
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cesareo Saiz Jimenez | + 3512 word(s) | 3512 | 2020-12-28 10:07:30 | | | |
| 2 | Peter Tang | Meta information modification | 3512 | 2021-01-07 11:03:18 | | |
Video Upload Options
Covering up to 25% of the land surface and acting as a rapid CH4 sink and alternately as a CO2 source or sink, karstic subterranean ecosystems play a decisive role in the carbon cycle in terms of their contribution to the global balance of greenhouse gases. Recent data indicate that microbiota must play a significant ecological role in the biogeochemical processes that control the composition of the subterranean atmosphere, as well as in the availability of nutrients for the ecosystem. Nevertheless, there are still essential gaps in our knowledge concerning the budgets of greenhouse gases at the ecosystem scale and the possible feedback mechanisms between environmental-microclimatic conditions and the rates and type of activity of microbial communities in subterranean ecosystems.
1. Introduction
Karst is the term used to describe terrains underlain by soluble rock and characterized by the occurrence of caves, sinkholes, sinking streams, and an assortment of other landforms carved on the bedrock. Shallow karst ecosystems cover up to 25% of the Earth's land surface [1] and differ from the surface environments because of their limited energy and available nutrients.
Caves, in general, are characterized by a constant temperature, humidity, and high carbon dioxide (CO2) concentration the year round, as well as absence of light and scarcity of nutrients. Microorganisms occupy all the niches of the biosphere, including the subsurface, as a part of the critical zone, the heterogeneous near surface environment in which complex interactions involve rock, soil, water, air, and living organisms [2].
Earth's subsurface contains an active microbiota colonizing rock surfaces. In this environment, microorganisms are forced to adapt their metabolism for surviving in extreme conditions, and the low input of carbon, nitrogen and phosphorus as well as the chemical composition of the rock has a direct impact on the community diversity. In fact, one of the main reservoirs of microbial life, even at great depths, where life is not dependent on solar energy and photosynthesis for its primary energy supply is the terrestrial subsurface [3].
The colonization of substrates in caves is not homogeneous. Microorganisms colonize speleothems, host rock, detrital sediments, and/or speleosols with different compositions (clays, carbonate minerals, etc.) and/or textures (crystal habit, grain size, permeability, etc.). Microbial colonization is ultimately a complex and dynamic process that is determined and controlled by physicochemical properties (temperature, pH, redox potential, salinity) and biochemical factors (bioreceptivity, nutrient or electron acceptor availability, carbon, nitrogen and phosphorous concentrations, etc.) [4]. Therefore, the collective metabolic processes of microorganisms are decisive in the biogeochemical cycles of the biosphere: C and N fixation, CH4 metabolism, S oxide reduction, etc.
It is well-known that dissolution and precipitation of carbonates are the main processes involved in the mobilization of carbon in subterranean environments. Cave microorganisms are able to induce the precipitation of carbonates, via biomineralization processes [5] and also dissolution processes due to the excretion of acids [6]. There is a wide array of literature on the study of bio-induced mineral formations in subterranean environments [7] and on the microbial–rock interaction related to the CO2 uptake or release processes [8]. In this context, previous studies have confirmed that Actinobacteria biofilms developing on cave walls promote uptake of CO2, dissolve the rock, and produce calcite crystals in periods of lower humidity and/or CO2 [8]. However, the interactions of microbes with the air–water–rock interfaces in subterranean ecosystems and the biological mechanisms by which microorganisms adjust to new environments or changes in their current environment are poorly understood.
Low energy subsurface environments are uniquely positioned for examining minimum energetic requirements and adaptations for chemolithotrophic life and become a suitable environment to study the origins of life on Earth and may also serve as analogs to explore subsurface life in extraterrestrial bodies [9]. Furthermore, the microbiota from shallow subsurface environments (karst cavities, lava tubes) are becoming a target of increasing interest in different research fields, including biodiversity [10], mineral formation and dissolution [7], cultural and natural heritage conservation [11], and paleoclimatology [12]. In addition, other important uses of microorganisms are the production of bioactive compounds valuable for medicine and enzymes for bioremediation [13].
The extensive literature about microbial diversity and activity of cave microorganisms has been reviewed by many authors. The books "Microbial Life of Cave Systems" by Engel [14] and "Cave Ecology" by Moldovan et al. [15] are a rich source of information. In addition, other book chapters and review articles are relevant [16][17][18][19][20][21].
2. The Control of Greenhouse Gas Fluxes by Cave Microorganisms
Global changes in the Earth's climate and its relationship to the increasing concentration of greenhouse gases (GHGs) in the atmosphere has received special attention since the last quarter of the 20th century. Etiope and Klusman [22] reported that the major sources for atmospheric methane (CH4) budget derive from the natural processes in the biosphere (modern microbial activity) and from fossil, radiocarbon-free CH4 emission, estimated at approximately 20% of atmospheric CH4, which is due to and mediated by anthropogenic activity. However, this estimation is higher than the estimates from statistical data of CH4 emission from fossil fuel and related anthropogenic sources. For these authors, geologic sources are more than enough to provide the amount of CH4 required to account for the suspected missing source of fossil CH4. In addition, Etiope and Lollar [23] distinguished between biotic and abiotic CH4, the latter produced in magmatic processes (volcanoes and high-temperature active hydrothermal vents) and postmagmatic processes at lower temperatures (gas–water–rock interactions).
