Catalytic membrane ozonation is a hybrid process that combines membrane filtration and catalytic ozonation. The membrane deposited with an appropriate solid material acts as catalyst. As a consequence, the catalytic membrane contactor can act simultaneously as contactor (i.e., improving the transfer/dissolution of gaseous ozone into the liquid phase), as well as reactor (i.e., oxidizing the organic compounds). It can be used in water and wastewater treatment limiting the disadvantages of membrane filtration (i.e., lower removal rates of emerging contaminants or fouling occurrence) and ozonation (i.e., selective oxidation, low mineralization rates, or bromate (BrO3−) formation). The catalytic membrane ozonation process can enhance the removal of micropollutants and bacteria, inhibit or decrease the BrO3− formation and additionally, restrict the membrane fouling (i.e., the major/common problem of membranes’ use). Nevertheless, the higher operational cost is the main drawback of these processes.
- membrane catalytic ozonation
- ozone
- membrane filtration
- hybrid process
- fouling control
- micropollutants
- bromate formation
- cost analysis
- water treatment
1. Introduction
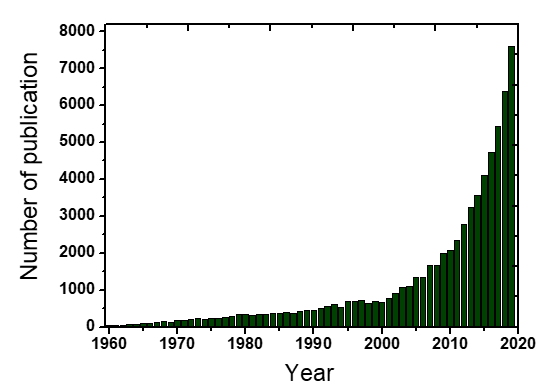
Clean water is very essential for the sustainable human future in planet Earth. However, water can be extensively contaminated and the development of innovate processes for its effective treatment and use constitutes a constantly evolving field [1]. Among the most recently detected pollution issues is considered the occurrence of emerging/persisting contaminants/chemicals in the aquatic ecosystems and especially, the presence of micropollutants. Micropollutants are termed the compounds of mostly anthropogenic origin that occurring usually in the aqueous environment in concentrations lower than mg/L (i.e., in the range of μg/L, or even ng/L). The evolution of analytical science and techniques especially during the recent years has permitted the accurate detection of them [1,2][1][2]. They can be divided into the following six main categories: (1) perfluorinated compounds (e.g., perfluorooctanesulfonate, perfluorooctanoic acid), (2) disinfection by-products (e.g., halomethanes, nitrosamines, hydroxyl acids), (3) gasoline additives (e.g., tert-butul ether, benzene, 1,3-butadiene), (4) manufactured nanomaterials (e.g., carbon nanotubes, silicon dioxide, titanium oxide), (5) human and veterinary pharmaceuticals (e.g., estradiol, 17α-ethinylestradiol, acetaminophen), and (6) sunscreens/ultraviolet filters (e.g., benzophenone-3, homosalate, 4-aminobenzoic acid) [2]. Micropollutants are omnipresent and generally contribute to improving the quality of human life, but they are consisting also an issue of major concern for the water and wastewater treatment plants. Micropollutants are mostly persist in the treated effluents, because the conventional systems are not specifically designed to remove them effectively [3]. The optimization of these treatment plants maybe a sufficient strategy for the efficient treatment of these problematic contaminants. Due to the respective developments the relevant scientific literature, regarding the treatment of emerging/persistent contaminants, has highly increased during the past few years, as shown in Figure 1.
The decontamination technologies generally have to be effective, cheap and eco-friendly in order to be widely applicable [1]. The Advanced Oxidation Processes (AOPs) present relevant advantages; among them belongs the membrane catalytic ozonation, a hybrid technique that can combine the processes of catalytic ozonation and of membrane filtration [4].
Figure 1. Yearly published scientific articles considering the emerging contaminants treatment from Science Direct [4].
2. Catalytic Ozonation
Catalytic ozonation is a highly studied advanced oxidation process (AOP) and it is based on the acceleration of hydroxyl radicals’ production (HO•) through the addition of appropriate materials (solids or dissolved), acting as catalysts. Ozone can be self-decomposed into hydroxyl radicals via a complex mechanism that can be summarized in the following reaction [5]:
|
3O3 + HO− + H+→ 2HO• + 4O2 |
(1) |
However, the addition of an appropriate catalyst can further increase the respective reaction rate and also, can decrease the relevant energy demands, which are considered as the main disadvantage of single ozonation process. Two main types of catalysts can be used in the catalytic ozonation technique, i.e., either several transition metals in dissolved form (leading to the "homogeneous" catalytic ozonation), or several solid materials, acting through their surface as potential catalysts (leading to the "heterogeneous" catalytic ozonation) [5]. Recently, the combination of membrane filtration with ozone has been studied extensively and this hybrid process can be performed via three modes, in which the ozonation can takes place before or after the membrane filtration, or both processes can operate simultaneously. In the catalytic ozonation the last (combined) mode is usually applied, while there are rather few studies, regarding the application of the other two alternative techniques [6][7][8].
When a solid material is used as catalyst, catalytic ozonation can be also divided in two sub-categories. Whether the catalyst is a solid material, suspended in the aqueous phase (usually in powdered form), the process is termed as "single heterogeneous catalytic ozonation"; whereas the process is commonly referred as "membrane catalytic ozonation", whether the solid material is appropriately immobilized onto the surface of membrane [4]. In this hybrid ozonation process the membrane can act simultaneously as catalyst for the acceleration of the hydroxyl radicals’ production, as well as a membrane contactor for the improvement of transfer and diffusion of ozone gas to the aqueous phase during treatment [9].
3. Catalytic Membrane Contactors
Membrane contactors (of polymeric or ceramic nature) nowadays are used in the gas-liquid processes, permitting the effective transfer of a constituent (usually gaseous) between a liquid and a gas phase [10]. In the case of ozonation process the ozone gas is diffused into the liquid phase most commonly by the application of injectors, bubble diffusers, or static mixers [11]. However, these techniques produce bubbles of rather larger diameters that are decreasing the respective mass-transfer rates, leading to unconsumed/unreacted ozone losses, and therefore to higher operational costs [12]. Alternatively, the application of membrane contactors is considered as a more eco-friendly technology [13] that can decrease the operational costs and increase the efficiency of the ozonation process, although the initial capital costs are higher, than the more conventional ones [10]. When solid materials are being used in ozonation processes (e.g., as catalysts) the nature of them should be carefully considered. Ozone is a strong oxidant agent that reacts and decomposes most organic compounds, including the polymeric materials [2]. As a consequence the use of common polymeric membrane contactors are quite limited [12]. It seems that only polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVDF), and polydimethylsiloxane (PDMS) polymeric membranes are presenting sufficient resistance, when contacting with ozone [4][14]. Alternatively, ceramic membranes can be often selected [12]. Considering the removal of micropollutants, the nano-filtration membranes are generally more efficient, mainly due to their smaller pore size and lower pressure operation, than, e.g., the reverse osmosis membranes [15].
