
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victoria Samanidou | + 2604 word(s) | 2604 | 2021-01-14 08:53:15 | | | |
| 2 | Vivi Li | Meta information modification | 2604 | 2021-01-15 03:09:24 | | |
Video Upload Options
Graphene oxide (GO) is a chemical compound with a form similar to graphene that consists of one-atom-thick two-dimensional layers of sp2-bonded carbon. Graphene oxide exhibits high hydrophilicity and dispersibility. Thus, it is difficult to be separated from aqueous solutions. Therefore, functionalization with magnetic nanoparticles is performed in order to prepare a magnetic GO nanocomposite that combines the sufficient adsorption capacity of graphene oxide and the convenience of magnetic separation. Moreover, the magnetic material can be further functionalized with different groups to prevent aggregation and extends its potential application. Until today, a plethora of magnetic GO hybrid materials have been synthesized and successfully employed for the magnetic solid-phase extraction of organic compounds from environmental, agricultural, biological, and food samples. The developed GO nanocomposites exhibit satisfactory stability in aqueous solutions, as well as sufficient surface area. Thus, they are considered as an alternative to conventional sorbents by enriching the analytical toolbox for the analysis of trace organic compounds.
1. Introduction
Solid-phase extraction (SPE) and liquid-liquid extraction (LLE) are two widely used and well-established techniques for the extraction of organic compounds. However, these conventional techniques tend to have many fundamental drawbacks such as complicated and time-consuming steps, requirement for a large amount of organic solvents and sample, as well as difficulties in automation [1][2][3][4]. Recent trends in sample preparation are focused on the progressive replacement of those techniques by miniaturized and environment-friendly techniques, such as solid-phase microextraction (SPME) [5], dispersive liquid–liquid microextraction (DLLME) [6], fabric phase sorptive extraction (FPSE) [7] and dispersive solid-phase extraction (d-SPE) [8].
Magnetic solid-phase extraction (MSPE) is a form of dispersive solid-phase extraction in which a magnetic sorbent is added into an aqueous sample in order to adsorb the target analytes. The sorbent is easily separated by applying an external magnetic field [9]. Subsequently, the analytes are eluted with the addition of an appropriate solvent and magnetic separation is performed again to collect the liquid phase, which is further analyzed. Compared with traditional SPE procedure, with magnetic sorbents there is no need to be packed into SPE cartridges, thus minimizing problems of column blocking and high pressure that are often observed in SPE. Meanwhile, the phase separation with an external magnetic field is a simple and rapid process compared to centrifugation and filtration steps. Sample and organic solvent consumption are also significantly decreased compared to classical SPE and LLE techniques [9][10].
Because of the evolution of technology and nanotechnology, novel extraction sorbents with improved chemical and physical properties have been synthesized and successfully used for magnetic solid-phase extraction of target analytes. Moreover, with the use of these materials, high extraction efficiency, good reproducibility in combination with low detection and quantification limits can be achieved [1][2]. Typical examples of MSPE sorbents are magnetic nanoparticles with surface modification by octadecyl (C18) [11], activated carbon [12], carbon-nanotubes [13], graphene [14], graphene oxide [15], metal-organic frameworks [16], covalent organic frameworks [17] and zeolitic imidazole frameworks [18].
Graphene oxide is the oxidized form of graphene that can be easily prepared from natural graphite powder with Hummer’s method after reaction with an anhydrous mixture of sulfuric acid, sodium nitrate and potassium permanganate [19][20][21][22][23][24]. Due to its superior properties such as good thermal and mechanical stability as well as its high surface area, graphene oxide has been used in multiple scientific fields including heterogenous catalysis, gas sorption, storage and separation, sensors and drug delivery [25].
In analytical chemistry, GO has been successfully employed for the sample preparation of a wide variety of samples including biological, food and environmental matrices [26][27][28]. Graphene oxide consists of one-atom-thick two-dimensional layers of sp2-bonded carbon and the material is rich in oxygen-containing groups including hydroxyl, carboxyl and epoxy groups, which assist the interaction between the sorbent and organic molecules through strong π-π stacking, hydrophobic interaction and hydrogen bonding [29][30][31].
Graphene oxide is an ultra-light material that poses high dispersibility in aqueous solutions as well as high hydrophilicity which makes its separation from this kind of solutions difficult. In order to improve the separation, GO can form magnetic nanocomposites with magnetite through electrostatic interaction between the negatively charged graphene oxide sheets and the positively charged surface of Fe3O4 [32]. The magnetic GO nanocomposites combine the high adsorption capacity of graphene oxide and the convenience of magnetic separation. Moreover, the hybrid material can be functionalized with different groups to prevent aggregation and extends its application [33][34].
