
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giorgia Ailuno | + 2487 word(s) | 2487 | 2021-01-11 02:47:41 | | | |
| 2 | Peter Tang | -96 word(s) | 2391 | 2021-01-23 13:24:17 | | |
Video Upload Options
Exosomes are endosome-derived nanovesicles produced by healthy as well as diseased cells. Their proteic, lipidic and nucleic acid composition is related to the cell of origin, and by vehiculating bioactive molecules they are involved in cell-to-cell signaling, both in healthy and pathologic conditions. Being nano-sized, non-toxic, biocompatible, scarcely immunogenic, and possessing targeting ability and organotropism, exosomes have been proposed as nanocarriers for their potential application in diagnosis and therapy. Among the different techniques exploited for exosome isolation, the sequential ultracentrifugation/ultrafiltration method seems to be the gold standard; alternatively, commercially available kits for exosome selective precipitation from cell culture media are frequently employed. To load a drug or a detectable agent into exosomes, endogenous or exogenous loading approaches have been developed, while surface engineering procedures, such as click chemistry, hydrophobic insertion and exosome display technology, allow for obtaining actively targeted exosomes.
1. Introduction
All prokaryotic and eukaryotic cells secrete, in an evolutionary conserved way, extracellular vesicles (EVs), i.e., membrane-derived nano- and microvesicles [1]. For a long time, these vesicles were supposed to be either a waste removal system, products of cellular damage, or experimental artefacts [2]. Nowadays, EVs are recognized as specific cellular components performing different biological functions [3][4].
EVs are classified on the basis of the different sizes, cellular compartment of origin and localization, either inside or outside the cells [1]. Among them, exosomes, microparticles, shedding vesicles, apoptotic bodies, tolerosomes, prostasomes and prominosomes have been distinguished [5]. Two main processes for EV formation have been identified: some EVs, such as exosomes, apparently derive from exocytosis of multivesicular bodies, a part of the endosomal system including primary endocytic vesicles, early and late endosomes, and lysosomes [6]; otherwise, EVs may form from the direct budding of the cell membrane [2].
Exosomes, firstly identified by Johnestone in 1987 [7], represent a homogenous class of EVs in terms of size (30–150 nm), density (1.13–1.19 g/mL [8][9]) and membrane composition, differently from the other classes of EVs, which are characterized by higher heterogeneity [10][11]. As exosomes derive from the membrane of late endosomes [1], their proteome is particularly rich in tetraspanins (CD9, CD63, CD81 and CD82) and heat shock proteins (HSP70, HSP90), but also includes transmembrane proteins that are specific to the parent cell; for example, exosomes deriving from platelets contain P-selectin and intercellular adhesion molecule-1, while α- and β-chains of integrins are expressed on the membrane of exosomes deriving from dendritic cells, reticulocytes and T-cells [12]. As for exosome lipid composition, phosphatidylserine, sphingomyelin, cholesterol, ceramides, and ganglioside GM3 are particularly abundant [1]. Sharing the same biogenesis, exosomes and apoptotic bodies present analogous membrane topology, with the interior side of the vesicle corresponding to the cytosolic side of the parent cell membrane, although phosphatidylserine is specifically exposed on the outer leaflet of the vesicle membrane, due to the activity of enzymes such as flippase, floppase and scramblase [11]. Moreover, exosomes carry nucleic acids such as mRNA, siRNA, miRNA and DNA fragments.
2. Biological Function
By vehiculating proteins and genetic material, exosomes are involved in cell-to-cell communication by molecule transfer from donor to nearby, as well as distant, recipient cells [13][14]. Some authors have already reviewed the physiologic functions of exosomes in the immune system [15][16]; for instance, an in vitro study on both human and murine models evidenced that exosomes deriving from lymphocytes stimulate CD4+ T cell clones, revealing that these EVs might be involved in the transfer of peptidic signals among immune cells [8].
The exosomal mechanism of interaction with recipient cells has not been clarified yet (Figure 1). Some studies reported that exosomes fuse with the plasma membrane of the recipient cell, releasing their content in the cytoplasm [17][18]; as observed by Parolini et al. [19], the fusion process might be facilitated under acidic pH, typical of the tumor intracellular environment.
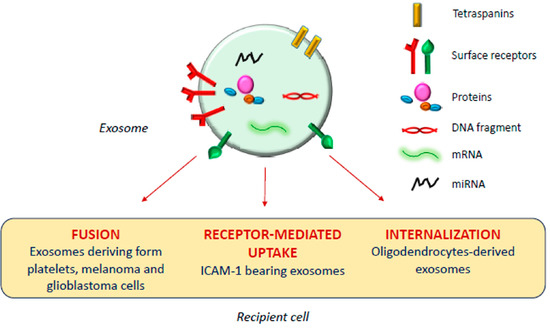
Figure 1. Exosomal mechanism of interaction with recipient cells.
