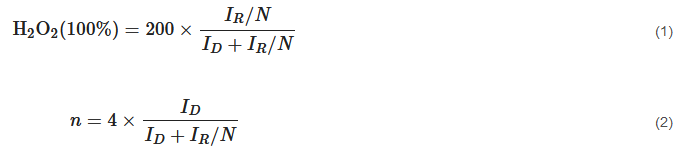| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Haoqi Yang | + 1499 word(s) | 1499 | 2020-12-29 08:41:41 | | | |
| 2 | Vicky Zhou | Meta information modification | 1499 | 2021-01-12 03:01:05 | | |
Video Upload Options
Oxygen reduction reaction (ORR) has attracted considerable attention for clean energy conversion technologies to reduce traditional fossil fuel consumption and greenhouse gas emissions. Although platinum (Pt) metal is currently used as an electrocatalyst to accelerate sluggish ORR kinetics, the scarce resource and high cost still restrict its further scale-up applications. In this regard, biomass-derived carbon electrocatalysts have been widely adopted for ORR electrocatalysis in recent years owing to their tunable physical/chemical properties and cost-effective precursors.
1. Introduction
The electrocatalytic activity of oxygen reduction reaction (ORR) significantly determines the performance of current energy conversion and storage devices, including various fuel cells and metal–air batteries [1][2][3]. Due to the sluggish kinetics of the ORR process, electrocatalysts have usually been required to accelerate the reaction rates and decrease the overpotentials [4]. Currently, platinum (Pt)-group metal (PGM) based materials have been broadly utilized and regarded as the most effective electrocatalysts for ORR catalysis [5][6]. However, the disadvantages of scarce resource, high-cost and poor durability greatly limit their further scale-up applications [7]. In this regard, a great number of efforts have concentrated on the development of cost-effective and high-performance candidates to replace the state-of-the-art PGM-based electrocatalysts [8][9][10].
Up to now, transition metal compound based and heteroatom doped metal-free carbon-based materials are two representative types of PGM-free electrocatalysts for ORR [11][12]. For example, transition-metal-nitrogen-carbon (M-N-C), transition metal oxides (TMOs), nitrides (TMNs), and phosphides (TMPs) based carbon hybrids [13][14][15][16], such as single-atom transition-metal-doped (Fe, Co, Mn, etc.) [17][18], have been well accepted as the promising candidates to replace PGM-based electrocatalysts. In these transition-metal-based electrocatalysts, Mx-Ny sites [19][20] or pyridinic and graphitic-N [21] have been recognized as the main active sites. Recently, different kinds of carbon materials (carbon nanotube, graphene, carbon nanofiber, etc.) have been developed as high-performance ORR electrocatalysts due to high electronic conductivity, excellent stability, tunable morphology, and facile functionality [22]. Although investigations into carbon nanotube and graphene as electrocatalysts have attracted significant attention for ORR electrocatalysis, scaled-up application of such carbon nanomaterials is still limited by high cost or deficient activity [23][24][25]. Fortunately, more and more inexpensive methods have been proposed to prepare graphene with high quality. For example, Tour and coworkers have developed a less expensive approach using six easily obtained raw-carbon-containing materials including cookies, chocolate, grass, plastics, cockroaches, and dog feces to grow graphene directly on the back of a Cu foil at 1050 °C under H2/Ar flow [26]. In recent years, designing efficient carbon electrocatalysts with sustainable and abundant biomass materials as precursors have been rapidly emerged owing to their cost-effective fabrication and environmentally friendly [27]. At present, various biomass materials have been reported as promising precursors to synthesize porous carbon, such as sugar [28], lignocellulose [29], animal biomass [30][31], natural cattail fibers [32], haddock peel [33], dandelion seeds [34], mulberry leaves [35], chitosan [36], gelatin [37], chitin [3] etc. Besides the commonly used method of thermal decomposition to prepare biomass-derived carbon electrocatalysts, several strategies including activation [38], hydrothermal carbonization [39][40], molten salt carbonization [41] and template method [42][43] have been proposed. Except for their renewable and sustainable properties, rich heteroatoms composition and inherited porous structure are two desirable features [30][32]. Biomass with natural pore structures and abundant active sites are promising to afford a tailorable template for electrocatalyst synthesis. However, it should be noted that the structural features and chemical composition of biomass would be different from region to region, thus resulting in a diverse performance.