Nevertheless, the applied contactors are mainly membranes and therefore, fouling may commonly occur, being among the main drawbacks of membrane application process. The fouling appears when organic particles/colloids and/or microorganisms are deposited on the surface of the membrane, increasing the trans membrane pressure (TMP). This incident acts as an additional resistance and decreases the efficiency of the process. It can be divided into four types: (a) complete pore blocking, (b) intermediate pore blocking, (c) standard pore blocking, and (d) surface cake layer. The formation of a cake layer in the surface of the membrane is usually observed [15].
The virgin membranes ordinarily do not present any particular catalytic activity; appropriate metal oxide particles have to be deposited (usually) onto them in order to accelerate the production of hydroxyl radicals. However, some materials, such as Al2O3, TiO2, and ZrO2, may present an inherent catalytic activity and can act as catalytic ceramic membrane contactors without further modification [16][17]. The most frequently used oxides are the iron and manganese oxides. Park et al. [18] coated a γ-Αl2O3 membrane with iron oxide nanoparticles and Chen et al. [19] coated a relevant membrane with a TiO2 and a Ti-Mn layer. In both cases the membrane fouling was significantly reduced through the increase of hydroxyl radicals’ production during the oxidation process. The same was observed also by Corneal et al. [20], when the manganese oxide (α-Mn2O3) was used as catalytic layer. The removal of micropollutants is based upon the same radicals’ production mechanism. Zhu et al. [21] deposited CeO2 on (inorganic) titania membrane to examine the removal of tetracycline by the application of catalytic ozonation. The concentration of micropollutant was reduced by 85%, due to the oxidation process, while the adsorption on the membrane surface contributed also to the removal. Furthermore, Guo et al. [22] deposited CuMn2O4 particles in a ceramic membrane, and observed that superoxide ions (O2•−) and hydrogen peroxide (H2O2) were produced during the oxidation process, which have the ability to accelerate further the production of hydroxyl radicals [5].
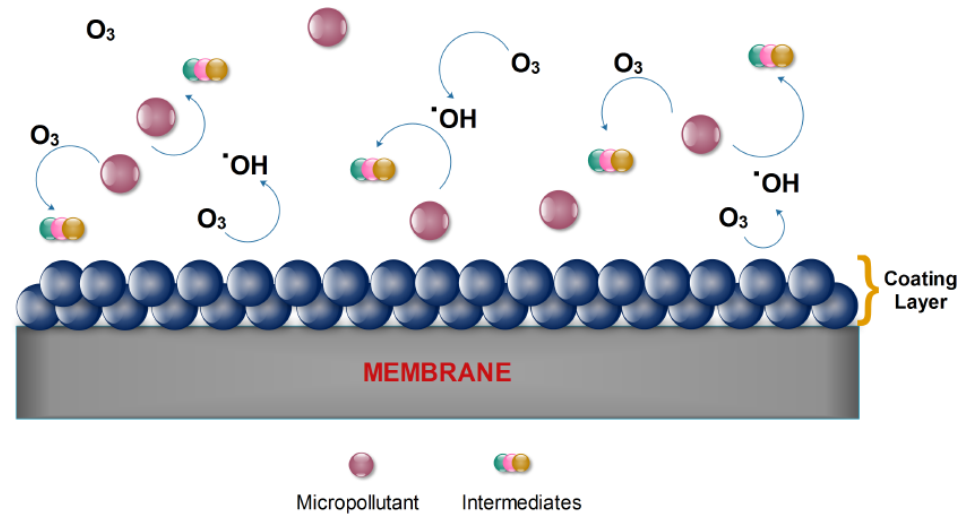
In most relevant ozonation cases the ceramic membranes have been commonly applied in the catalytic ozonation systems, because they are fully ozone resistant and the substrate material is simpler to be used as coating substrate. However, Yu et al. [23] who have coated a PVDF membrane with MnO2 nanoparticles; they reported that the presence of these oxide particles lowered the fouling rate and the presence of ozone has limited the bio-accumulation of several components onto the membranes’ surface, hence restricting the fouling problem. Furthermore, Sun et al. [24] have doped a similar membrane with TiO2 nanoparticles to examine the removal of nitrobenzene. Under acidic pH values the removal efficiency of this pollutant was rather low, but it was further increased with the increase of pH values up to 10, due to the higher production of hydroxyl radicals. Nevertheless, a further increase of pH value leaded to a quick decompose of hydroxyl radicals into simple ions, hence reducing the removal rate. Zhu et al., [25] used for the ozonation treatment of municipal wastewater the same catalytic membrane and observed that only the larger molecular weight compounds were degraded into smaller ones. The produced micro-molecules could not be removed effectively by this membrane, and thus the overall COD value of the wastewater was reduced only by 27.6%. Figure 2 shows schematically the fundamental mechanism of catalytic membrane ozonation process.
Figure 2. Fundamental mechanism of catalytic membrane ozonation process (adapted from [4]).
In order the catalytic effect to be employed the ozone dosing must be continuous. When only a single ozone dose at the beginning of the process is applied, then the oxidation takes place at the first min of the reaction and the ozone concentration in the system is quickly substantially decreasing; as a result membrane filtration takes over and the membrane fouling can occur [16,26][16][26].
There are five main factors that influence the catalytic performance of a membrane: (1) surface charge; (2) coating times; (3) pore size; (4) preparation method regarding the metal oxide deposition; and (5) adsorption capacity [15,18,27][15][18][27]. More specific information about how these factors are influencing the catalytic membrane ozonation can be found in the respective reference of Psaltou and Zouboulis [4].
Almost all researchers, studying this process, have observed that the catalytic membrane ozonation follows a mechanism based on the production of radicals. The prominent radical species in most cases were the hydroxyl ones, noting also that in some cases both hydroxyl and superoxide radicals were found to participate in the oxidation process [27]. However, Scarati et al. [28] have noticed that when CuO was used as (catalytic) coating for membranes, then the primary radical species were the superoxide radicals, which however did not lead to the further production of hydroxyl radicals. Although, the superoxide radicals are part of the ozone decomposition mechanism towards the formation of hydroxyl radicals [29[29][30],30], it seems that in the aforementioned case the decomposition pathway was different. However, the obtained results were not very promising and the catalytic ozonation with the use of this coated membrane may not be sufficiently effective, when applied for the removal/treatment of micropollutants.
Except for the removal of micropollutants and the prevention of membrane fouling some researchers have used the membrane catalytic ozonation process also for the removal of bacteria. The relevant studies have shown that bacteria adhere to the metal oxide surface, increasing their retention time at the membrane and subsequently, their contact with ozone will be prolonged, improving the efficiency of oxidation. The inactivation of bacteria occurs by the presence of radical species and especially by the hydroxyl radicals, similar with the case of micropollutants removal. Therefore, the radical mechanism can enhance also the disinfection behavior of this treatment system [31,32][31][32]. All the aforementioned studies are summarized in Table 1.