Actually, besides shuttling bioactive molecules among healthy cells, exosomes also play a crucial role under pathologic conditions such as cancer, and neurodegenerative, cardiovascular, infectious and respiratory diseases [14][20][21][22][23].
Tumor cells release high amounts of EVs, and several studies report a possible involvement of tumor-derived exosomes in different phases of tumor formation and progression, as reviewed in [24], although opposing pieces of evidence have been reported by different research groups. Al-Nedawi et al. [25] observed that glioblastoma cell-derived EVs membranes are enriched in a mutant form of the epidermal growth factor receptor, which promotes anti-apoptotic pathways and increases the anchorage-independent growth capacity; on the other hand, Ristorcelli et al. [26] evidenced that EVs secreted by pancreatic tumor cells, due to their high membrane content of cholesterol and sphingomyelin, induce activation of the mitochondrial apoptotic pathway in tumor cells, and Zitvogel and his group [27] even demonstrated the capability of dendritic cell-deriving exosomes to inhibit tumor growth in vivo.
Besides membrane composition, the exosomal content may also influence tumor progression. Some studies evidenced the role of miRNA present in tumor-derived exosomes in promoting neovascularization and angiogenesis [28][29]; also, a recent work of Gerloff et al. [30] evidenced that cutaneous melanoma-derived exosomes are enriched in miR-125b-5p, which induces a tumor-promoting phenotype in tumor-associated macrophages, while Kurahashi et al. [31] observed increased miR-204-5p levels in urinary exosomes of transgenic mice used as a model of a rare form of renal cancer.
An important aspect of tumor progression is the capacity of cells to elude the immune system, and several studies demonstrated that tumor-derived exosomes are involved in this process. Chalmin et al. [32] isolated exosomes deriving from different murine and human cancer cell lines and identified the interaction between Hsp-72, associated with the exosome membranes, and Stat3, expressed by the parent cells, as the key factor inducing the immunosuppressing activity of both mouse and human myeloid-derived suppressor cells.
Moreover, several papers highlighted the involvement of tumor-derived exosomes in the metastastic process [33][34][35][36]. For example, Peinado et al. [37] demonstrated that the highly metastatic behavior of primary melanomas might be ascribed to the abundant generation of exosomes influencing bone marrow progenitors, although different pieces of evidence were obtained in a replication study in 2018 [38]. Ramteke et al. [39] demonstrated that prostate cancer cell-derived exosomes, secreted under the hypoxic conditions typical of the malignancy, enhance the invasiveness of the tumor through induction of the cleavage of E-cadherin, a protein involved in the adherens junctions among epithelial cells. Another study [40] demonstrated that exosomes deriving from bladder cancer cells promote lymphatic metastasis through the action of a long non-coding RNA vehiculated by the vesicles; similarly, miR-105, miR-122 and miR-200-containing EVs promote breast cancer cell metastasis [41][42][43] and miR-221-containing exosomes derived from gastric cancer mesenchymal stem cells were found to promote migration of human gastric cancer cells in vitro [44].
3. Applications in Therapy
Exosomes are endowed with several characteristics suitable for drug delivery: they are nano-sized, non-toxic, biocompatible, scarcely immunogenic, and possess targeting ability and organotropism [45]. Indeed, exosomes are similar to small unilamellar liposomes in terms of size and capacity to carry both hydrophilic and lipophilic molecules, but the asymmetrical lipid distribution and specific protein composition of exosome membranes justify their organotropism and homing ability [46], confirmed by the evidence that cancer-derived exosomes fuse preferentially with their parent cells [47].
However, the clinical translation of exosomes as drug carriers is affected by several technical issues, including low production yield, considerable structural heterogeneity and complexity, difficulties in drug loading and in developing standard, scalable, and cost-effective GMP procedures for exosome isolation and purification [48]. To overcome these issues, bioinspired exosome-like vesicles have emerged as an alternative to naturally derived exosomes. Most of the artificial exosome-mimetic systems proposed to date stem from liposomes—the so-called hybrid exosomes derive from the fusion of exosome and liposome membranes [49]—or are obtained by serial extrusion of a parent cell suspension through decreasing pore size membranes [50].
Many research groups have developed exosomes and exosome-mimetic systems as nanocarriers for cancer treatment [45], proposing different techniques for vesicle isolation and purification, drug loading and surface functionalization [51][52]. The drug loading methods, in particular, can be classified in two main classes, endogenous and exogenous loading [52]. Endogenous loading includes the genetic modification of the parent cells, to have them to express specific proteins or nucleic acids to be included in the released vesicles, or their simple incubation with the drug to be loaded; exogenous loading implicates the incorporation of the drug in exosomes previously isolated from cell culture media or body fluids (urine, blood, saliva, breast milk, etc.).
Various active principles have been loaded into vesicles developed for the treatment of different types of cancer, such as doxorubicin [53][54], paclitaxel [55], gemcitabine [56], but also aspirin [57], imperialine [58], several miRNA [59][60][61][62][63][64] and mRNA molecules [65], tumor necrosis factor-α [66] and recombinant methioninase [67].