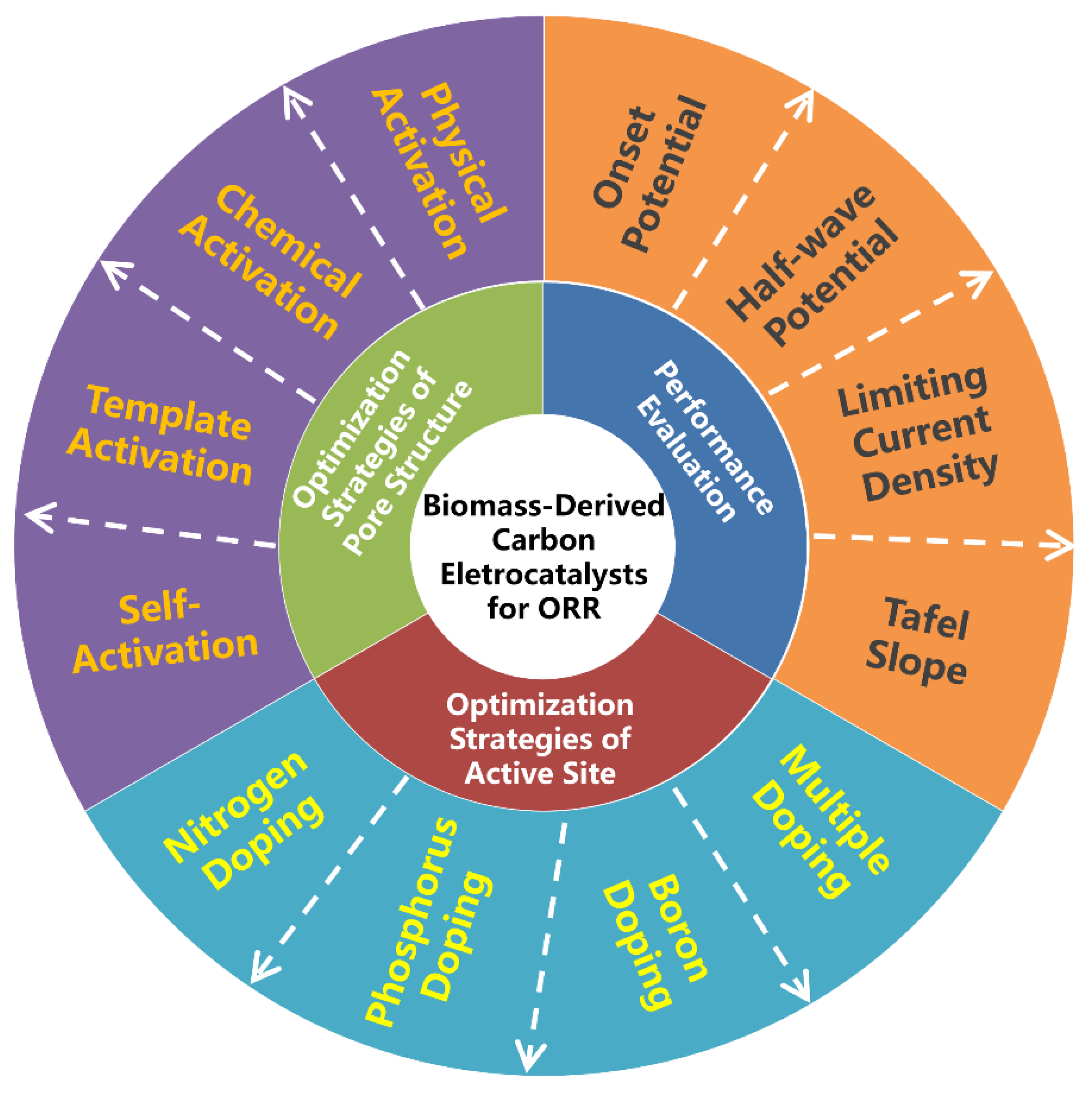
There have been several valuable reviews on the achievements of biomass-derived carbon electrocatalysts including their preparation, physicochemical properties and ORR applications [25][44][45][46][47]. However, the progress focusing on the optimization strategies of pore structure and active site for oxygen electroreduction has not been specifically summarized yet. Moreover, this research field is developing rapidly. Therefore, it is highly essential to provide a timely review as well as in-depth understanding of optimization strategies on ORR performance by considering them in entirety (Figure 1).