Table 1. Summary of studies regarding wastewater treatment by the application of membrane catalytic ozonation.
|
Catalytic Membrane |
Target Parameter/Pollutant |
Efficiency |
Reference |
|
α-Al2O3 |
Color TOC (WWTP effluent) |
68% 21% |
[16] |
|
TiO2 |
88% 43% |
||
|
Cement |
p-chloro-nitro-benzene |
90% |
[17] |
|
IONs*/α-Al2O3 |
p-chlorobenzoic acid (River water) |
56% |
[18] |
|
Ti-Mn/TiO2/Al2O3 |
NOM (aquaculture wastewater) |
52.1% |
[19] |
|
α-Mn2O3/CéRAM |
Flux (Lake water) |
95% (flux recovery) |
[20] |
|
CeO2-TiO2/α-Al2O3 |
Tetracycline |
>80% |
[21] |
|
CuMn2O4/ZrO2/α-Al2O3 |
Benzophenone |
76.6% |
[22] |
|
MnO2/PVDF |
Model raw water |
Prevention of membrane fouling |
[23] |
|
Nano-TiO2/PVDF |
Nitrobenzene |
59.5% |
[24] |
|
Nano-TiO2/PVDF |
COD (Municipal wastewater after primary treatment) |
27.6% |
[25] |
|
TiO2/α-Al2O3 |
Color |
88.5% |
[26] |
|
TOC (Municipal wastewater treatment) |
48.7% |
||
|
CuO/α-Al2O3-ZrO2 |
1,4-dioxane |
65% |
[28] |
|
Iron Oxide/CéRAM |
E. Coli (Lake water) |
>99%(mortality) |
[31] |
*IONs: Iron Oxide Nanoparticles.
4. Important Issues of Consideration, When Applying the Ozonation Process
4.1. Bromate Formation
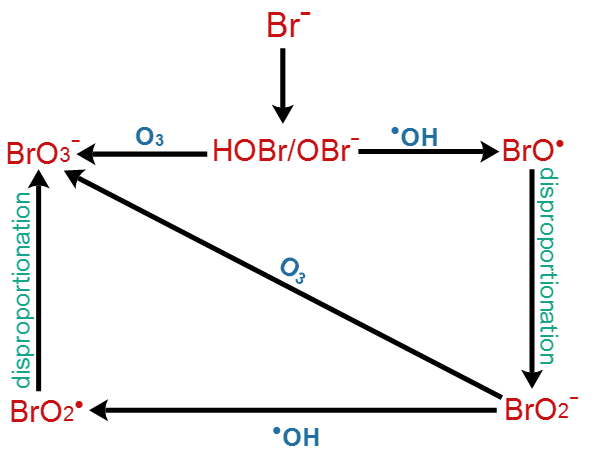
Although, ozone has been applied widely in drinking water and in wastewater treatment, its use may cause secondary problems, when the water to be treated contains bromide (Br−) at concentrations higher than 50 μg/L. The Br− concentrations in natural waters may usually range between 4 and 1000 μg/L [33]. Due to salt intrusion, Br− is detected in surface waters, as well as in the influent of wastewater treatment plants at concentrations up to 400 μg/L [34]. The main by-product of the reaction between ozone and Br- is bromate (BrO3−). BrO3− once formed, it is neither biodegradable nor removable by the application of conventional treatment [33]. It is considered as a potential carcinogen (Group 2B) by the International Agency for Research on Cancer (IARC) [34]. The maximum permissible contaminant level for BrO3− is 10 μg/L, according to USEPA and Drinking Water Commission of Europe [35]. Due to that the minimization or the prevention of its production in water treatment is crucial [33]. Figure 3 shows the formation of BrO3− via the direct ozonation and through the radical mechanism, noting that BrO3− is mostly produced by the 2nd (radical) pathway. It is reported that in Milli-Q water experiments, almost 70% of BrO3− formation occurs through the hydroxyl radicals oxidation reactions and the remaining 30% depends on the molecular ozone reactions [36]. More specific details about the reaction pathways, regarding the production of BrO3− in ozonation processes can be found in Fischbacher et al. 2015 [37].
Figure 3. Comparison of the molecular ozone mechanism and the •OH mechanism in the presence of bromide anions (Br−) for the production of bromates (adapted from [35]).
There are four main factors, affecting BrO3- formation, in ozonation processes [33]:
- pH value: In alkaline pH region the BrO3- formation is higher. The reaction rate constants of Br
3
-/OBr- with molecular ozone and with hydroxyl radicals are 160 M−1s−1/330 M−1s−1 and 1.1 * 109 M−1s−1/4.5 * 109 M−1s−1, respectively. When the solution pH increased, more hydroxyl radicals are formed, leading to more Br- formation is higher. The reaction rate constants of Br-
- and hypobromide ions (OBr/OBr- with molecular ozone and with hydroxyl radicals are 160 M
-), which are subsequently oxidized to form BrO−1s−1/330 M−1s−1 and 1.1 * 109 M−1s−1/4.5 * 109 M−1s
3- [34].pH value: In alkaline pH region the BrO−1, respectively. When the solution pH increased, more hydroxyl radicals are formed, leading to more Br- and hypobromide ions (OBr-), which are subsequently oxidized to form BrO3- [34].
- Initial bromide concentration: The production of BrO3- increase with the increase of Br-
Initial bromide concentration: The production of BrO3- increase with the increase of Br-
- Ozone dose: The BrO3- concentration increases with an increased ozone dose. In membrane ozonation there is a strong linear relationship between the BrO3- concentration in the permeate and the dissolved ozone concentration [36].
Ozone dose: The BrO3- concentration increases with an increased ozone dose. In membrane ozonation there is a strong linear relationship between the BrO3- concentration in the permeate and the dissolved ozone concentration [36].
- Temperature: The BrO3- formation [38]. Table 2 shows the ozone escape to the gas phase at the third min of ozonation process, as well as the respective half-lives at various temperatures.
Temperature: The BrO3
-concentration increases with increasing temperature up to 25 °C, because at relatively higher temperature (>25-concentration increases with increasing temperature up to 25 °C, because at relatively higher temperature (>25 °C) the ozone stability also becomes a major factor. Additionally, according, to Henry’s law, the efficiency of ozone solubility in an ozonation system decreases with the increase of temperature. Under these conditions the ozone concentration could be even below the respective threshold limit required for BrO3- formation [
°C) the ozone stability also becomes a major factor. Additionally, according, to Henry’s law, the efficiency of ozone solubility in an ozonation system decreases with the increase of temperature. Under these conditions the ozone concentration could be even below the respective threshold limit required for BrO38]. Table 2 shows the ozone escape to the gas phase at the third min of ozonation process, as well as the respective half-lives at various temperatures.