2. Preparation and Applications of GO for the MSPE of Organic Compounds
2.1. Nanocomposites of GO with Fe3O4 Nanoparticles
Due to its high surface area and its superparamagnetic properties GO/Fe3O4 has been employed for the extraction of a wide variety of organic compounds from various samples. The surface of magnetic graphene oxide is rich in hydroxyl and carboxyl groups, which assist the interaction between the sorbent and the target analytes through strong π-π stacking, hydrophobic interaction as well as hydrogen bonding [26][27][28][29][30][31]. Figure 1 shows the structure of graphite, graphene oxide, reduced graphene oxide, and magnetic graphene oxide.
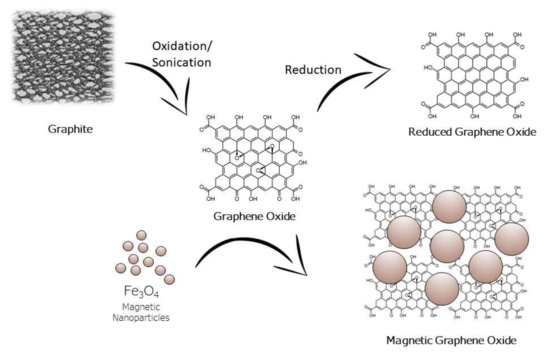
Figure 1. Structure of graphite, graphene oxide, reduced graphene oxide, and magnetic graphene oxide.
The one-step co-precipitation approach is the most common synthetic route for the preparation of magnetic GO. In this approach, graphene oxide is dispersed in water. Subsequently, salts of Fe2+ (e.g., ferrous chloride) and of Fe3+ (e.g., ferric chloride) are added, the mixture is heated and ammonium hydroxide is slowly added and the magnetite nanoparticles are formed [26].
2.2. Nanocomposites of Reduced GO with Fe3O4 Nanoparticles
Reduced graphene oxide (RGO) is a nanomaterial obtained by chemical reduction of graphene oxide that contains less oxygen groups and has properties closer to those of graphene [25][35]. RGO has various applications such as removal of metals and dyes [36][37], catalysts [38], electroanalytical sensors [39] etc. Magnetic nanocomposites of reduced graphene oxide have been successfully applied for the MSPE of various analytes from different sample matrices. Due to the combination of the magnetic Fe3O4 nanoparticles and the graphene sheets, the magnetic RGO sorbent shows distinguished properties including good dispersity, high surface area, high adsorption efficiency and good super-paramagnetism [40].
There are different synthetic procedures for the fabrication of RGO/Fe3O4 sorbents. The co-precipitation approach is a multi-step procedure in which GO/Fe3O4 previously prepared by precipitating Fe2+ and Fe3+ in the presence of GO is reduced with the addition of hydrazine hydrate [41][42]. For the solvothermal approach, graphite oxide is exfoliated in diethylene glycol under sonication to produce graphene oxide while ferric chloride with sodium acetate are also dissolved in diethylene glycol. In this case, diethylene glycol was both solvent and reducing agent. Accordingly, the GO dispersion is added into the second solution and the mixture was sonicated and heated at 190 °C in an autoclave [41][43]. The RGO/Fe3O4 can be also prepared through the hydrothermal method, which is similar to the solvothermal but instead of organic solvents that are used in the solvothermal method, water is used as a solvent in the hydrothermal approach. For this purpose, a salt of Fe3+ and sodium hydroxide is added to an aqueous solution of graphene oxide and the mixture is heated in an autoclave for a certain time span [41][44]. Figure 2 shows the scanning electron micrographs (SEM) of RGO/Fe3O4 prepared by solvothermal (a), hydrothermal (b) and co-precipitation (c) methods.
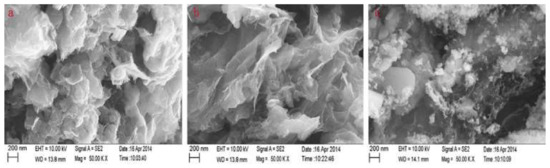
Figure 2. Scanning electron micrographs of reduced graphene oxide (RGO)/Fe3O4 prepared by solvothermal (a), hydrothermal (b) and co-precipitation (c) methods. Reproduced with permission from [41]. Copyright Elsevier, 2015.