Figure 1. Schematic overview of three important aspects for the development of advanced biomass-derived carbon electrocatalysts for oxygen reduction reaction (ORR).
2. Performance Evaluation of Biomass-Derived Carbon Electrocatalysts for ORR
To reliably evaluate the ORR performance of biomass-derived carbon electrocatalysts [37], constructing a general and standard procedure is highly necessary. So far, the widely adopted method to assess the intrinsic activity of catalysts is based on the rotating disk electrode (RDE) and rotating ring disk electrode (RRDE) measurement [48]. Such method is capable of avoiding the mass transfer concerns and thus affording a stable ORR kinetic current density (jk), because the working electrode rotates at a high speed during the evaluation process (Figure 2a) [44]. Typically, ORR measurement is carried out in a three-electrode system, in which the electrocatalyst is the working electrode, an Ag/AgCl or Hg/HgO electrode is the reference electrode, and a Pt wire is the counter electrode [49]. The electrocatalysts are usually dispersed in solvent with Nafion as binders to form a uniform slurry. Then the slurry is drop-casted on glassy carbon (GC)-based RDE or RRDE [50]. Of note, the quality of drop-casting film and loading amount of electrocatalyst directly affects the ORR kinetics. Therefore, uniform catalyst films and appropriate catalyst mass loadings on the electrode are essential to obtain an accurate ORR performance, including onset potential (Eonset), half-wave potential (E1/2), limiting current density (jL) and Tafel slope parameters [51]. A positive shift of Eonset or E1/2, large jL, and small Tafel slope indicate high ORR activity and fast ORR kinetics, respectively [52].
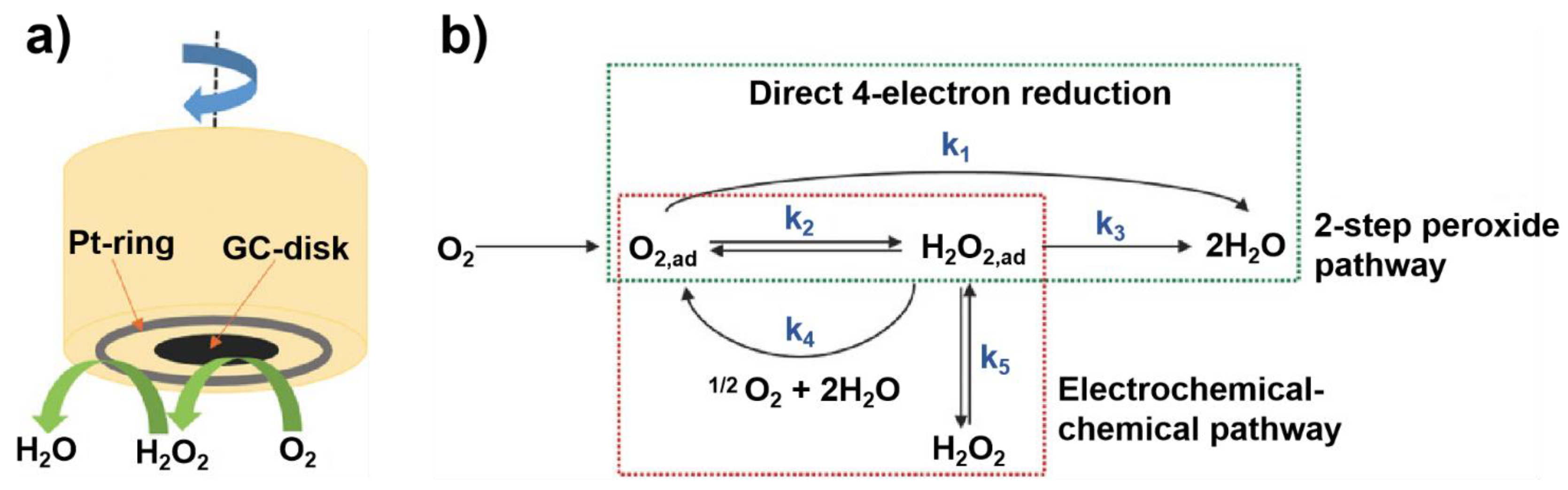
Generally, two ORR pathways have been proposed: one is the direct four-electron (4e−) reduction pathway, the other is the indirect two-electron (2e−) peroxide pathway (Figure 2b) [44]. O2 can be directly reduced to H2O through the 4e− pathway with a rate constant k1; alternatively, it can also be reduced to adsorbed hydrogen peroxide (H2O2,ad) through the 2e− pathway with a rate constant k2 (HO2− in alkaline medium and H2O2 in acidic medium). The amount of H2O2 was calculated according to Equation (1). Subsequently, H2O2,ad could either be electrochemically reduced to H2O with a rate constant k3, or could be chemically decomposed to O2,ad