3
Table 2. Ozone escape to the gas phase at the 3rd min of ozonation process and its typical half-life at various temperatures [39,40][39][40].
|
Temperature (°C) |
Ozone Escape (%) |
Half-Life (min) |
|
15 |
7.7 |
30 |
|
25 |
9.4 |
15 |
|
35 |
11.1 |
8 |
In terms of BrO3- formation control, the application of heating process is financially unprofitable. Cooling down the ozonation system is preferable, if there is already an adequate cooling device in the treatment system [38]. The reduction of pH/temperature or the addition of carbonate alkalinity are more effective ways to control the BrO3- production; however, it is neither cost effective, nor practical considering full-scale applications [34,36][34][36]. Furthermore, the control of these parameters may present also negative effects regarding the micropollutants removal, e.g., by slowing down the production of hydroxyl radicals [39]. Therefore, a key challenge is to develop a process that can effectively degrades micropollutants and simultaneously minimizes the formation of BrO3- [36]. To overcome these drawbacks, the heterogeneous catalytic ozonation process was introduced as a potential solution [34]. However, catalytic ozonation can enhance the production of hydroxyl radicals and because the hydroxyl radicals are mainly responsible for the BrO3- formation, its application must be carefully studied [41].
Liu et al. [38] applied the catalytic ozonation process with the use of different oxides to control BrO3- formation. The addition of magnesium (MgO) and titanium oxide (TiO2) promoted the formation of BrO3-, while the presence of KMnO4 inhibited that production. More particularly, by increasing the KMnO4 dose the noted inhibition was further enhanced. Increased production of hydroxyl radicals was observed in the MgO/O3 and TiO2/O3 systems. On the contrary, the ozone decomposition, but not towards hydroxyl radicals, was promoted by the KMnO4 addition and as a consequence, the oxidation ability of the treatment system was reduced.
The most applicable catalyst, as reported in the literature for the elimination of BrO3- formation, is CeO2. Wang et al. [42] showed that CeO2 can be a good inhibitor for the BrO3- production. However, the organic micropollutants removal efficiency by using the O3/CeO2 process was rather small. The same results were reported by Zhang et al. [43], who observed that the inhibition effect was optimal at slightly acidic conditions, while at the neutral pH value the efficiency of process was decreased considerably. This limitation significantly restricts the application of this catalyst in drinking water treatment processes.
Ye et al. [41] showed that the BrO3- formation rate by using graphene oxide (GO) as catalyst was almost twice, than the case where the single ozonation was applied. Moreover, by adding Tert-Butyl-Alcohol (TBA), the BrO3- formation was decreased to 25%, because the hydroxyl radicals’ production was limited. However, when the 0.36CeO2/RGO (reduced graphene oxide) material was used as catalyst, the degradation efficiency of DEET (N,N-Diethyl-meta-toluamide) was enhanced during the ozonation process, but adsorption experiments were not conducted, aiming to eliminate the possibility this micropollutant (DEET) to be simply adsorbed by GO. Alongside, Ye et al. were compared the formation of BrO3- by the addition of various metal oxides in the ozonation system. After 10 min, the BrO3- production during the O3, O3/CeO2, O3/FeOOH, O3/Fe2O3, or O3/ZnO processes were found 1.41, 0.59, 1.34, 0.92, and 1.74 μM, respectively. All these concentrations were lower than the BrO3- produced during the application of single ozonation except for the O3/ZnO process, in which 1.74 μM of BrO3- was formed. CeO2 achieved an inhibition efficiency of 58% for BrO3- formation; ozone adsorbed to CeO2 active sites and the decomposition of ozone to hydroxyl radicals was decreased. Furthermore, as Shen et al. [44] observed, the use of a relevant catalyst was found to decreased the degradation efficiency of p-CNB (p-chloro-nitro-benzene), as this compound can be practically removed only by hydroxyl radicals’ mechanism.
The material LaCoO3 was also examined by Zhang et al. [34], as catalyst for the elimination of BrO3- formation and for the removal of organic compounds during the application of catalytic ozonation. When the concentration of benzotriazole (BEN) was 0.148 mg/L, the formed BrO3-reached the highest concentration and the Br- conversion rate was 89.4%. However, when the BEN concentration increased, then the formed BrO3- decreased significantly. This might be due to the competition reactions taken place between BEN and Br- with the ozone molecules and the hydroxyl radicals. The formed BrO3- in catalytic ozonation was lower than that formed during the application of single ozonation at each pH value, suggesting that catalytic ozonation is more efficient process for the reduction of both BrO3- and micropollutant (BEN). At the pH value 7.22 (i.e., a typical value for a drinking water treatment effluent) the degradation of BEN was enhanced and the BrO3- formation was reduced simultaneously [34]. However, this study did not show whether the BEN reduction can be considered enough for a micropollutant treatment process. In general, as von Gunten et al. [35] reported, BrO3- formation can be lower in an advanced oxidation processes, e.g., when compared to single ozonation, if the ozone dosage is kept constant.
Another way (and probably more effective) to lower the BrO3- formation is the use of membranes. Moslemi et al. [36] observed that under constant operating pressure, as the molecular weight cut-off (MWCO) of the membrane increased, the permeate flux also increased, while the residence time in the treatment system decreased. Therefore, for the membranes presenting higher MWCOs, the ozone exposure (i.e., the retention time multiplied by the dissolved ozone concentration) was reduced. Therefore, in such hybrid systems there is a another (6th) factor that affects the BrO3- formation, which is the contact (residence) time of ozone with Br- in the membrane and the entire treatment system.
Hamid et al. [45] studied also the formation of BrO3-using a hybrid filtration-ozonation system. The results suggested that the BrO3- formation can be minimized when using ceramic membranes. The BrO3-formed by the application of membrane ozonation was 50% lower, than that of the ozonation using a conventional diffuser. The reduced BrO3- formation observed in this study may be due to the lower ozone exposure with Br-, due to the catalytic formation of hydroxyl radicals (even in small extent) at the surface of the membrane. The reduction of BrO3- production could also explained by the surface properties of ceramic membrane that can cause the attraction or repulsion of ions. When a charged membrane is in contact with an electrolyte solution, the electrostatic repulsion will repel ions with the same charge, because the concentration of these ions will be generally lower near the membrane surface and within the membrane pores; inversely, the membrane surface will attract ions having the opposite charge, resulting in higher concentrations (locally) of them. The surface properties of ceramic membranes can play an important role and are worthy to be explored further as an additional mechanism for BrO3-control.
Merle et al. [46] combined homogeneous catalytic ozonation with membranes in the so-called MEMBRO3X process, noting also that for the efficient removal of p-chlorobenzoic acid (p-CBA) are required large H2O2 dosages (5.67 mg/L). At this dose and for an ozone concentration 1.0 g/Nm−3 around 98% of p-CBA was degraded and about 6 μg/L of BrO3- was formed, whereas the BrO3-formation were about 79% higher in comparison with the conventional peroxone reaction. The key feature of the MEMBRO3X process is the addition of ozone over multiple injection points. In ozone-based processes for BrO3- formation can be limited by keeping the O3 concentration low enough, which is achieved by dosing it in small quantities over the membrane contactor device and transforming it efficiently to hydroxyl radicals, which can further react with the micropollutants [35].
