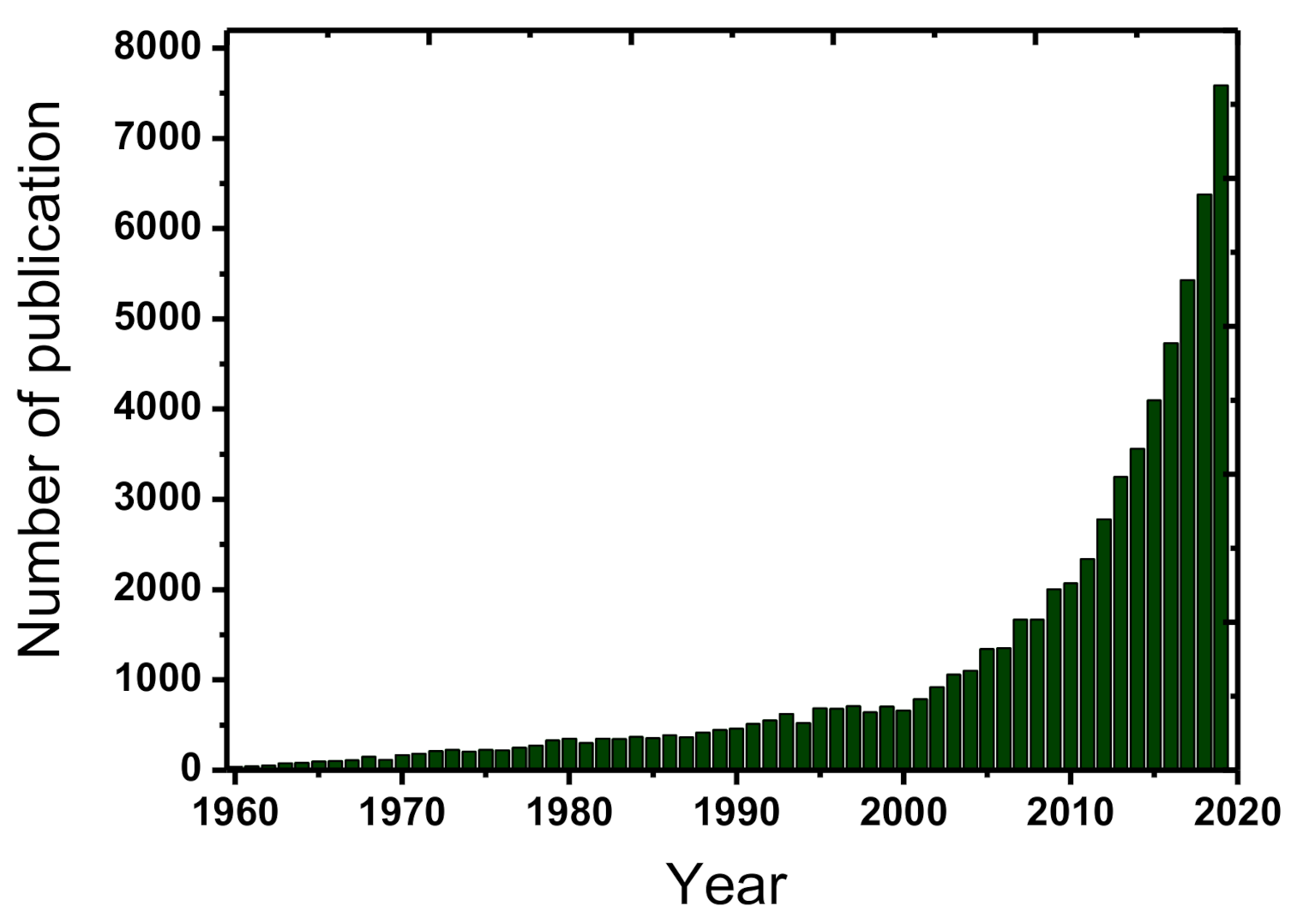
Clean water is very essential for the sustainable human future in planet Earth. However, water can be extensively contaminated and the development of innovate processes for its effective treatment and use constitutes a constantly evolving field [1]. Among the most recently detected pollution issues is considered the occurrence of emerging/persisting contaminants/chemicals in the aquatic ecosystems and especially, the presence of micropollutants. Micropollutants are termed the compounds of mostly anthropogenic origin that occurring usually in the aqueous environment in concentrations lower than mg/L (i.e., in the range of μg/L, or even ng/L). The evolution of analytical science and techniques especially during the recent years has permitted the accurate detection of them [1][2]. They can be divided into the following six main categories: (1) perfluorinated compounds (e.g., perfluorooctanesulfonate, perfluorooctanoic acid), (2) disinfection by-products (e.g., halomethanes, nitrosamines, hydroxyl acids), (3) gasoline additives (e.g., tert-butul ether, benzene, 1,3-butadiene), (4) manufactured nanomaterials (e.g., carbon nanotubes, silicon dioxide, titanium oxide), (5) human and veterinary pharmaceuticals (e.g., estradiol, 17α-ethinylestradiol, acetaminophen), and (6) sunscreens/ultraviolet filters (e.g., benzophenone-3, homosalate, 4-aminobenzoic acid) [2]. Micropollutants are omnipresent and generally contribute to improving the quality of human life, but they are consisting also an issue of major concern for the water and wastewater treatment plants. Micropollutants are mostly persist in the treated effluents, because the conventional systems are not specifically designed to remove them effectively [3]. The optimization of these treatment plants maybe a sufficient strategy for the efficient treatment of these problematic contaminants. Due to the respective developments the relevant scientific literature, regarding the treatment of emerging/persistent contaminants, has highly increased during the past few years, as shown in Figure 1.
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and Technology for Water Purification in the Coming Decades. Nature 2008, 452, 301–310.
- Lei, M.; Zhang, L.; Lei, J.; Zong, L.; Li, J.; Wu, Z.; Wang, Z. Overview of Emerging Contaminants and Associated Human Health Effects. BioMed Res. Int. 2015, 2015, 404796.
- Kim, M.-K.; Zoh, K.-D. Occurrence and Removals of Micropollutants in Water Environment. Environ. Eng. Res. 2016, 21, 319–332.
- Psaltou, S.; Zouboulis, A. Catalytic Ozonation and Membrane Contactors—A Review Concerning Fouling Occurrence and Pollutant Removal. Water 2020, 12, 2964.