Sudan dyes have been extracted from tomato sauce and chili-containing foods with RGO/Fe3O4 prior to their determination by HPLC-DAD [45]. The developed MSPE procedure was simple, economic and provided satisfactory extraction recoveries and LOD values. Magnetic RGO/Fe3O4 has been also used for the MSPE of bisphenol A from water samples prior to its determination by HPLC-UV. The MSPE technique was coupled with dispersive liquid–liquid microextraction (DLLME) in order to utilize the benefits of both sample preparation techniques. The magnetic sorbent was separated conveniently and rapidly from the sample matrix and it was found to be reusable for at least 12 repeated cycles [46].
In 2016, Mehdinia et al. developed a microwave-assisted synthesis of reduced graphene oxide decorated with magnetite and gold nanoparticles. Gold nanoparticles offer the benefits of chemical stability, biocompatibility, satisfactory magnetic properties and possibility for chemical modification. The RGO/Fe3O4@Au sorbent was used for the MSPE of organochlorine pesticides from seawater samples prior to their determination with GC-MS [47].
A magnetic polyethyleneimine functionalized reduced graphene oxide nanocomposite was synthesized and used for the MSPE of polar non-steroidal anti-inflammatory drugs from water samples [48] and polar acidic herbicides from rice [49]. The modification of RGO with polyethyleneimine changed the polarity of RGO to some extent and offered more active sites for the adsorption of the polar target analytes.
2.3. Functionalized Nanocomposites of GO with Fe3O4 Nanoparticles
The main disadvantage of graphene oxide-based adsorbents is the important π–π stacking interactions between graphene oxide nanosheets, which are responsible for serious aggregation and restacking of the nanosheets, resulting in a potential block of the active adsorption sites of the sorbent and a decrease of its specific surface area. In order to overcome this problem, functionalization of the sorbent with different molecules that can enter between the GO nanosheet and prevent them from aggregation and restacking can take place [50].
In order to overcome this limitation, Yilmaz et al. developed a magnetic nanodiamond/graphene oxide hybrid and used it for the MSPE of sildenafil from alleged herbal aphrodisiacs by HPLC-DAD system. Functionalization with nanodiamond successfully prevented the aggregation and restacking of GO nanosheets [50].
Functionalization of graphene oxide-based adsorbents can also take place to enhance the extraction efficiency of the material by introducing compatible chemical molecules with high surface area and abundant functional groups in the structure of GO.
Polyamidoamine (PAMAM) dendrimer has been used to develop amino-terminated hyper-branched PAMAM polymer grafted magnetic graphene oxide nanosheets for the MSPE of selective serotonin reuptake inhibitors from plasma samples [51]. Due to the large number of terminal groups of polyamidoamine dendrimer, the structural characteristics as well as the internal spaces between their branches, which can trap the target analyte, the functionalized GO/Fe3O4 sorbent exhibited higher extraction efficiency compared to the conventional GO/Fe3O4 nanocomposite.
Functionalization with soluble eggshell membrane protein (SEP) was found to increase the stability and adsorption performance as well as accuracy and recoveries of GO/Fe3O4 due to the high density of surface functional groups such as amines, amides and carboxylic groups of SEP [52].
Porphyrin has been also used for the functionalization of GO/Fe3O4 nanocomposite and the sorbent was used for the MSPE of sulfonamides from tap and river water samples [53]. Due to the π-π stacking and electrostatic attraction between the negatively charged functionalized nanocomposite and the positively charged sulfonamides, the extraction process was accelerated. The novel sorbent showed higher adsorption capacity than the conventional GO/Fe3O4.
A co-polymer of divinylbenzene (DVB) and glycidylmethacrylate (GMA) was used for the functionalization of GO/Fe3O4 in order to develop a sorbent for the MSPE of chlorophenols from environmental water prior to their determination by HPLC-MS/MS [54]. Due to π-π stacking and hydrogen-bonding interactions between the analytes and the functionalized adsorbent, good extraction efficiency was observed.
Polystyrene (PS) [55] and poly(pyrrole-co-aniline) [56] are two examples of functional groups that were employed to prepare magnetic graphene functionalized nanocomposites, which were used for the MSPE of PAHs from water samples. Polystyrene is rich in phenyl and alkyl groups. Therefore, functionalization with PS enhanced the extraction efficiency by increasing the active surface sites of the material. The sorbent exhibited sufficient surface area, excellent magnetic properties and resulted in good extraction efficiencies and low detection limits [55]. Similarly, the poly(pyrrole-co-aniline) functionalized graphene oxide nanocomposite combined the properties of the polypyrrole and polyaniline co-polymer, the GO, and the magnetic nanoparticles. As a result, the developed nanocomposite exhibited a significant enhancement of extraction efficiency due to the increased number of active surface sites on the sorbent as well as the protection of the Fe3O4 nanoparticles [56].