4. Applications in Diagnosis
Beyond their possible use as therapeutic active carriers, exosomes can be employed in the diagnostic field with two different approaches. Passive diagnostic applications involve the use of naturally derived tumor exosomes as cancer diagnostic and prognostic biomarkers [68][69], since, differently from circulating cancer cells, their abundance in blood allows for easy detection in small volumes of frozen plasma or serum [70]. By analyzing the proteomic and genomic profile of these exosomes, including mRNA, miRNA and mitochondrial RNA, it is possible to determine the type of tumor and its stage [68][71][72][73][74]. As an example, Zong et al. [75] developed silicon quantum dots (Si-QD) decorated with a CD63 aptamer to bind CD63 expressed on exosomes isolated from human breast SKBR3 cancer cells [12], thus obtaining a nanoprobe for super-resolution microscopy suitable for trafficking studies in live cells and for the investigation of exosome role in cancer metastasis. Moreover, Chen et al. [76] developed an exosome-based system for super-resolution microscopy, demonstrating the possibility of simultaneous dual-color imaging by immunofluorescent labeling of CD63 and HER2 molecules expressed on SKBR3-derived exosomes.
On the other hand, some researchers proposed to exploit exosomes as an active diagnostic tool, by manipulating them with compounds or nanoparticles (NP) detectable using different imaging techniques, such as optical fluorescence, computed tomography (CT), positron emission tomography (PET), single photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI), for their use in the diagnosis of some forms of cancer that are difficult to reach, such as brain tumors, or in the early detection of cancer recurrences and metastasis, within a precision medicine approach [77].
On the wave of growing interest in exosome and EVs applications, the aim of this review is to summarize the diagnostic or theranostic platforms based on exosomes (Table 1) or exosome-mimetic vesicles (Table 2) that have been developed so far, classified on the basis of the labeling probes; in particular, the review focuses on the diverse manufacturing (Figure 2), loading and surface modification procedures, and on applications in oncology; the final paragraph briefly reports on exosome applications in other pathologic conditions.
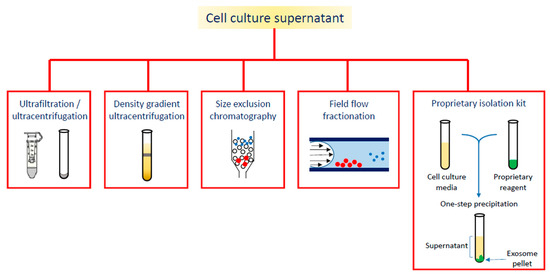
Figure 2. Different strategies for exosome isolation.
Table 1. Theranostic platforms based on exosomes, classified by loading strategy.
|
Ref. |
Labeling Strategy |
Parent Cells |
Exosome Isolation Method |
Labeling Compound |
Therapeutic Compound |
Loading/Labeling Procedure |
Surface Engineering |
Detection Technique |
Tests |
|
[78] |
Nanoparticle-loaded exosomes |
Raw264.7 mouse macrophages |
Sequential centrifugation |
SPION |
Curcumin |
Exogenous (electroporation) |
NRP-1 binding peptide by click chemistry |
MRI |
In vitro: U251 cells In vivo: BALB/c nude mice transplanted with U251 cells |
|
[79] |
SKBR3 breast cancer cells |
Exosome isolation kit |
Gold-carbon QD |
Exogenous (incubation exploiting targeted loading through anti-HER2 antibodies) |
Fluorescence imaging |
In vitro: HeLa cells |
|||
|
[80] |
MCF-7 breast cancer cells |
Exosome isolation kit |
Vanadium carbide QD |
Exogenous (electroporation) |
RGD peptide introduced by incubating exosomes with DSPE-PEG-RGD |
Photoacoustic imaging |
In vitro: MCF-7, A549, NHDF cells In vivo: tumor-bearing BALB/c nude mice |
||
|
[81] |
Urine of gastric cancer patients |
Sequential centrifugation |
Chlorine-6 labeled gold NP |
Exogenous (electroporation) |
Fluorescence imaging |
In vitro: MGC-803, Raw264.7 cells In vivo: MGC-803 tumor-bearing BALB/c-nude mice |
|||
|
Murine adipose stem cells |
Exosome isolation kit |
USPION |
|
Endogenous (cell incubation) |
|
MRI |
In vitro: exosomes immobilized in an agarose matrix In vivo: C57BL/6 mice |
||
|
[84] |
Mesenchymal stem cells |
Sequential centrifugation |
Gold NP |
Exogenous (incubation) |
CT |
In vivo: C57bl/6 mice |
|||
|
[85] |
Transition metal-labeled exosomes |
Human umbilical cord mesenchymal stem cells |
Sequential centrifugation |
68Gd (complexed by DOTA) |
|
Exogenous (lipid insertion technique with Gd-DOTA-DSPE) |
|
MRI |
In vitro: K7M2 mouse and 14B human osteosarcoma cells In vivo: immunodeficient NU/NU nude mice implanted with K7M2 cells |
|
[86] |
Human umbilical cord blood mononuclear cells |