A better understanding of the carbon cycle in the Earth-climate system is nowadays a crucial knowledge gap. The main research efforts are focused on identifying and characterizing all possible sources, reservoirs, and sinks of GHGs, mainly CO2 and CH4, in order to more accurately calculate the budgets, especially in the carbon cycle [24]. This issue is critical to understand the effects of changes in the carbon cycle on Earth's climate, and to assess the level of effort required in order to adapt and mitigate climate change.
The interactions between geological, microbiological, and chemical processes are responsible for the physical-chemical properties of the atmosphere and especially for changes in its composition. Caves and other shallow vadose environments are populated by methanotrophic microorganisms and thus represent a CH4 sink. This subterranean CH4 sink is largely overlooked in the scientific literature. Understanding how cave microbiomes influence the systems in which they inhabit is proving to be an exceptional research challenge [25].
Methane is consumed from the atmosphere by methanotrophs in forests, grasslands, paddy, and other unsaturated soils, which represent the major terrestrial sinks. Environmental CH4 oxidation by bacteria is mainly carried out by Gammaproteobacteria, Alphaproteobacteria, and Verrucomicrobia [26], though there is also recent evidence for methanotrophy in Rokubacteria [27].
The presence of methanotrophic bacteria in caves has been widely studied in Movile Cave, Romania, by using isolation techniques, 13CH4-labelling, and 13C-DNA analysis, and the significant importance to the ecosystem development and primary productivity has been remarked upon [28][29][30][31]. Evidence of the occurrence of methanotrophs has also been found in other caves [10][32][33]. However, in these studies the microorganisms were not related with the sink of GHGs in caves.
Specific studies, both on the environmental-driven controls on microbial activity and, in turn, on the microbial role in composition changes of natural subterranean ecosystems, constitute a new research area of the highest potential with a pool of questions to solve. The starting hypothesis was that the subterranean microbiome plays a significant ecological role in the biogeochemical processes controlling the composition of the underground atmosphere, as well as in the availability of nutrients for the rest of the ecosystem's biota.
Fernandez-Cortes et al. [34] evidenced for the first time that cave ecosystems act as effective natural sinks of atmospheric CH4 on seasonal and daily scales and this phenomenon may thus be relevant on a global scale in terms of its contribution to the global balance of GHGs. The potential methanotrophy in four Spanish caves was assessed by tracking the presence of methane-oxidizing bacteria using the particulate methane monooxygenase gene pmoA, which is a phylogenetic marker for identifying methanotroph-specific DNA sequences in the environment [35]. The study revealed the presence of the proteobacteria Methylocapsa aurea, Methylomicrobium album, Methylococcus capsulatus, and methanotrophs of the K1-1 and K3-16 groups in samples from Altamira, Sidron, and Ojo Guareña caves, mainly in locations where CH4 usually reaches concentrations near to the atmospheric background levels. These soil bacteria oxidize the atmospheric CH4 [36].
However, the analyses did not detect methanotrophs in remote subterranean locations or poorly ventilated caves, such as Castañar de Ibor Cave, where CH4 is absent or present in minimal concentrations (below the accuracy threshold) throughout the year. Fernandez-Cortes et al. [34] suggested that complete consumption of CH4 was favored in the subsurface atmosphere under near vapor-saturation conditions without significant intervention of methanotrophic bacteria. This led to the assumption that CH4 oxidation was induced by ions and •OH generated by the radioactive decay of radon (222Rn). In fact, one of the important •OH sources in cave air may be from radioactive 222Rn decay [34]. However, further research verified that the mechanism of CH4 consumption was seasonally changing and methane-oxidizing bacteria were primarily responsible for the widespread observations of CH4 depletion in subterranean environments, discarding any evidence of radiolysis contribution [37][38][39].
Schimmelmann et al. [37] tested, in controlled laboratory experiments, whether radiolysis could rapidly oxidize CH4 in sealed air with different relative humidity and elevated levels of radiation from Rn isotopes. No evidence of CH4 oxidation by radiolysis was found. On the contrary, a rapid loss of CH4 was found when moist soil in the absence of Rn was added to the container. This was consistent with the presence of methane-oxidizing bacteria, which were responsible for the widespread observations of CH4 depletion in subterranean environments.
Since the pioneering work of Fernandez-Cortes et al. [34], a few authors, based on studies in caves from Australia, the USA, Vietnam, and Spain, additionally supported CH4 oxidation by methanotrophic bacteria [38][39][40][41][42].