Until now there is no study conducted about the control of BrO3- formation during the catalytic membrane ozonation process. This process, due to the dual role played by the membrane (i.e., acting as a diffuser and as a reactor), has the potential to remove efficiently the micropollutants, as well as to minimize the BrO3-formation and simultaneously, to disinfect the treated water; therefore, presenting certain significant advantages. Table 3 summarizes the studies about bromate formation during catalytic ozonation process.
Table 3. Bromate formation during the application of catalytic ozonation process.
|
Used Catalyst |
Experimental Conditions |
Bromate Formation (μg/L) |
Reference |
|
LaCoO3 |
[Br−] = 100 μg/L [O3] = 2 mg/L [catalyst] = 0.25 g/L pH = 6.41 |
84.4 |
34 |
|
MgO |
[Br−] = 50 mg/L |
≈80 |
38 |
|
TiO2 |
[O3] = 0.4 mg/L |
≈135 |
|
|
KMnO4 |
[catalyst] ≈ 4 mg/L |
≈35 |
|
|
CeO2 |
[Br-] = 1 mg/L [O3] = 10 mg/L*min [catalyst] = 0.5 g/L pH = 7 time = 10 min |
75.5 |
41 |
|
FeOOH |
171.4 |
||
|
Fe2O3 |
117.7 |
||
|
ZnO |
222.5 |
||
|
CeO2 |
[Br−] = 1.8 mg/L [O3] = 5.21 mg/L [catalyst] = 0.1 g/L pH = 6.30 T = 18 °C Time = 30 min |
205.58 |
42 |
|
MgO |
471.01 |
||
|
FeOOH |
351.53 |
||
|
CeO2 |
[Br−] = 2 mg/L [O3] = 4.51 mg/L [catalyst] = 0.1 g/L pH = 6.20 T = 15 °C Time = 30 min |
<300 |
43 |
|
α-FeOOH |
≈350 |
||
|
γ-FeOOH |
<350 |
||
|
α-Fe2O3 |
≈450 |
||
|
MEMBRO3X |
[Br−] = 180 ± 4 μg/L [O3] = 5 g/m3 [catalyst] = 5.67 mg/L pH = 8.1 T = 20 °C time = 25 min |
≈35 |
46 |
4.2. Operational Cost Evaluation
Except for the BrO3- formation another major drawback of ozonation is the relatively high energy consumption that leads to higher operation costs. In general there are two main parameters that can make a technology suitable for the industry: (1) Technical, and (2) Economical feasibility [47]. High capital, operation and maintenance costs reflect negatively on any applicable method [48]. High energy consumption leads to a costly unit with a larger footprint treatment [49] and influences the operational cost. In the single ozonation process ozone dose is the main factor that influences in large extent the relevant cost. Table 4 shows, the operational costs, according to different ozone doses, as well as the micropollutant degradation efficiency obtained for each dose. Increasing ozone dosages from 2 to 6 mg/L leads to 64% cost increase.
Table 4. Electrical Energy and Operating Cost required for the ozonation process (adapted from [50]).
|
Ozone Concentration (mg/L) |
Electrical Energy (kWhm−3) |
No. of PPCPs Removed by ≥90% Efficiency from a Total Number 37 |
Operational Cost (Yen m−3) |
|
2 |
0.03 |
24 |
0.5 |
|
4 |
0.06 |
32 |
0.9 |
|
6 |
0.09 |
35 |
1.4 |
The composition of the influent to be treated also affects the operational cost of the process, although indirectly, through the determination of the necessarily applied ozone dosage, because some pollutants are less degradable than others and they need higher oxidant concentrations to be efficiently treated. Furthermore, the applied ozone concentration is related to the initial micropollutant concentration [48]. Table 5 shows the removal efficiency of different micropollutants with the use of different ozone dosages and the respective operational cost of the treatment system. As becomes obvious, phenol having 100 times higher initial concentration, needs 3 times lower ozone dose to be effectively removed, than the tri-chloro-ethylene (TCE). TCE with reaction rate constant with ozone (kO3) equal to 17 M−1·s−1 [51] is more difficult to be oxidized, than phenol presenting much higher kO3, equal to 1.3 * 103 M−1·s−1 [52].
Table 5. Cost estimation for the removal of different pollutants by the application of ozonation (adapted from [47]).
|
Pollutant |
Ozone Concentration (mg/L) |
Pollutant Concentration (mg/L) |
Efficiency (%) |
Operational Cost ($/1000 gal) |
|
Phenol |
2 |
235.28 |
90 |
1.2023 |
|
Reactive azo dye |
12.4 |
20 |
90 |
4.0839 |
|
TCE |
6 |
2.2 |
40.9 |
2.3549 |
Advanced oxidation processes (AOPs) are generally more complex to handle (e.g., for ozone generation, light sources, pumps, etc.) and are presenting usually higher energy costs. For that reason, they are still categorized as rather expensive processes [53]. However, among the various AOPs the application costs differs widely. Catalytic and photocatalytic ozonation are two of the main processes in this category. Table 6 shows the energy consumption and the treatment cost for the removal of oxalic acid from aqueous solutions by the application of three different AOPs. All these processes in the study were performed with the use of TiO2 as catalyst for comparison reasons. The TiO2 proved to be inefficient catalyst for the catalytic ozonation process and the removal of the micropollutant was quite small, noting also that the treatment cost of catalytic ozonation may have been decreased with the use of an optimum catalyst. Furthermore, considering the oxidation of oxalic acid, 25 mg/L and 70 mg/L of O3 was applied in the catalytic and photocatalytic ozonation processes respectively (in the gas phase), which means that the ozone concentration in the liquid phase was about 10 mg/L and 25 mg/L [53]. Thus, the photocatalytic ozonation seems more cost efficient treatment process because the relevant economic parameters were calculated based on the cost per micropollutant removal and not per treated volume.
Table 6. Specific energy consumption and treatment costs of photocatalytic oxidation, photocatalytic ozonation and catalytic ozonation for the removal of oxalic acid (adapted from [53]).
|
AOPs |
Specific Energy Consumption (kWh/mM) |
Treatment Costs (euro/m3) |
|
Catalytic ozonation |
0.017 |
3.65 |
|
Photocatalytic oxidation |
0.063 |
13.55 |
|
Photocatalytic ozonation |
0.007 |
1.51 |
Another parameter that can affect the cost of catalytic ozonation process is the specific type of the catalyst and its potential reusability [55][54]. For example, Biard et al., [54][55] select ruthenium among other metals (Co, Pd, Pt, and Rh) not only for its better reactivity, but also for its lower cost (about 30 euros/g). From the same perspective Martins et al. [55][54] examined three low-cost materials (i.e., volcanic rock, sepiolite, and iron shavings) as potential catalysts for the treatment of olive mill wastewater by the application of heterogeneous catalytic ozonation. Iron shavings proved to be the most efficient catalyst among the others, when the same ozone dose was applied; therefore the ozonation system can achieve the lower operational cost.