with a rate constant k4 on surface, and desorbed into electrolyte with a rate constant k5. The suitable electrocatalyst drives the ORR process towards direct 4e− reduction pathway, which requires high current efficiency and fewer peroxide species [53]. The selectivity of the electrocatalyst is usually described with the number (n) of transferred electrons, which can be calculated by K-L equation and RRDE measurement [54]. The number (n) was calculated according to Equation (2).
where ID is the disk current, IR is the ring current, and N is the collection efficiency (≈0.24–0.5).
3. Conclusion and Perspective
The ORR activity significantly determines the performance of various energy-conversion devices, such as polymer electrolyte membrane fuel cells, microbial fuel cells and metal-air batteries. Therefore, biomass materials from different sources have been widely considered as scalable and sustainable catalysts [55][56]. That pore structure and active sites affect the physicochemical properties and electrocatalytic performance has been emphasized, and thus giving a guideline for rational design of biomass-derived carbon materials. Compared with the state-of-the-art Pt/C catalyst, biomass-derived carbons exhibit some intriguing advantages of lower production cost, better methanol and CO tolerance, as well as better stability [57][58]. However, several great challenges still remain for designing active electrocatalysts that exhibit comparable activity to Pt/C in acidic environment.
Although biomass-derived carbon electrocatalysts show excellent ORR activity in RDE measurements, it is still difficult to integrate such catalysts into energy conversion devices, especially in H2-O2 fuel cells. Biomass materials are highly abundant and economical, but there has not been much progress yet in large-scale applications. Using biomass-derived carbon materials as substrates to reduce the noble metal loading is a promising strategy, which can simultaneously enhance the electrocatalytic activity and electrochemically active surface area. Here, we have demonstrated that the rational design of pore structure and active site plays a crucial role for synthesizing biomass-derived carbon electrocatalysts with satisfactory physicochemical and electrochemical properties. It is believed that developing effective and green synthetic methods will be a promising strategy to achieve more sustainable platforms.