Polythionine was also employed for the functionalization of magnetic graphene oxide through an oxidative polymerization reaction of thionine on the surface of GO/Fe3O4 [57][58]. This surface modification significantly improved the merits of GO/Fe3O4, providing satisfactory extraction efficiency. The functionalized nanocomposite was used for the MSPE of chlorpheniramine [57] and duloxetine [58] from human plasma prior to their determination by HPLC-UV.
In order to enhance the dispersibility of magnetic GO in hydrophobic media, functionalization with phytic acid has been reported. Phytic acid-stabilized GO/Fe3O4 was applied for extraction of PAHs from vegetable oils. Due to the super-amphiphilicity of phytic acid, the dispersibility of the conventional GO/Fe3O4 sorbent increased. [59].
Functionalization of GO/Fe3O4 can also be performed for the enhancement of its selectivity towards the target analytes. In 2015, Abdolmohammad-Zadeh and Talleb synthesized a β-cyclodextrin (β-CD) grafted GO/Fe3O4 nano-hybrid and used it for the MSPE of gemfibrozil from human serum and pharmaceutical waste-water samples followed by determination using spectrofluorometry. This chemical compound can selectively bind with various organic, inorganic and biological guest molecules into its cavity to form stable host–guest inclusion complexes by a series of forces such as hydrophobic and van der Waals interactions. Therefore, due to the surface modification of graphene oxide with β-cyclodextrin, selective separation of the target analyte from complex sample matrices was achieved [60].
2.4. Functionalized Nanocomposites of Magnetic GO with MOFs
Metal-organic frameworks are mixed organic-inorganic supramolecular materials that became popular in 1995, when Yaghi and Li reported the synthesis of a MOF with large rectangular channels [61]. These materials are based on the coordination of metal ions or clusters with bi- or multidentate organic linkers [62][63]. Metal-organic frameworks exhibit various extraordinary properties including luminosity, tunable pore size, flexibility and thermal stability as well as high surface areas [64][65].
The combination of graphene oxide and metal organic frameworks enhances the merits of sorbent including its reusability, its pore volume, its dispersion capability, its extraction capacity, its mechanical strength as well as its surface area [66][67][68].
Liu et al. developed a sorbent based on magnetic graphene oxide functionalized MOF-199 with the aim to combine the benefits of GO/Fe3O4 and MOFs for the rapid separation and highly selective adsorption of triazole pesticides [69]. The sorbent was employed for the MSPE of triazole pesticides from environmental water samples prior to their determination by HPLC-MS/ΜS. High adsorption capacity for the target analytes was observed due to the high surface area and pore volume of the novel nanocomposite.
A high-affinity graphene oxide-encapsulated magnetic zirconium-MOF was developed for the MSPE of photosensitizers hematoporphyrin and hematoporphyrin monomethyl ether from human urine prior to their determination by ultra-performance liquid chromatography-high resolution mass spectrometry (UPLC-HRMS) [70]. The novel sorbent combined the advantages of GO, magnetic nanoparticles with the advantages of large surface area, high porosity, and easy modification of metal-organic frameworks.
Wang et al. synthesized magnetic Cu-MOFs embedded within graphene oxide nanocomposites and used it for the MSPE of benzenoid-containing insecticides prior to their determination by HPLC-UV [71]. For this purpose, GO nanosheets were functionalized with silica-coated Fe3O4 nanoparticles with core-shell structured through covalent bonding and subsequently the GO surfaces were modified with Cu-MOFs. The silica shells prevented the oxidation and agglomeration of Fe3O4 nanoparticles and served as a platform to integrate the Fe3O4 particles and GO nanosheets through covalent bonds. Finally, the functionalization with the Cu-MOFs enhanced the extraction efficiency by providing more active sites for adsorption because of the high porosity and tunability of MOFs. Figure 3 shows the SEM images of TEM Cu-MOFs (a) and Fe3O4@SiO2-GO-MOFs (b) as well as the TEM Cu-MOFs (c) and Fe3O4@SiO2-GO-MOFs (d).