Sequential centrifugation |
64Cu (complexed by DOTA) |
|
Exogenous (reaction between the maleimide group of DOTA and thiol groups on exosome surface) |
|
PET/MRI |
In vitro: HUVEC In vivo: C57BL/6J mice |
|
|
[87] |
4T1 breast cancer cells |
Sequential centrifugation |
64Cu (complexed by NOTA) |
|
Exogenous (reaction of NOTA with exosome surface proteins) |
PEG decoration using PEG5k/NHS |
PET |
In vivo: 4T1 tumor-bearing BALB/c mice |
|
|
[88] |
Mouse macrophage HEK293T cells |
Sequential centrifugation |
99mTc |
Exogenous (incubation with fac-[99mTc(CO)3(H2O)3]+) |
DARPin G3 functionalization by transfection of the parent cells |
Radioactive signal by gamma-counter |
In vitro: SKOV-3, MCF-7, U87-MG, HT-29, A549 cells In vivo: BALB/c mice, SKOV-3 xenografted C57 nude mice |
||
|
[89] |
Human embryonic kidney HEK293 cells |
Sequential centrifugation |
111In |
Exogenous (incubation with 111In -oxine) |
CSPGAKVRC peptide, functionalized by transfection of the parent cells |
CT/SPECT |
In vitro: Raw264.7 cells In vivo: 4T1 tumor-bearing Balb/c mice |
||
|
[90] |
Bioluminescently labeled exosomes |
Human embryonic kidney 293T cells |
Sequential centrifugation |
Gaussia princeps luciferase (Gluc) |
Endogenous (transfection of the parent cells with a gene encoding for Gluc bound to a membrane protein) |
IVIS imaging |
In vivo: immunodeficient athymic nude mice |
||
|
[91] |
Human embryonic kidney 293T cells |
Sequential centrifugation |
GFP, tandem dimer Tomato |
Endogenous (transfection of the parent cells with a gene encoding for palmGFP/palmtdTomato) |
Multiphoton intravital microscopy |
In vitro: 293T cells In vivo: C57BL6 (B6) mice implanted with mouse thymoma EL-4 cells |
|||
|
[92] |
Nanocluster loaded exosomes |
HepG2 human hepatocellular carcinoma |
Sequential centrifugation |
Ag-nanoclusters and Fe3O4 NP |
Endogenous (parent cells cultured in the presence of AgNO3 and FeCl2 forming the nanoclusters) |
Flurescence bioimaging, CT, MRI |
In vitro: HepG2, U87 cells |
||
|
[93] |
Metabolic labeled exosomes |
MDA-MB-231 breast cancer cells |
Ultracentrifugation and size exclusion chromatography |
Deuterium |
Endogenous (parent cells cultured in presence of D2O/d-Gluc/d-Chol) |
Raman spectroscopic imaging |
In vitro: MDA-MB-231, MCF10A cells |
Table 2. Theranostic platforms based on exosome-mimetic vesicles.
|
Ref. |
Cell Line |
Labeling Compound |
Therapeutic Compound |
Vesicle Preparation Method |
Loading/Labeling Procedure |
Detection Technique |
Tests |
|
[94] |
Bel-7402 human hepatoma cancer cells |
NP-encapsulated doxorubicin |
NP-encapsulated doxorubicin |
Coating of the NP with cell membranes through extrusion |
Incubation |
Fluorescence imaging |
In vitro: Bel-7402, MCF-7, L-O2 cells |
|
[95] |
J774A.1 mouse macrophages |
Gd-conjugated liposomes |
|
Sonication and extrusion of the exosome/liposome mixture |
Obtained during vesicle preparation procedure |
MRI |
In vitro: K7M2, NIH/3T3 cells In vivo: osteosarcoma—bearing NU/NU immunodeficient mice |
|
[96] |
Raw264.7 mouse macrophages, HB1.F3 human neural stem cells |
99mTc-HMPAO |
Sequential extrusion of parent cells and density gradient centrifugation |
Incubation |
SPECT/CT |
In vivo: BALB/c mice |
References
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289.
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51.
- Hristov, M.; Erl, W.; Linder, S.; Weber, P.C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 2004, 104, 2761–2766.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383.
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochimica Biophysica Acta Gen. Subj. 2012, 1820, 940–948.
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.G.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113, 3365–3374.
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation—Association of plasma-membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420.
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Leijendekker, R.; Harding, C.V.; Melief, C.J.M.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172.
- Thery, C.; Regnault, A.; Garin, J.; Wolfers, J.; Zitvogel, L.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Molecular characterization of dendritic cell-derived exosomes: Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 1999, 147, 599–610.
- Simpson, R.J.; Jensen, S.S.; Lim, J.W.E. Proteomic profiling of exosomes: Current perspectives. Proteomics 2008, 8, 4083–4099.