On the other hand, in order to increase the competitiveness of biomass-derived carbon electrocatalysts for ORR, enhancing the intrinsic activity through optimizing active sites is urgently required. Consequently, understanding the catalytic mechanism of active sites and the relationship between mass transfer and pore structure for ORR process is important [59]. The in-depth investigation on these mechanisms helps to decide what types of biomass precursors should be chosen and what preparation methods should be performed. With the rapid development of materials science and technology, the community should further broaden the practical applications of biomass-derived carbon electrocatalysts in ORR-related devices, such as water splitting, supercapacitors [60][61], lithium-ion batteries [62][63], and CO2 reduction, etc. In this way, large volumes of biomass and biowaste can be truly turned into a valuable resource and a sustainable society can be really established.
References
- Wang, D.-W.; Su, D. Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 576.
- Wang, Z.L.; Xu, D.; Xu, J.J.; Zhang, X.B. Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786.
- Wang, M.; Ma, J.; Yang, H.; Lu, G.; Yang, S.; Chang, Z. Nitrogen and Cobalt Co-Coped Carbon Materials Derived from Biomass Chitin as High-Performance Electrocatalyst for Aluminum-Air Batteries. Catalysts 2019, 9, 954.
- Liu, M.; Zhang, R.; Chen, W. Graphene-supported nanoelectrocatalysts for fuel cells: Synthesis, properties, and applications. Chem. Rev. 2014, 114, 5117–5160.
- Luo, M.; Zhao, Z.; Zhang, Y.; Sun, Y.; Xing, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.; Qin, Y.; et al. PdMo bimetallene for oxygen reduction catalysis. Nature 2019, 574, 81–85.
- Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.M.; Lou, X.W.; et al. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856.
- Wang, H.; Wu, Z.; Plaseied, A.; Jenkins, P.; Simpson, L.; Engtrakul, C.; Ren, Z. Carbon nanotube modified air-cathodes for electricity production in microbial fuel cells. J. Power Source 2011, 196, 7465–7469.
- Zhang, H.; Zhou, Y.; Li, C.; Chen, S.; Liu, L.; Liu, S.; Yao, H.; Hou, H. Porous nitrogen doped carbon foam with excellent resilience for self-supported oxygen reduction catalyst. Carbon 2015, 95, 388–395.
- Zhang, H.; Ji, X.; Liu, N.; Zhao, Q. Synergy effect of carbon nanotube and graphene hydrogel on highly efficient quantum dot sensitized solar cells. Electrochim. Acta 2019, 327.
- Wu, M.; Zhang, G.; Qiao, J.; Chen, N.; Chen, W.; Sun, S. Ultra-long life rechargeable zinc-air battery based on high-performance trimetallic nitride and NCNT hybrid bifunctional electrocatalysts. Nano Energy 2019, 61, 86–95.
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760.
- Wang, W.; Jia, Q.; Mukerjee, S.; Chen, S. Recent Insights into the Oxygen-Reduction Electrocatalysis of Fe/N/C Materials. ACS Catal. 2019, 2019.
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447.
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Cai, Q.; Vasileff, A.; Li, L.H.; Han, Y.; Chen, Y.; Qiao, S.-Z. Molecule-Level g-C3N4 Coordinated Transition Metals as a New Class of Electrocatalysts for Oxygen Electrode Reactions. J. Am. Chem. Soc. 2017, 139, 3336–3339.
- Jiang, W.-J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.-J.; Wang, J.-Q.; Hu, J.-S.; Wei, Z.; Wan, L.-J. Understanding the High Activity of Fe–N–C Electrocatalysts in Oxygen Reduction: Fe/Fe3C Nanoparticles Boost the Activity of Fe–Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578.
- Strickland, K.; Miner, E.; Jia, Q.; Tylus, U.; Ramaswamy, N.; Liang, W.; Sougrati, M.T.; Jaouen, F.; Mukerjee, S. Highly active oxygen reduction non-platinum group metal electrocatalyst without direct metal-nitrogen coordination. Nat. Commun. 2015, 6, 7343.
- Yang, X.F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740.