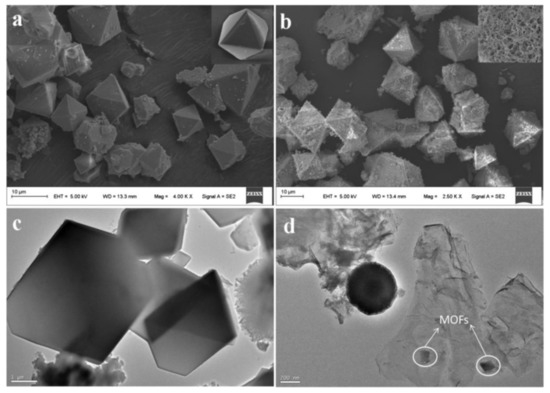
Figure 3. SEM images of Cu-MOFs (a) and Fe3O4@SiO2-GO-MOFs (b) and TEM images of Cu-MOFs (c) and Fe3O4@SiO2-GO-MOFs (d). Reproduced with permission from [71]. Copyright Elsevier, 2018.
References
- Samanidou, V. Trends in Microextraction Techniques for Sample Preparation. Separations 2017, 5, 1.
- Manousi, N.; Raber, G.; Papadoyannis, I. Recent Advances in Microextraction Techniques of antipsychotics in Biological Fluids Prior to Liquid Chromatography Analysis. Separations 2017, 4, 18.
- Filippou, O.; Bitas, D.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B 2017, 1043, 44–62.
- Kissoudi, M.; Samanidou, V. Recent Advances in Applications of Ionic Liquids in Miniaturized Microextraction Techniques. Molecules 2018, 23, 1437.
- Manousi, N.; Zachariadis, G. Determination of Volatile Compounds in Nut-Based Milk Alternative Beverages by HS-SPME Prior to GC-MS Analysis. Molecules 2019, 24, 3091.
- Anthemidis, A.; Ioannou, K. Sequential injection ionic liquid dispersive liquid–liquid microextraction for thallium preconcentration and determination with flame atomic absorption spectrometry. Anal. Bioanal. Chem. 2012, 404, 685–691.
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436.
- Manousi, N.; Gomez-Gomez, B.; Madrid, Y.; Deliyanni, E.; Zachariadis, G. Determination of rare earth elements by inductively coupled plasma-mass spectrometry after dispersive solid phase extraction with novel oxidized graphene oxide and optimization with response surface methodology and central composite design. Microchem. J. 2019, 152, 104428.
- Giakisikli, G.; Anthemidis, A. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Analyt. Chim. Acta 2013, 789, 1–16.
- Hemmati, M.; Rajabi, M.; Asghari, A. Magnetic nanoparticle based solid-phase extraction of heavy metal ions: A review on recent advances. Microchim. Acta 2018, 185.
- Ahmadi, F.; Rajabi, M.; Faizi, F.; Rahimi-Nasrabadi, M.; Maddah, B. Magnetic solid-phase extraction of Zineb by C18-functionalised paramagnetic nanoparticles and determination by first-derivative spectrophotometry. Int. J. Environ. Anal. Chem. 2014, 94, 1123–1138.
- Filippou, O.; Deliyanni, E.; Samanidou, V. Fabrication and evaluation of magnetic activated carbon as adsorbent for ultrasonic assisted magnetic solid phase dispersive extraction of bisphenol A from milk prior to high performance liquid chromatographic analysis with ultraviolet detection. J. Chromatogr. A 2017, 1479, 20–31.
- Li, W.; Shi, Y. Recent advances and applications of carbon nanotubes based composites in magnetic solid-phase extraction. Trac Trends Anal. Chem. 2019, 118, 652–665.
- Chatzimitakos, T.; Samanidou, V.; Stalikas, C.D. Graphene-functionalized melamine sponges for microextraction of sulfonamides from food and environmental samples. J. Chromatogr. A 2017, 1522, 1–8.
- Ziaei, E.; Mehdinia, A.; Jabbari, A. A novel hierarchical nanobiocomposite of graphene oxide–magnetic chitosan grafted with mercapto as a solid phase extraction sorbent for the determination of mercury ions in environmental water samples. Anal. Chim. Acta 2014, 850, 49–56.
- Lin, S.; Gan, N.; Qiao, L.; Zhang, J.; Cao, Y.; Chen, Y. Magnetic metal-organic frameworks coated stir bar sorptive extraction coupled with GC-MS for determination of polychlorinated biphenyls in fish samples. Talanta 2015, 144, 1139–1145.
- Wang, M.; Gao, M.; Zhang, K.; Wang, L.; Wang, W.; Fu, Q.; Xia, Z.; Gao, D. Magnetic covalent organic frameworks with core-shell structure as sorbents for solid phase extraction of fluoroquinolones, and their quantitation by HPLC. Microchim. Acta 2019, 186.