- Hugel, B.; Carmen, M.; Martinez, M.C.; Kunzelmann, C.; Freyssinet, J.M. Membrane microparticles: Two sides of the coin. Physiology 2005, 20, 22–27.
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579.
- Pontecorvi, G.; Bellenghi, M.; Puglisi, R.; Care, A.; Mattia, G. Tumor-derived extracellular vesicles and microRNAs: Functional roles, diagnostic, prognostic and therapeutic options. Cytokine Growth Factor Rev. 2020, 51, 75–83.
- Bang, C.; Thum, T. Exosomes: New players in cell-cell communication. Int. J. Biochem. Cell Biol. 2012, 44, 2060–2064.
- Bobrie, A.; Colombo, M.; Raposo, G.; Thery, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668.
- Chaput, N.; Thery, C. Exosomes: Immune properties and potential clinical implementations. Semin. Immunopathol. 2011, 33, 419–440.
- Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018, 28, R435–R444.
- Bebelman, M.P.; Bun, P.; Huveneers, S.; van Niel, G.; Pegtel, D.M.; Verweij, F.J. Real-time imaging of multivesicular body-plasma membrane fusion to quantify exosome release from single cells. Nat. Protoc. 2020, 15, 102–121.
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A., et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222.
- Fleming, A.; Sampey, G.; Chung, M.C.; Bailey, C.; van Hoek, M.L.; Kashanchi, F.; Hakami, R.M. The carrying pigeons of the cell: Exosomes and their role in infectious diseases caused by human pathogens. Pathog. Dis. 2014, 71, 107–118.
- Kimura, K.; Hohjoh, H.; Fukuoka, M.; Sato, W.; Oki, S.; Tomi, C.; Yamaguchi, H.; Kondo, T.; Takahashi, R.; Yamamura, T. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat. Commun. 2018, 9, doi:10.1038/s41467-017-02406-2.
- Gon, Y.; Shimizu, T.; Mizumura, K.; Maruoka, S.; Hikichi, M. Molecular techniques for respiratory diseases: MicroRNA and extracellular vesicles. Respirology 2020, 25, 149–160, doi:10.1111/resp.13756.
- Sasaki, R.; Kanda, T.; Yokosuka, O.; Kato, N.; Matsuoka, S.; Moriyama, M. Exosomes and hepatocellular carcinoma: From bench to bedside. Int. J. Mol. Sci. 2019, 20, doi:10.3390/ijms20061406.
- Abak, A.; Abhari, A.; Rahimzadeh, S. Exosomes in cancer: Small vesicular transporters for cancer progression and metastasis, biomarkers in cancer therapeutics. PEERJ 2018, 6, e4763.
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFrvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624.
- Ristorcelli, E.; Beraud, E.; Verrando, P.; Villard, C.; Lafitte, D.; Sbarra, V.; Lombardo, D.; Verine, A. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J. 2008, 22, 3358–3369.
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600.
- Umezu, T.; Ohyashiki, K.; Kuroda, M.; Ohyashiki, J.H. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 2013, 32, 2747–2755.
- Gajos-Michniewicz, A.; Duechler, M.; Czyz, M. MiRNA in melanoma-derived exosomes. Cancer Lett. 2014, 347, 29–37.
- Gerloff, D.; Lutzkendorf, J.; Moritz, R.K.C.; Wersig, T.; Mader, K.; Muller, L.P.; Sunderkotter, C. Melanoma-derived exosomal miR-125b-5p educates tumor associated macrophages (TAMs) by targeting lysosomal acid lipase A (LIPA). Cancers 2020, 12, 464.
- Kurahashi, R.; Kadomatsu, T.; Baba, M.; Hara, C.; Itoh, H.; Miyata, K.; Endo, M.; Morinaga, J.; Terada, K.; Araki, K., et al. MicroRNA-204-5p: A novel candidate urinary biomarker of Xp11.2 translocation renal cell carcinoma. Cancer Sci. 2019, 110, 1897–1908, doi:10.1111/cas.14026.
- Chalmin, F.; Ladoire, S.; Mignot, G.; Vincent, J.; Bruchard, M.; Remy-Martin, J.P.; Boireau, W.; Rouleau, A.; Simon, B.; Lanneau, D., et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Investig. 2010, 120, 457–471.
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495.
- Jung, T.; Castellana, D.; Klingbeil, P.; Hernandez, I.C.; Vitacolonna, M.; Orlicky, D.J.; Roffler, S.; Brodt, P.; Zoller, M. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia 2009, 11, 1093–1150.
- Lima, L.G.; Chammas, R.; Monteiro, R.Q.; Moreira, M.E.C.; Barcinski, M.A. Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett. 2009, 283, 168–175.
- Hood, J.L.; Roman, S.S.; Wickline, S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011, 71, 3792–3801.
- Peinado, H.; Kovic, M.A.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.M., et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET (vol 18, pg 883, 2012). Nat. Med. 2016, 22, 1502–1502.