5. Limitations and Future Perspectives
Although the catalytic membrane ozonation seems to be a quite promising technology, the stability of applied coatings may be considered as an important issue of concern. In several cases the initially deposited metals can be leached back into the aqueous solution during the reaction procedure, leading to a secondary pollution problem. Even for the metals that specific regulation limits have not been yet established (e.g., for the case of Sn) their secondary occurrence in drinking water supply may raise health issues for the living organisms that should be carefully addressed [56,57][56][57]. Furthermore, the formation of by-products during the oxidation process must be studied in depth, as it possibly constitutes an additional pollution source. These by-products can be produced due to the incomplete mineralization of organic pollutants and their acute toxicity in some cases is even higher than the initially oxidized compounds [58]. Gomes et al., [58] studied the ecotoxicity of a five parabens mixture (methyl-, ethyl-, propyl-, benzyl-, and butylparaben) and their by-products. They found that although catalytic ozonation could degrade efficiently the parabens, the treated sample was more toxic than the initial one. It presented higher toxicity over D. Magna, R. subcapitata and L. savitum. The most toxic by-products were hydroquinone and 1,4-benzoquinone. Hydroquinone is also one of the by-products of quaiacol oxidation, a well-known chemical intermediate for food materials and perfumery products [59]. Therefore, the conduct of toxicity assays is essential.
In terms of materials science, the coating techniques should be further developed to facilitate the further use of catalytic membranes. New solid materials, deposited into the membranes surface to convert them into catalytic ones, should be also examined. Additionally, ozone-resistant polymeric membranes or coated materials that make them more stable against the contact with ozone should be developed and accompanied with new techniques that make easier the deposition of catalytic materials. These developments are expected to lead to an increase of catalyst ranges/options, raising further the effective/economic applications of catalytic membrane ozonation process.
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and Technology for Water Purification in the Coming Decades. Nature 2008, 452, 301–310, doi:10.1038/nature06599.
- Lei, M.; Zhang, L.; Lei, J.; Zong, L.; Li, J.; Wu, Z.; Wang, Z. Overview of Emerging Contaminants and Associated Human Health Effects. BioMed Res. Int. 2015, 2015, 1–12, doi:10.1155/2015/404796.
- Kim, M.-K.; Zoh, K.-D. Occurrence and Removals of Micropollutants in Water Environment. Environ. Eng. Res. 2016, 21, 319–332, doi:10.4491/eer.2016.115.
- Psaltou, S.; Zouboulis, A. Catalytic Ozonation and Membrane Contactors—A Review Concerning Fouling Occurrence and Pollutant Removal. Water 2020, 12, 2964, doi:10.3390/w12112964.
- Gottschalk, C.; Libra, J.A.; Saupe, A. Ozonation of Water and Waste Water. A Practical Guide to Understanding Ozone and Its Application; Wiley-VCH: Weinheim, Germany, 2010.
- Zhang, Y.; Zhao, P.; Li, J.; Hou, D.; Wang, J.; Liu, H. A Hybrid Process Combining Homogeneous Catalytic Ozonation and Membrane Distillation for Wastewater Treatment. Chemosphere 2016, 160, 134–140, doi:10.1016/j.chemosphere.2016.06.070.
- Gu, L.; Tang, X.; Sun, Y.; Kou, H. Bioavailability of Dissolved Organic Matter in Biogas Slurry Enhanced by Catalytic Ozonation Combined with Membrane Separation. Ecotoxicol. Environ. Safety 2020, 196, 110547, doi:10.1016/j.ecoenv.2020.110547.
- Chen, J.; Tian, S.; Kong, L.; Tu, Y.; Lu, J.; Xiong, Y. Efficient Degradation of Nitrobenzene by an Integrated Heterogeneous Catalytic Ozonation and Membrane Separation System with Active MgO(111) Catalyst. Desalination Water Treat. 2015, 56, 2168–2180, doi:10.1080/19443994.2014.961561.
- Ozdemir, S.; Buonomenna, M.; Drioli, E. Catalytic Polymeric Membranes: Preparation and Application. Appl. Catal. A Gen. 2006, 307, 167–183, doi:10.1016/j.apcata.2006.03.058.
- Stylianou, S.K.; Szymanska, K.; Katsoyiannis, I.A.; Zouboulis, A.I. Novel Water Treatment Processes Based on Hybrid Membrane-Ozonation Systems: A Novel Ceramic Membrane Contactor for Bubbleless Ozonation of Emerging Micropollutants. J. Chem. 2015, 2015, 214927, doi:10.1155/2015/214927.
- Berry, M.; Taylor, C.; King, W.; Chew, Y.; Wenk, J. Modelling of Ozone Mass-Transfer through Non-Porous Membranes for Water Treatment. Water 2017, 9, 452, doi:10.3390/w9070452.
- Li, K.; Xu, L.; Zhang, Y.; Cao, A.; Wang, Y.; Huang, H.; Wang, J. A Novel Electro-Catalytic Membrane Contactor for Improving the Efficiency of Ozone on Wastewater Treatment. Appl. Catal. B Environ. 2019, 249, 316–321, doi:10.1016/j.apcatb.2019.03.015.
- Janknecht, P.; Picard, C.; Larbot, A.; Wilderer, P.A. Membrane Ozonation in Wastewater Treatment. Acta Hydrochim. Hydrobiol. 2004, 32, 33–39, doi:10.1002/aheh.200300521.
- Zoumpouli, G.; Baker, R.; Taylor, C.; Chippendale, M.; Smithers, C.; Xian, S.; Mattia, D.; Chew, Y.; Wenk, J. A Single Tube Contactor for Testing Membrane Ozonation. Water 2018, 10, 1416, doi:10.3390/w10101416.
- Madsen, H.T. Membrane Filtration in Water Treatment—Removal of Micropollutants. In Chemistry of Advanced Environmental Purification Processes of Water; Elsevier: Amsterdam, The Netherlands, 2014; pp. 199–248, doi:10.1016/B978-0-444-53178-0.00006-7.
- Zhu, B.; Hu, Y.; Kennedy, S.; Milne, N.; Morris, G.; Jin, W.; Gray, S.; Duke, M. Dual Function Filtration and Catalytic Breakdown of Organic Pollutants in Wastewater Using Ozonation with Titania and Alumina Membranes. J. Membr. Sci. 2011, 378, 61–72, doi:10.1016/j.memsci.2010.11.045.
- Wang, Z.; Chen, Z.; Chang, J.; Shen, J.; Kang, J.; Chen, Q. Fabrication of a Low-Cost Cementitious Catalytic Membrane for p-Chloronitrobenzene Degradation Using a Hybrid Ozonation-Membrane Filtration System. Chem. Eng. J. 2015, 262, 904–912, doi:10.1016/j.cej.2014.10.033.