- Chen, Y.; Ji, S.; Wang, Y.; Dong, J.; Chen, W.; Li, Z.; Shen, R.; Zheng, L.; Zhuang, Z.; Wang, D.; et al. Innenrücktitelbild: Isolated Single Iron Atoms Anchored on N-Doped Porous Carbon as an Efficient Electrocatalyst for the Oxygen Reduction Reaction (Angew. Chem. 24/2017). Angew. Chem. 2017, 129, 7041–7045.
- Ferrero, G.A.; Preuss, K.; Marinovic, A.; Jorge, A.B.; Mansor, N.; Brett, D.J.L.; Fuertes, A.B.; Sevilla, M.; Titirici, M.-M. Fe–N-Doped Carbon Capsules with Outstanding Electrochemical Performance and Stability for the Oxygen Reduction Reaction in Both Acid and Alkaline Conditions. ACS Nano 2016, 10, 5922–5932.
- Sa, Y.J.; Seo, D.-J.; Woo, J.; Lim, J.T.; Cheon, J.Y.; Yang, S.Y.; Lee, J.M.; Kang, D.; Shin, T.J.; Shin, H.S.; et al. A General Approach to Preferential Formation of Active Fe–Nx Sites in Fe–N/C Electrocatalysts for Efficient Oxygen Reduction Reaction. J. Am. Chem. Soc. 2016, 138, 15046–15056.
- Saidi, W.A. Oxygen Reduction Electrocatalysis Using N-Doped Graphene Quantum-Dots. J. Phys. Chem. Lett. 2013, 4, 4160–4165.
- Singh, H.; Zhuang, S.; Ingis, B.; Nunna, B.B.; Lee, E.S. Carbon-based catalysts for oxygen reduction reaction: A review on degradation mechanisms. Carbon 2019, 151, 160–174.
- Dai, L. Metal-Free Carbon Electrocatalysts: Recent Advances and Challenges Ahead. Adv. Mater. 2019, 31, 1900973.
- Du, L.; Prabhakaran, V.; Xie, X.; Park, S.; Wang, Y.; Shao, Y. Low-PGM and PGM-Free Catalysts for Proton Exchange Membrane Fuel Cells: Stability Challenges and Material Solutions. Adv. Mater. 2020, 1908232.
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799.
- Ruan, G.; Sun, Z.; Peng, Z.; Tour, J.M. Growth of graphene from food, insects, and waste. ACS Nano 2011, 5, 7601–7607.
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854.
- Deng, J.; Li, M.; Wang, Y. ChemInform Abstract: Biomass-Derived Carbon: Synthesis and Applications in Energy Storage and Conversion. ChemInform 2016, 47.
- De, S.; Balu, A.M.; Van der Waal, J.C.; Luque, R. ChemInform Abstract: Biomass-Derived Porous Carbon Materials: Synthesis and Catalytic Applications. ChemInform 2015, 46.
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2017, 129, 695–716.
- Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016.
- Liu, Z.; Li, Z.; Ma, J.; Dong, X.; Ku, W.; Wang, M.; Sun, H.; Liang, S.; Lu, G. Nitrogen and cobalt-doped porous biocarbon materials derived from corn stover as efficient electrocatalysts for aluminum-air batteries. Energy 2018, 162, 453–459.
- Lu, L.; Yu, J.; Wu, Z.; Fan, J.; Lei, W.; Ouyang, Y.; Xia, X.; He, G.; Hao, Q. Shaddock peel derived nitrogen and phosphorus dual-doped hierarchical porous carbons as high-performance catalysts for oxygen reduction reaction. Int. J. Hydrogen Energy 2019, 44, 26982–26991.
- Tang, J.; Wang, Y.; Zhao, W.; Zeng, R.J.; Liu, T.; Zhou, S. Biomass-derived hierarchical honeycomb-like porous carbon tube catalyst for the metal-free oxygen reduction reaction. J. Electroanal. Chem. 2019, 847, 113230.