- Huang, X.; Liu, Y.; Liu, H.; Liu, G.; Xu, X.; Li, L.; Lv, J.; Liu, Z.; Zhou, W.; Xu, D. Magnetic Solid-Phase Extraction of Dichlorodiphenyltrichloroethane and Its Metabolites from Environmental Water Samples Using Ionic Liquid Modified Magnetic Multiwalled Carbon Nanotube/Zeolitic Imidazolate Framework-8 as Sorbent. Molecules 2019, 24, 2758.
- Kyzas, C.Z.; Deliyanni, E.A.; Bikiaris, D.N.; Mitropoulos, A.C. Graphene composites as dye adsorbents: Review. Chem. Eng. Res. Des. 2018, 129, 75–88.
- Travlou, N.A.; Kyzas, C.Z.; Lazaridis, N.K.; Deliyanni, E.A. Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir. 2013, 29, 1657–1668.
- Hummers, W.; Offeman, R. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339.
- Kyzas, G.Z.; Travlou, N.A.; Kalogirou, O.; Deliyanni, E. Magnetic graphene oxide: Effect of preparation route on Reactive Black 5 adsorption. Materials 2013, 6, 1360–1376.
- Kyzas, G.Z.; Deliyanni, E.; Matis, K.A. Graphene oxide and its application as adsorbent to waste water treatment. J. Chem. Technol. Biotechnol 2014, 89, 196–205.
- Rekos, K.; Kampouraki, Z.C.; Sarafidis, C.; Samanidou, V.; Deliyanni, E. Graphene Oxide Based Magnetic Nanocomposites with Polymers as Effective Bisphenol–A Nanoadsorbents. Materials 2019, 12, 1987.
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano. Mater. Sci. 2019, 1, 31–47.
- Sun, J.; Liang, Q.; Han, Q.; Zhang, X.; Ding, M. One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid phase extraction of heavy metal ions from biological samples. Talanta 2015, 132, 557–563.
- Kazemi, E.; Dadfarnia, S.; Haji Shabani, A. Dispersive solid phase microextraction with magnetic graphene oxide as the sorbent for separation and preconcentration of ultra-trace amounts of gold ions. Talanta 2015, 141, 273–278.
- AlKinani, A.; Eftekhari, M.; Gheibi, M. Ligandless dispersive solid phase extraction of cobalt ion using magnetic graphene oxide as an adsorbent followed by its determination with electrothermal atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2019, 1–18.
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814.
- Babaei, A.; Zeeb, M.; Es-haghi, A. Magnetic dispersive solid-phase extraction based on graphene oxide/Fe3O4@polythionine nanocomposite followed by atomic absorption spectrometry for zinc monitoring in water, flour, celery and egg. J. Sci. Food Agric. 2018, 98, 3571–3579.
- Wen, Y.; Niu, Z.; Ma, Y.; Ma, J.; Chen, L. Graphene oxide-based microspheres for the dispersive solid-phase extraction of non-steroidal estrogens from water samples. J. Chromatogr. A 2014, 1368, 18–25.
- Han, Q.; Wang, Z.; Xia, J.; Chen, S.; Zhang, X.; Ding, M. Facile and tunable fabrication of Fe3O4/graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Talanta 2012, 101, 388–395.
- Wu, J.; Xiao, D.; Zhao, H.; He, H.; Peng, J.; Wang, C.; Zhang, C.; He, J. A nanocomposite consisting of graphene oxide and Fe3O4 magnetic nanoparticles for the extraction of flavonoids from tea, wine and urine samples. Microchim. Acta 2015, 182, 2299–2306.
- Zeng, S.; Gan, N.; Weideman-Mera, R.; Cao, Y.; Li, T.; Sang, W. Enrichment of polychlorinated biphenyl 28 from aqueous solutions using Fe3O4 grafted graphene oxide. Chem. Eng. J. 2013, 218, 108–115.
- Bo, Z.; Shuai, X.; Mao, S.; Yang, H.; Qian, J.; Chen, J.; Yan, J.; Cen, K. Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci. Rep. 2014, 4.
- Yuan, Y.; Sun, N.; Yan, H.; Han, D.; Row, K. Determination of indometacin and acemetacin in human urine via reduced graphene oxide-based pipette tip solid-phase extraction coupled to HPLC. Microchim. Acta 2015, 183, 799–804.
- Chandra, V.; Park, J.; Chun, Y.; Lee, J.W.; Hwang, I.; Kim, K.S. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 2010, 4, 3979–3986.
- Ai, L.; Zhang, C.; Chen, Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J. Hazard. Mater. 2011, 192, 1515–1524.