- Park, H.; Kim, Y.; An, B.; Choi, H. Characterization of Natural Organic Matter Treated by Iron Oxide Nanoparticle Incorporated Ceramic Membrane-Ozonation Process. Water Res. 2012, 46, 5861–5870, doi:10.1016/j.watres.2012.07.039.
- Chen, S.; Yu, J.; Wang, H.; Yu, H.; Quan, X. A pilot-scale coupling catalytic ozonation- membrane filtration system for recirculating aquaculture wastewater treatment. Desalination 2015, 363, 37–43, doi:10.1016/j.desal.2014.09.006.
- Corneal, L.M.; Baumann, M.J.; Masten, S.J.; Davies, S.H.R.; Tarabara, V.V.; Byun, S. Mn oxide coated catalytic membranes for hybrid ozonation-membrane filtration: Membrane microstructural characterization. J. Membr. Sci. 2011, 369, 182–187, doi:10.1016/j.memsci.2010.11.071.
- Zhu, Y.; Quan, X.; Chen, F.; Fan, X.; Feng, Y. CeO2-TiO2 Coated Ceramic Membrane with Catalytic Ozonation Capability for Treatment of Tetracycline in Drinking Water. Sci. Adv. Mater. 2012, 4, 1191–1199, doi:10.1166/sam.2012.1413.
- Guo, Y.; Song, Z.; Xu, B.; Li, Y.; Qi, F.; Croue, J.-P.; Yuan, D. A Novel Catalytic Ceramic Membrane Fabricated with CuMn2O4 Particles for Emerging UV Absorbers Degradation from Aqueous and Membrane Fouling Elimination. J. Hazard. Mater. 2018, 344, 1229–1239, doi:10.1016/j.jhazmat.2017.11.044.
- Yu, W.; Brown, M.; Graham, N.J.D. Prevention of PVDF Ultrafiltration Membrane Fouling by Coating MnO2 Nanoparticles with Ozonation. Sci. Rep. 2016, 6, 30144, doi:10.1038/srep30144.
- Sun, M.J.; Zhang, C.; Yang, C.; Zhang, T. Degradation of Nitrobenzene by Nano-TiO2/PVDF Membrane Catalytic Ozonation. In Advances in Intelligent and Soft Computing, Proceedings of the 2011 International Conference on Informatics, Cybernetics, and Computer Engineering (ICCE2011), Melbourne, Australia, 19–20 November 2011; Jiang, L., Ed., Springer: Berlin/Heidelberg, Germany, 2011; Volume 112, doi:10.1007/978-3-642-25194-8_89.
- Zhu, Y.; Zhang, H.; Zhang, X. Study on Catalytic Ozone Oxidation with Nano-TiO2 Modified Membrane for Treatment of Municipal Wastewater. Asian J. Chem. 2014, 26, 3871–3874, doi:10.14233/ajchem.2014.15980.
- Hu, Y.; Milne, N.; Gray, S.; Morris, G.; Jin, W.; Duke, M.; Zhu, B. Combined TiO2 membrane filtration and ozonation for efficient water treatment to enhance the reuse of wastewater. Desalination Water Treat. 2011, 34, 57–62, doi:10.5004/dwt.2011.2867.
- Lee, W.J.; Bao, Y.; Hu, X.; Lim, T.-T. Hybrid Catalytic Ozonation-Membrane Filtration Process with CeOx and MnOx Impregnated Catalytic Ceramic Membranes for Micropollutants Degradation. Chem. Eng. J. 2019, 378, 121670, doi:10.1016/j.cej.2019.05.031.
- Scaratti, G.; De Noni Júnior, A.; José, H.J.; de Fatima Peralta Muniz Moreira, R. 1,4-Dioxane Removal from Water and Membrane Fouling Elimination Using CuO-Coated Ceramic Membrane Coupled with Ozone. Environ. Sci. Pollut. Res. 2020, 27, 22144–22154, doi:10.1007/s11356-019-07497-6.
- Von Sonntag, C.; von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment. From Basic Principals to Applications; IWA Publishing: London, UK, 2012.
- Popiel, S.; Nalepa, T.; Dzierżak, D.; Stankiewicz, R.; Witkiewicz, Z. Rate of Dibutylsulfide Decomposition by Ozonation and the O3/H2O2 Advanced Oxidation Process. J. Hazard. Mater. 2009, 164, 1364–1371, doi:10.1016/j.jhazmat.2008.09.049.
- Karnik, B.S.; Davies, S.H.; Baumann, M.J.; Masten, S.J. Removal of Escherichia Coli after Treatment Using Ozonation-Ultrafiltration with Iron Oxide-Coated Membranes. Ozone Sci. Eng. 2007, 29, 75–84, doi:10.1080/01919510601139492.
- Davies, S.H.; Baumann, M.J.; Byun, S.; Corneal, L.M.; Tarabara, V.V.; Masten, S.J. Fabrication of Catalytic Ceramic Membranes for Water Filtration. Water Supply 2010, 10, 81–86, doi:10.2166/ws.2010.789.
- Gounden, A.N.; Jonnalagadda, S.B. Advances in Treatment of Brominated Hydrocarbons by Heterogeneous Catalytic Ozonation and Bromate Minimization. Molecules 2019, 24, 3450, doi:10.3390/molecules24193450.
- Zhang, Y.; Ji, H.; Liu, W.; Wang, Z.; Song, Z.; Wang, Y.; Liu, C.; Xu, B.; Qi, F. Synchronous Degradation of Aqueous Benzotriazole and Bromate Reduction in Catalytic Ozonation: Effect of Matrix Factor, Degradation Mechanism and Application Strategy in Water Treatment. Sci. Total Environ. 2020, 727, 138696, doi:10.1016/j.scitotenv.2020.138696.
- von Gunten, U.; Bruchet, A.; Costentin, E. Bromate Formation in Advanced Oxidation Processes. J. Am. Water Works Assoc. 1996, 88, 53–65, doi:10.1002/j.1551-8833.1996.tb06571.x.
- Moslemi, M.; Davies, S.H.; Masten, S.J. Bromate Formation in a Hybrid Ozonation-Ceramic Membrane Filtration System. Water Res. 2011, 45, 5529–5534, doi:10.1016/j.watres.2011.08.015.
- Fischbacher, A.; Löppenberg, K.; von Sonntag, C.; Schmidt, T.C. A New Reaction Pathway for Bromite to Bromate in the Ozonation of Bromide. Environ. Sci. Technol. 2015, 49, 11714–11720, doi:10.1021/acs.est.5b02634.
- Liu, Z.; Cui, Y.; Chen, J.; Yan, Z. The Control of Bromate Formation in Ozonation of Bromide-Containing Water. Desalination Water Treat. 2014, 52, 4942–4946, doi:10.1080/19443994.2013.809994.