- He, D.; Zhao, W.; Li, P.; Sun, S.; Tan, Q.; Han, K.; Liu, L.; Liu, L.; Qu, X. Bifunctional biomass-derived N, S dual-doped ladder-like porous carbon for supercapacitor and oxygen reduction reaction. J. Alloys Compd. 2019, 773, 11–20.
- Zhao, J.; Liu, Y.; Quan, X.; Chen, S.; Yu, H.; Zhao, H. Nitrogen-doped carbon with a high degree of graphitization derived from biomass as high-performance electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2017, 396, 986–993.
- Yang, H.; Kou, S.; Li, Z.; Chang, Z.; Wang, M.; Liu, Z.; Lu, G. 3D interconnected nitrogen-self-doped carbon aerogels as efficient oxygen reduction electrocatalysts derived from biomass gelatin. RSC Adv. 2019, 9, 40301–40308.
- Zhao, C.; Liu, G.; Sun, N.; Zhang, X.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Biomass-derived N-doped porous carbon as electrode materials for Zn-air battery powered capacitive deionization. Chem. Eng. J. 2018, 334, 1270–1280.
- Wu, X.L.; Wen, T.; Guo, H.L.; Yang, S.; Wang, X.; Xu, A.W. Biomass-Derived Sponge-like Carbonaceous Hydrogels and Aerogels for Supercapacitors. ACS Nano 2013, 7, 3589–3597.
- Brun, N.; Wohlgemuth, S.A.; Osiceanu, P.; Titirici, M.M. Original design of nitrogen-doped carbon aerogels from sustainable precursors: Application as metal-free oxygen reduction catalysts. Green Chem. 2013, 15, 2514–2524.
- Yin, H.; Lu, B.; Xu, Y.; Tang, D.; Mao, X.; Xiao, W.; Wang, D.; Alshawabkeh, A.N. Harvesting Capacitive Carbon by Carbonization of Waste Biomass in Molten Salts. Environ. Ence Technol. 2014, 48, 8101–8108.
- Ling, Z.; Wang, Z.; Zhang, M.; Yu, C.; Wang, G.; Dong, Y.; Liu, S.; Wang, Y.; Qiu, J. Sustainable Synthesis and Assembly of Biomass-Derived B/N Co-Doped Carbon Nanosheets with Ultrahigh Aspect Ratio for High-Performance Supercapacitors. Adv. Funct. Mater. 2016, 26, 111–119.
- Tang, J.; Liu, J.; Li, C.; Li, Y.; Tade, M.O.; Dai, S.; Yamauchi, Y. Synthesis of Nitrogen-Doped Mesoporous Carbon Spheres with Extra-Large Pores through Assembly of Diblock Copolymer Micelles. Angew. Chem. 2015, 127, 598–603.
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced Biomass-Derived Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2018, 30, 1703691.
- Kaur, P.; Verma, G.; Sekhon, S.S. Biomass derived Hierarchical Porous Carbon Materials as Oxygen Reduction Reaction Electrocatalysts in Fuel Cells. Prog. Mater. Sci. 2018, 102, 1–71.
- Wang, M.; Wang, S.; Yang, H.; Ku, W.; Yang, S.; Liu, Z.; Lu, G. Carbon-Based Electrocatalysts Derived From Biomass for Oxygen Reduction Reaction: A Minireview. Front. Chem. 2020, 8.
- Du, L.; Zhang, G.; Liu, X.; Hassanpour, A.; Dubois, M.; Tavares, A.C.; Sun, S. Biomass-derived nonprecious metal catalysts for oxygen reduction reaction: The demand-oriented engineering of active sites and structures. Carbon Energy 2020.
- Yang, H.; Li, Z.; Kou, S.; Lu, G.; Liu, Z. A complex-sequestered strategy to fabricate Fe single-atom catalyst for efficient oxygen reduction in a broad pH-range. Appl. Catal. B-Environ. 2020, 278.
- Wang, X.; Du, J.; Zhang, Q.; Gu, L.; Cao, L.; Liang, H.-P. In situ synthesis of sustainable highly efficient single iron atoms anchored on nitrogen doped carbon derived from renewable biomass. Carbon 2020, 157, 614–621.