- Gao, Y.; Ma, D.; Wang, C.; Guan, J.; Bao, X. Reduced graphene oxide as a catalyst for hydrogenation of nitrobenzene at room temperature. Chem. Commun. 2011, 47, 2432–2434.
- Rowley-Neale, S.; Randviir, E.; Abo Dena, A.; Banks, C. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226.
- Qi, T.; Huang, C.; Yan, S.; Li, X.; Pan, S. Synthesis, characterization and adsorption properties of magnetite/reduced graphene oxide nanocomposites. Talanta 2015, 144, 1116–1124.
- Luo, X.; Wang, C.; Luo, S.; Dong, R.; Tu, X.; Zeng, G. Adsorption of As (III) and As (V) from water using magnetite Fe3O4-reduced graphite oxide–MnO2 nanocomposites. Chem. Eng. J. 2012, 187, 45–52.
- Yan, S.; Qi, T.; Chen, D.; Li, Z.; Li, X.; Pan, S. Magnetic solid phase extraction based on magnetite/reduced graphene oxide nanoparticles for determination of trace isocarbophos residues in different matrices. J. Chromatogr. A 2014, 1347, 30–38.
- Wang, Q.; Jiao, L.; Du, H.; Wang, Y.; Yuan, H. Fe3O4 nanoparticles grown on graphene as advanced electrode materials for supercapacitors. J. Power Sources 2014, 245, 101–106.
- Zhang, M.; Wang, M.; Hao, Y.; Shi, X.; Wang, X. Effective extraction and simultaneous determination of Sudan dyes from tomato sauce and chili-containing foods using magnetite/reduced graphene oxide nanoparticles coupled with high-performance liquid chromatography. J. Sep. Sci. 2016, 39, 1749–1756.
- Li, D.; Ma, X.; Wang, R.; Yu, Y. Determination of trace bisphenol A in environmental water by high-performance liquid chromatography using magnetic reduced graphene oxide based solid-phase extraction coupled with dispersive liquid–liquid microextraction. Anal. Bioanal.l Chem. 2016, 409, 1165–1172.
- Mehdinia, A.; Rouhani, S.; Mozaffari, S. Microwave-assisted synthesis of reduced graphene oxide decorated with magnetite and gold nanoparticles, and its application to solid-phase extraction of organochlorine pesticides. Microchim. Acta 2016, 183, 1177–1185.
- Li, N.; Chen, J.; Shi, Y. Magnetic polyethyleneimine functionalized reduced graphene oxide as a novel magnetic sorbent for the separation of polar non-steroidal anti-inflammatory drugs in waters. Talanta 2019, 191, 526–534.
- Li, N.A.; Chen, Y.; Shi, Y. Magnetic polyethyleneimine functionalized reduced graphene oxide as a novel magnetic solid-phase extraction adsorbent for the determination of polar acidic herbicides in rice. Anal. Chim. Acta 2017, 949, 23–34.
- Yilmaz, E.; Ulusoy, H.; Demir, Ö.; Soylak, M. A new magnetic nanodiamond/graphene oxide hybrid (Fe3O4@ND@GO) material for pre-concentration and sensitive determination of sildenafil in alleged herbal aphrodisiacs by HPLC-DAD system. J. Chromatogr. B 2018, 1084, 113–121.
- Musevi, S.; Mohammad-Rezaei, R.; Razmi, H. Magnetic solid-phase extraction of malachite green using soluble eggshell membrane protein doped with magnetic graphene oxide nanocomposite. Int. J. Environ. Anal. Chem. 2018, 98, 1242–1252.
- Lotfi, Z.; Mousavi, H.; Sajjadi, S. Amino-terminated hyper-branched polyamidoamine polymer grafted magnetic graphene oxide nanosheets as an efficient sorbent for the extraction of selective serotonin reuptake inhibitors from plasma samples. Anal. Methods 2017, 9, 4504–4513.
- Shi, P.; Ye, N. Investigation of the adsorption mechanism and preconcentration of sulfonamides using a porphyrin-functionalized Fe3O4-graphene oxide nanocomposite. Talanta 2015, 143, 219–225.
- Pan, S.; Zhou, L.; Zhao, Y.; Chen, X.; Shen, H.; Cai, M.; Jin, M. Amine-functional magnetic polymer modified graphene oxide as magnetic solid-phase extraction materials combined with liquid chromatography-tandem mass spectrometry for chlorophenols analysis in environmental water. J. Chromatogr. A 2014, 1362, 34–42.