- Psaltou, S.; Kaprara, E.; Kalaitzidou, K.; Mitrakas, M.; Zouboulis, A. The Effect of Thermal Treatment on the Physicochemical Properties of Minerals Applied to Heterogeneous Catalytic Ozonation. Sustainability 2020, 12, 10503, doi:10.3390/su122410503.
- Miller, F.A.; Silva, C.L.M.; Brandão, T.R.S. A Review on Ozone-Based Treatments for Fruit and Vegetables Preservation. Food Eng. Rev. 2013, 5, 77–106, doi:10.1007/s12393-013-9064-5.
- Ye, B.; Chen, Z.; Li, X.; Liu, J.; Wu, Q.; Yang, C.; Hu, H.; Wang, R. Inhibition of Bromate Formation by Reduced Graphene Oxide Supported Cerium Dioxide during Ozonation of Bromide-Containing Water. Front. Environ. Sci. Eng. 2019, 13, 86, doi:10.1007/s11783-019-1170-z.
- Wang, Q. Study on the Mechanism of Cerium Oxide Catalytic Ozonation for Controlling the Formation of Bromate in Drinking Water. Desalination Water Treat. 2016, 57, 15533–15546.
- Zhang, T.; Chen, W.; Ma, J.; Qiang, Z. Minimizing Bromate Formation with Cerium Dioxide during Ozonation of Bromide-Containing Water. Water Res. 2008, 42, 3651–3658, doi:10.1016/j.watres.2008.05.021.
- Shen, J.; Chen, Z.; Xu, Z.; Li, X.; Xu, B.; Qi, F. Kinetics and Mechanism of Degradation of P-Chloronitrobenzene in Water by Ozonation. J. Hazard. Mater. 2008, 152, 1325–1331, doi:10.1016/j.jhazmat.2007.08.009.
- Ibn Abdul Hamid, K.; Scales, P.J.; Allard, S.; Croue, J.-P.; Muthukumaran, S.; Duke, M. Ozone Combined with Ceramic Membranes for Water Treatment: Impact on HO Radical Formation and Mitigation of Bromate. J. Environ. Manag. 2020, 253, 109655, doi:10.1016/j.jenvman.2019.109655.
- Merle, T.; Pronk, W.; von Gunten, U. MEMBRO3X, a Novel Combination of a Membrane Contactor with Advanced Oxidation (O3/H2O2) for Simultaneous Micropollutant Abatement and Bromate Minimization. Environ. Sci. Technol. Lett. 2017, 4, 180–185, doi:10.1021/acs.estlett.7b00061.
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced Oxidation Processes (AOPs) Involving Ultrasound for Waste Water Treatment: A Review with Emphasis on Cost Estimation. Ultrason. Sonochemistry 2010, 17, 990–1003, doi:10.1016/j.ultsonch.2009.09.005.
- Fast, S.A.; Gude, V.G.; Truax, D.D.; Martin, J.; Magbanua, B.S. A Critical Evaluation of Advanced Oxidation Processes for Emerging Contaminants Removal. Environ. Process. 2017, 4, 283–302, doi:10.1007/s40710-017-0207-1.
- Stylianou, S.K.; Kostoglou, M.; Zouboulis, A.I. Ozone Mass Transfer Studies in a Hydrophobized Ceramic Membrane Contactor: Experiments and Analysis. Ind. Eng. Chem. Res. 2016, 55, 7587–7597, doi:10.1021/acs.iecr.6b01446.
- Kim, I.; Tanaka, H. Energy Consumption for PPCPs Removal by O3 and O3/UV. Ozone Sci. Eng. 2011, 33, 150–157, doi:10.1080/01919512.2011.549427.
- Glaze, W.H.; Kang, J.-W. Advanced Oxidation Processes for Treating Groundwater Contaminated With TCE and PCE: Laboratory Studies. J. Am. Water Works Assoc. 1988, 80, 57–63, doi:10.1002/j.1551-8833.1988.tb03038.x.
- Ramseier, M.K.; Gunten, U. von. Mechanisms of Phenol Ozonation—Kinetics of Formation of Primary and Secondary Reaction Products. Ozone Sci. Eng. 2009, 31, 201–215, doi:10.1080/01919510902740477.
- Mehrjouei, M.; Müller, S.; Möller, D. Energy Consumption of Three Different Advanced Oxidation Methods for Water Treatment: A Cost-Effectiveness Study. J. Clean. Prod. 2014, 65, 178–183, doi:10.1016/j.jclepro.2013.07.036.
- Biard, P.-F.; Werghi, B.; Soutrel, I.; Orhand, R.; Couvert, A.; Denicourt-Nowicki, A.; Roucoux, A. Efficient Catalytic Ozonation by Ruthenium Nanoparticles Supported on SiO2 or TiO2: Towards the Use of a Non-Woven Fiber Paper as Original Support. Chem. Eng. J. 2016, 289, 374–381, doi:10.1016/j.cej.2015.12.051.Martins, R.C.; Ramos, C.M.; Quinta-Ferreira, R.M. Low-Cost Catalysts To Enhance Ozone Action on the Depuration of Olive Mill Wastewaters. Ind. Eng. Chem. Res. 2014, 53, 15357–15368, doi:10.1021/ie501187e.
- Martins, R.C.; Ramos, C.M.; Quinta-Ferreira, R.M. Low-Cost Catalysts To Enhance Ozone Action on the Depuration of Olive Mill Wastewaters. Ind. Eng. Chem. Res. 2014, 53, 15357–15368, doi:10.1021/ie501187e.Biard, P.-F.; Werghi, B.; Soutrel, I.; Orhand, R.; Couvert, A.; Denicourt-Nowicki, A.; Roucoux, A. Efficient Catalytic Ozonation by Ruthenium Nanoparticles Supported on SiO2 or TiO2: Towards the Use of a Non-Woven Fiber Paper as Original Support. Chem. Eng. J. 2016, 289, 374–381, doi:10.1016/j.cej.2015.12.051.
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human Health Risk Assessment for Aluminium, Aluminium Oxide, and Aluminium Hydroxide. J. Toxicol. Environ. Health Part B 2007, 10, 1–269, doi:10.1080/10937400701597766.
- Pines, D.S.; Reckhow, D.A. Effect of Dissolved Cobalt(II) on the Ozonation of Oxalic Acid. Environ. Sci. Technol. 2002, 36, 4046–4051, doi:10.1021/es011230w.
- Gomes, J.F.; Frasson, D.; Pereira, J.L.; Gonçalves, F.J.M.; Castro, L.M.; Quinta-Ferreira, R.M.; Martins, R.C. Ecotoxicity Variation through Parabens Degradation by Single and Catalytic Ozonation Using Volcanic Rock. Chem. Eng. J. 2019, 360, 30–37, doi:10.1016/j.cej.2018.11.194.
- Bertaud, F.; Croue, J.P.; Lebube, B. Ozonation of a β-0-4 Dimer Lignin Model: By-Product identification and Reaction Pathways. Ozone Sci. Eng. 2001, 23, 139–148, doi:10.1080/01919510108961996.