- Li, B.-Q.; Zhao, C.-X.; Chen, S.; Liu, J.-N.; Chen, X.; Song, L.; Zhang, Q. Framework-Porphyrin-Derived Single-Atom Bifunctional Oxygen Electrocatalysts and their Applications in Zn-Air Batteries. Adv. Mater. 2019, 31.
- Wang, C.; Chen, W.; Xia, K.; Xie, N.; Wang, H.; Zhang, Y. Silk-Derived 2D Porous Carbon Nanosheets with Atomically-Dispersed Fe-N-x-C Sites for Highly Efficient Oxygen Reaction Catalysts. Small 2019, 15.
- Wan, C.; Duan, X.; Huang, Y. Molecular Design of Single-Atom Catalysts for Oxygen Reduction Reaction. Adv. Energy Mater. 2020, 10.
- Wu, M.; Zhang, G.; Wu, M.; Prakash, J.; Sun, S. Rational design of multifunctional air electrodes for rechargeable Zn-Air batteries: Recent progress and future perspectives. Energy Storage Mater. 2019, 21, 253–286.
- Wang, D.; Xiao, L.; Yang, P.; Xu, Z.; Lu, X.; Du, L.; Levin, O.; Ge, L.; Pan, X.; Zhang, J. Dual-Nitrogen-Source Engineered Fe-Nx Moieties as A Booster to Oxygen Electroreduction. J. Mater. Chem. A 2019, 7, 11007–11015.
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and Nitrogen Dual-Doped Mesoporous Graphene Electrocatalyst for Oxygen Reduction with Synergistically Enhanced Performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500.
- Guo, C.-Z.; Liao, W.-L.; Chen, C.-G. Design of a non-precious metal electrocatalyst for alkaline electrolyte oxygen reduction by using soybean biomass as the nitrogen source of electrocatalytically active center structures. J. Power Source 2014, 269, 841–847.
- Guo, Z.; Xiao, Z.; Ren, G.; Xiao, G.; Zhu, Y.; Dai, L.; Jiang, L. Natural tea-leaf-derived, ternary-doped 3D porous carbon as a high-performance electrocatalyst for the oxygen reduction reaction. Nano Res. 2016, 9, 1244–1255.
- Zhu, C.; Zhai, J.; Dong, S. Bifunctional fluorescent carbon nanodots: Green synthesis via soy milk and application as metal-free electrocatalysts for oxygen reduction. Chem. Commun. 2012, 48, 9367–9369.
- Liu, J.; Song, P.; Xu, W. Structure-activity relationship of doped-nitrogen (N)-based metal-free active sites on carbon for oxygen reduction reaction. Carbon 2017, 115, 763–772.
- Yang, H.; Kou, S. Recent Advances of Flexible Electrospun Nanofibers-based Electrodes for Electrochemical Supercapacitors: A Minireview. Int. J. Electrochem. Sci. 2019, 14, 7811–7831.
- Shang, Y.; Yang, H.; Qin, Z.; Yin, S.; Yang, L.; Xu, M.; Li, Z.; Jin, Z.; Sun, H. Arbitrary-shaped reduced graphene oxide aerogels via an unsaturated water vapor reduction. Carbon 2020, 168, 169–179.
- Yang, H.; Liu, S.; Cao, L.; Jiang, S.; Hou, H. Superlithiation of non-conductive polyimide toward high-performance lithium-ion batteries. J. Mater. Chem. A 2018, 6, 21216–21224.
- Liu, S.; Yang, H.; Sui, L.; Jiang, S.; Hou, H. Self-Adhesive Polyimide (PI)@Reduced Graphene Oxide (RGO)/PI@Carbon Nanotube (CNT) Hierarchically Porous Electrodes: Maximizing the Utilization of Electroactive Materials for Organic Li-Ion Batteries. Energy Technol. 2020, 8.