- Amiri, A.; Baghayeri, M.; Sedighi, M. Magnetic solid-phase extraction of polycyclic aromatic hydrocarbons using a graphene oxide/Fe3O4@polystyrene nanocomposite. Microchim. Acta 2018, 185.
- Amiri, A.; Baghayeri, M.; Hamidi, E. Poly(pyrrole-co-aniline)@graphene oxide/Fe3O4 sorbent for the extraction and preconcentration of polycyclic aromatic hydrocarbons from water samples. New, J. Chem. 2018, 42, 16744–16751.
- Daryakenary, M.; Zeeb, M. Trace determination of chlorpheniramine in human plasma using magnetic dispersive solid-phase extraction based on a graphene oxide/Fe3O4@polythionine nanocomposite combined with high-performance liquid chromatography. RSC Adv. 2017, 7, 53210–53218.
- Zeeb, M.; Farahani, H. Graphene oxide/Fe3O4@polythionine nanocomposite as an efficient sorbent for magnetic solid-phase extraction followed by high-performance liquid chromatography for the determination of duloxetine in human plasma. Chem. Pap. 2017, 72, 15–27.
- Ji, W.; Zhang, M.; Duan, W.; Wang, X.; Zhao, H.; Guo, L. Phytic acid-stabilized super-amphiphilic Fe3O4-graphene oxide for extraction of polycyclic aromatic hydrocarbons from vegetable oils. Food Chem. 2017, 235, 104–110.
- Abdolmohammad-Zadeh, H.; Talleb, Z. Magnetic solid phase extraction of gemfibrozil from human serum and pharmaceutical wastewater samples utilizing a β-cyclodextrin grafted graphene oxide-magnetite nano-hybrid. Talanta 2015, 134, 387–393.
- Yaghi, O.M.; Li, H. Hydrothermal synthesis of a metal-organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402.
- Hashemi, B.; Zohrabi, P.; Raza, N.; Kim, K. Metal-organic frameworks as advanced sorbents for the extraction and determination of pollutants from environmental, biological, and food media. Trends Anal. Chem. 2017, 97, 65–82.
- Giannakoudakis, D.A.; Bandosz, T.J. Building MOF Nanocomposites with Oxidized Graphitic Carbon Nitride Nanospheres: The Effect of Framework Geometry on the Structural Heterogeneity. Molecules 2019, 24, 4529.
- Zhou, H.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674.
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yagh, O.M. The chemistry and applications of metal-organic frameworks. Science. 2013, 341.
- Pourbahman, F.; Zeeb, M.; Monzavi, A.; Homami, S. Simultaneous trace monitoring of prokinetic drugs in human plasma using magnetic dispersive micro-solid phase extraction based on a new graphene oxide/metal–organic framework-74/Fe3O4/polytyramine nanoporous composite in combination with HPLC. Chem. Pap. 2019, 73, 3135–3150.
- Cao, X.; Jiang, Z.; Wang, S.; Hong, S.; Li, H.; Shao, Y.; She, Y.; Wang, J.; Jin, F.; Jin, M. One-pot synthesis of magnetic zeolitic imidazolate framework/grapheme oxide composites for the extraction of neonicotinoid insecticides from environmental water samples. J. Sep. Sci. 2017, 40, 4747–4756.
- Zhang, S.; Yao, W.; Zhou, C.; Wang, J.; Zhao, H. Fabrication of magnetic zeolitic imidazolate framework-7 supported graphene oxide for the extraction of fungicides from environmental water and soil samples. Int. J. Environ. Anal. Chem. 2019, 1–10.
- Liu, G.; Li, L.; Huang, X.; Zheng, S.; Xu, D.; Xu, X.; Zhang, Y.; Lin, H. Determination of triazole pesticides in aqueous solution based on magnetic graphene oxide functionalized MOF-199 as solid phase extraction sorbents. Micropor. Mesopor. Mater. 2018, 270, 258–264.
- Hua, X.; Gao, G.; Pan, S. High-affinity graphene oxide-encapsulated magnetic Zr-MOF for pretreatment and rapid determination of the photosensitizers hematoporphyrin and hematoporphyrin monomethyl ether in human urine prior to UPLC-HRMS. Anal. Bioanal. Chem. 2018, 410, 7749–7764.
- Wang, X.; Ma, X.; Huang, P.; Wang, J.; Du, P.; Du, X.; Lu, X. Magnetic Cu-MOFs embedded within graphene oxide nanocomposites for enhanced preconcentration of benzenoid-containing insecticides. Talanta 2018, 181, 112–117.