- Kim, J.; Afshari, A.; Sengupta, R.; Sebastiano, V.; Gupta, A.; Kim, Y.H.; Biol, R.P.C. Replication study: Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Elife 2018, 7, e39944.
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2015, 54, 554–565.
- Chen, C.H.; Luo, Y.M.; He, W.; Zhao, Y.; Kong, Y.; Liu, H.W.; Zhong, G.Z.; Li, Y.T.; Li, J.; Huang, J., et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J. Clin. Investig. 2020, 130, 404–421.
- Zhou, W.Y.; Fong, M.Y.; Min, Y.F.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R., et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515.
- Fong, M.Y.; Zhou, W.Y.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.S.; Chin, A.R., et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015, 17, 183–194.
- Le, M.T.N.; Hamar, P.; Guo, C.Y.; Basar, E.; Perdigao-Henriques, R.; Balaj, L.; Lieberman, J. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Investig. 2014, 124, 5109–5128.
- Wang, M.; Zhao, C.; Shi, H.; Zhang, B.; Zhang, L.; Zhang, X.; Wang, S.; Wu, X.; Yang, T.; Huang, F., et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: Novel biomarkers and a mechanism for gastric cancer. Br. J. Cancer 2014, 110, 1199–1210.
- Lim, W.; Kim, H.S. Exosomes as Therapeutic Vehicles for Cancer. Tissue Eng. Regen. Med. 2019, 16, 213–223.
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics 2018, 10, 218.
- Qiao, L.; Hu, S.Q.; Huang, K.; Su, T.; Li, Z.H.; Vandergriff, A.; Cores, J.; Dinh, P.U.; Allen, T.; Shen, D.L., et al. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics 2020, 10, 3474–3487.
- Lu, M.; Huang, Y.Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 2020, 242, 119925.
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933.
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.S.; Roh, T.Y.; Park, J.; Nilsson, J.; Lotvall, J.; Kim, Y.K.; Gho, Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013, 7, 7698–7710.
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochimica Biophysica Acta Rev. Cancer 2014, 1846, 75–87.
- Zhang, Y.F.; Shi, J.B.; Li, C. Small extracellular vesicle loading systems in cancer therapy: Current status and the way forward. Cytotherapy 2019, 21, 1122–1136.
- Srivastava, A.; Amreddy, N.; Babu, A.; Panneerselvam, J.; Mehta, M.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Razaq, M.; Riedinger, N., et al. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci. Rep. 2016, 6, 38541.
- Ye, H.; Wang, K.Y.; Lu, Q.; Zhao, J.; Wang, M.L.; Kan, Q.M.; Zhang, H.T.; Wang, Y.J.; He, Z.G.; Sun, J. Nanosponges of circulating tumor-derived exosomes for breast cancer metastasis inhibition. Biomaterials 2020, 242, 119932.
- Li, Y.J.; Wu, J.Y.; Wang, J.M.; Hu, X.B.; Cai, J.X.; Xiang, D.X. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomaterialia 2020, 101, 519–530.
- Haney, M.J.; Zhao, Y.L.; Jin, Y.S.; Li, S.M.; Bago, J.R.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Macrophage-derived extracellular vesicles as drug delivery systems for triple negative breast cancer (TNBC) therapy. J. Neuroimmune Pharmacol. 2020, 15, 487–500.
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Nguyen, T.N.G.; Lee, B.J.; Duan, W. Aspirin-loaded nanoexosomes as cancer therapeutics. Int. J. Pharm. 2019, 572, 118786.
- Lin, Q.; Qu, M.K.; Zhou, B.J.; Patra, H.K.; Sun, Z.H.; Luo, Q.; Yang, W.Y.; Wu, Y.C.; Zhang, Y.; Li, L., et al. Exosome-like nanoplatform modified with targeting ligand improves anti-cancer and anti-inflammation effects of imperialine. J. Control Release 2019, 311, 104–116.
- Baldari, S.; Di Rocco, G.; Magenta, A.; Picozza, M.; Toietta, G. Extracellular vesicles-encapsulated MicroRNA-125b produced in genetically modified mesenchymal stromal cells inhibits hepatocellular carcinoma cell proliferation. Cells 2019, 8, E1560a.
- Nie, H.F.; Xie, X.D.; Zhang, D.D.; Zhou, Y.; Li, B.F.; Li, F.Q.; Li, F.Y.; Cheng, Y.L.; Mei, H.; Meng, H., et al. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale 2020, 12, 877–887.
- Wang, H.B.; Wei, H.; Wang, J.S.; Li, L.; Chen, A.Y.; Li, Z.G. MicroRNA-181d-5p-containing exosomes derived from CAFs promote EMT by regulating CDX2/HOXA5 in breast cancer. Mol. Ther. Nucleic Acids 2020, 19, 654–667.
- Liang, G.F.; Zhu, Y.L.; Ali, D.J.; Tian, T.; Xu, H.T.; Si, K.; Sun, B.; Chen, B.A.; Xiao, Z.D. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 2020, 18, 10.
- Chen, X.J.; Liu, J.Q.; Zhang, Q.L.; Liu, B.X.; Cheng, Y.; Zhang, Y.L.; Sun, Y.N.; Ge, H.; Liu, Y.Q. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J. Exp. Clin. Cancer Res. 2020, 39, 65.
- Kobayashi, M.; Sawada, K.; Miyamoto, M.; Shimizu, A.; Yamamoto, M.; Kinose, Y.; Nakamura, K.; Kawano, M.; Kodama, M.; Hashimoto, K., et al. Exploring the potential of engineered exosomes as delivery systems for tumor-suppressor microRNA replacement therapy in ovarian cancer. Biochem. Biophys. Res. Commun. 2020, 527, 153–161.
- Forterre, A.V.; Wang, J.H.; Delcayre, A.; Kim, K.; Green, C.; Pegram, M.D.; Jeffrey, S.S.; Matin, A.C. Extracellular vesicle-mediated in vitro transcribed mRNA delivery for treatment of HER2(+) breast cancer xenografts in mice by prodrug CB1954 without general toxicity. Mol. Cancer Ther. 2020, 19, 858–867.
- Zhuang, M.J.; Chen, X.L.; Du, D.; Shi, J.M.; Deng, M.; Long, Q.; Yin, X.F.; Wang, Y.Y.; Rao, L. SPION decorated exosome delivery of TNF-alpha to cancer cell membranes through magnetism. Nanoscale 2020, 12, 173–188.
- Xin, L.; Yuan, Y.W.; Liu, C.; Zhou, L.Q.; Liu, L.; Zhou, Q.; Li, S.H. Preparation of internalizing RGD-modified recombinant methioninase exosome active targeting vector and antitumor effect evaluation. Dig. Dis. Sci. 2020, doi:10.1007/s10620-020-06262-x.
- Huang, T.; Deng, C.X. Current progresses of exosomes as cancer diagnostic and prognostic biomarkers. Int. J. Biol. Sci. 2019, 15, 1–11.
- van der Meel, R.; Krawczyk-Durka, M.; van Solinge, W.W.; Schiffelers, R.M. Toward routine detection of extracellular vesicles in clinical samples. Int. J. Lab. Hematol. 2014, 36, 244–253.
- Shao, H.L.; Chung, J.; Lee, K.; Balaj, L.; Min, C.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Lee, H.; Weissleder, R. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat. Commun. 2015, 6, 6999.
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092.
- Li, Y.; Zheng, Q.P.; Bao, C.Y.; Li, S.Y.; Guo, W.J.; Zhao, J.; Chen, D.; Gu, J.R.; He, X.H.; Huang, S.L. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984.
- Choi, D.S.; Kim, D.K.; Kim, Y.K.; Gho, Y.S. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom. Rev. 2015, 34, 474–490.
- Verma, M.; Lam, T.K.; Hebert, E.; Divi, R.L. Extracellular vesicles: Potential applications in cancer diagnosis, prognosis, and epidemiology. BMC Clin. Pathol. 2015, 15, 6.
- Zong, S.F.; Zong, J.Z.; Chen, C.; Jiang, X.Y.; Zhang, Y.Z.; Wang, Z.Y.; Cui, Y.P. Single molecule localization imaging of exosomes using blinking silicon quantum dots. Nanotechnology 2018, 29, 065705.
- Chen, C.; Zong, S.F.; Wang, Z.Y.; Lu, J.; Zhu, D.; Zhang, Y.Z.; Cui, Y.P. Imaging and intracellular tracking of cancer-derived exosomes using single-molecule localization-based super-resolution microscope. ACS Appl. Mater. Interfaces 2016, 8, 25825–25833.
- Shang, M.Y.; Ji, J.S.; Song, C.; Gao, B.J.; Jin, J.G.; Kuo, W.P.; Kang, H.J. Extracellular vesicles: A brief overview and its role in precision medicine. In Extracellular Vesicles: Methods and Protocols; Kuo, W.P., Jia, S., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1660, pp. 1–14.
- Jia, G.; Han, Y.; An, Y.L.; Ding, Y.A.; He, C.; Wang, X.H.; Tang, Q.S. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316.
- Jiang, X.Y.; Zong, S.F.; Chen, C.; Zhang, Y.Z.; Wang, Z.Y.; Cui, Y.P. Gold-carbon dots for the intracellular imaging of cancer-derived exosomes. Nanotechnology 2018, 29, 175701.
- Cao, Y.; Wu, T.T.; Zhang, K.; Meng, X.D.; Dai, W.H.; Wang, D.D.; Dong, H.F.; Zhang, X.J. Engineered exosome-mediated near-infrared-II region V2C quantum dot delivery for nucleus-target low-temperature photothermal therapy. ACS Nano 2019, 13, 1499–1510.
- Pan, S.J.; Pei, L.J.; Zhang, A.M.; Zhang, Y.H.; Zhang, C.L.; Huang, M.; Huang, Z.C.; Liu, B.; Wang, L.R.; Ma, L.J., et al. Passion fruit-like exosome-PMA/Au-BSA@Ce6 nanovehicles for real-time fluorescence imaging and enhanced targeted photodynamic therapy with deep penetration and superior retention behavior in tumor. Biomaterials 2020, 230, 119606.
- Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, D.; Malatesta, M.; Sbarbati, A.; Marzola, P.; Mariotti, R. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: A new method to obtain labeled exosomes. Int. J. Nanomed. 2016, 11, 2481–2490.
- Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, D.; Malatesta, M.; Sbarbati, A.; Marzola, P.; Mariotti, R. Labeling and magnetic resonance imaging of exosomes isolated from adipose stem cells. Curr. Protoc. Cell Biol. 2017, 75, doi:10.1002/cpcb.23.
- Betzer, O.; Perets, N.; Ange, A.; Motiei, M.; Sadan, T.; Yadid, G.; Offen, D.; Popovtzer, R. In Vivo Neuroimaging of Exosomes Using Gold Nanoparticles. ACS Nano 2017, 11, 10883–10893.
- Abello, J.; Nguyen, T.D.T.; Marasini, R.; Aryal, S.; Weiss, M.L. Biodistribution of gadolinium- and near infrared-labeled human umbilical cord mesenchymal stromal cell-derived exosomes in tumor bearing mice. Theranostics 2019, 9, 2325–2345.
- Banerjee, A.; Alves, V.; Rondao, T.; Sereno, J.; Neves, A.; Lino, M.; Ribeiro, A.; Abrunhosa, A.J.; Ferreira, L.S. A positron-emission tomography (PET)/magnetic resonance imaging (MRI) platform to track in vivo small extracellular vesicles. Nanoscale 2019, 11, 13243–13248.
- Shi, S.X.; Li, T.T.; Wen, X.F.; Wu, S.Y.; Xiong, C.Y.; Zhao, J.; Lincha, V.R.; Chow, D.S.; Liu, Y.Y.; Sood, A.K., et al. Copper-64 labeled PEGylated exosomes for in vivo positron emission tomography and enhanced tumor retention. Bioconjugate Chem. 2019, 30, 2675–2683.
- Molavipordanjani, S.; Khodashenas, S.; Abedi, S.M.; Moghadam, M.F.; Mardanshahi, A.; Hosseinimehr, S.J. Tc-99m-radiolabeled HER2 targeted exosome for tumor imaging. Eur. J. Pharm. Sci. 2020, 148, 105312.
- Rashid, M.H.; Borin, T.F.; Ara, R.; Alptekin, A.; Liu, Y.T.; Arbab, A.S. Generation of novel diagnostic and therapeutic exosomes to detect and deplete protumorigenic M2 macrophages. Adv. Ther. 2020, 3, 1900209.
- Lai, C.P.; Tannous, B.A.; Breakefield, X.O. Noninvasive in vivo monitoring of extracellular vesicles. Biolumin. Imaging Methods Protoc. 2014, 1098, 249–258.
- Lai, C.P.; Kim, E.Y.; Badr, C.E.; Weissleder, R.; Mempel, T.R.; Tannous, B.A.; Breakefield, X.O. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat. Commun. 2015, 6, 7029.
- Tayyaba; Rehman, F.U.; Shaikh, S.; Tanziela; Semcheddine, F.; Du, T.Y.; Jiang, H.; Wang, X.M. In situ self-assembled Ag-Fe3O4 nanoclusters in exosomes for cancer diagnosis. J. Mater. Chem. B 2020, 8, 2845–2855.
- Horgan, C.C.; Nagelkerke, A.; Whittaker, T.E.; Nele, V.; Massi, L.; Kauscher, U.; Penders, J.; Bergholt, M.S.; Hood, S.R.; Stevens, M.M. Molecular imaging of extracellular vesicles in vitro via Raman metabolic labelling. J. Mater. Chem. B 2020, 8, 4447–4459.
- Wu, Y.F.; Zhang, F.; Wang, K.; Luo, P.C.; Wei, Y.Q.; Liu, S.Q. Activatable fluorescence imaging and targeted drug delivery via extracellular vesicle-like porous coordination polymer nanoparticles. Anal. Chem. 2019, 91, 14036–14042.
- Rayamajhi, S.; Marasini, R.; Nguyen, T.D.T.; Plattner, B.L.; Biller, D.; Aryal, S. Strategic reconstruction of macrophage-derived extracellular vesicles as a magnetic resonance imaging contrast agent. Biomater. Sci. 2020, 8, 2887–2904.
- Hwang, D.W.; Choi, H.; Jang, S.C.; Yoo, M.Y.; Park, J.Y.; Choi, N.E.; Oh, H.J.; Ha, S.; Lee, Y.S.; Jeong, J.M., et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using Tc-99m-HMPAO. Sci. Rep. 2015, 5, 15636.