Webster et al. [38] reported that the concentrations and stable isotopic compositions of CH4, CO2, and Rn in cave air overlapped and diverged from those of the atmosphere, as the majority of cave air samples were depleted in CH4 and enriched in CO2 relative to the local atmosphere. These differences indicate that atmospheric and internal cave processes influenced the composition of cave air. Therefore, the authors, on the basis of CH4 concentrations, δ13CCH4, and δ2HCH4 values measured in 33 caves in the USA and three caves in New Zealand, suggested that microbial methanotrophy within caves is the primary CH4 consumption mechanism. Furthermore, the stable isotopic composition of CH4 in the studied caves suggested that, in addition to atmospheric CH4, at least two additional CH4 sources were present in some caves: CH4 produced from acetate fermentation, and from CO2 reduction, processes occurring over a wide scale in the environment.
Lennon et al. [39] also proposed that biological processes, largely oxidation by methanotrophic bacteria, cause a depletion of CH4 in caves. They conducted a field mesocosm experiment to test whether or not microbial methanotrophy has the potential to act as a daily sink for CH4 in two fairly well-ventilated Vietnamese caves with low Rn concentrations (75–115 Bq/m3), temperatures of 19–21 °C, and relative humidity ranging between 85 and 95%, depending on the airflow and location within the cave. The data suggested that biological processes have the potential to deplete atmospheric levels of CH4 (~2 ppmv) via methanotrophy on a daily basis, as an 87% reduction in CH4 concentrations was observed.
It appears that CH4 depletion is a seasonal phenomenon, as reported by several authors. Fernandez-Cortes et al. [34] found significant seasonal and even daily variations in the gas composition of cave air, which involves the exchange of large amounts of other GHGs, in addition to CO2(g), with the lower troposphere. Waring et al. [40] performed a continuous 3-year record of CH4 and other trace gases in an Australian cave and found a seasonal cycle of extreme CH4 depletion, from ambient ~1775 ppb to near zero during summer and to ~800 ppb in winter.
Ojeda et al. [41] found methanotrophic bacteria from the families Methylococcaceae (Gammaproteobacteria) and Methylocystaceae (Alphaproteobacteria) in 67% of the samples collected in Nerja Cave, Spain. In a recent innovative research, Cuezva et al. [42] confirmed that microbial action in caves plays a crucial role in the processes of production, consumption and storage of GHGs (CO2 and CH4) and largely determines the strong variations of these major GHGs in natural underground ecosystems. This study was developed in three Spanish caves (Pindal, Castañar de Ibor and La Garma) as a first approach to systematically characterize the role of cave sediments in the production and transport of CO2 and CH4 in the subterranean environment.
Monitoring and sampling for more than two years in La Garma Cave showed that during the stages with greater ventilation, air circulates daily and there is a continual contribution of external air to the cave, which has lower CO2 content and CH4 levels close to the atmospheric background. Therefore, CH4 depletion rises with slight changes in CO2. Conversely, in stages with a low ventilation rate, CO2 reaches high concentrations in the cave because air exchange with the external atmosphere is negligible. Thus, the removed CH4 is not rapidly replenished. As a result, CH4 depletion rate tends to become negligible as the CO2 content of cave air rises (Figure 1a).
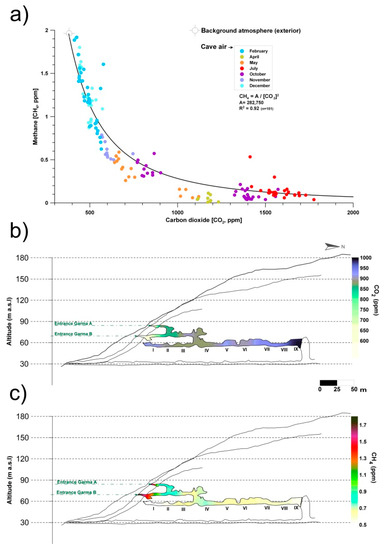
Figure 1. (a) Monthly co-variations in the concentrations of carbon dioxide (CO2) and methane (CH4) in La Garma Cave (Cantabria, northern Spain), a dynamically ventilated cave. (b) Spatial distribution of average concentrations of CO2. (c) Spatial distribution of average concentrations of CH4. Data from October 2014 to July 2017.
Figure 1b,c shows the spatial distribution of air CO2 and CH4 concentrations, respectively, in the air from La Garma Cave. Data for each contour map correspond to mean values from a set of bimonthly spot air samplings, conducted from October 2014 to July 2017, in a pre-established network of 11 points covering up to three levels of cave passages along an altitude gradient.
The average CO2 and CH4 concentrations of cave air were 894 and 0.65 ppm, respectively. Both GHGs depend on the rate of cave air exchange with the local atmosphere, which is controlled by climate-driven processes (primarily advection), and it is a very good indicator of the levels of matter and energy exchange with the exterior, showing the isolated areas and those with a prevailing connection with the exterior. Thus, a remarkable spatial pattern is distinguished; the highest average values of CO2 concentration and the lowest CH4 were found in the sectors of the lower gallery furthest from the main cave entrances (Garma A and Garma B, Figure 1b,c). Therefore, these cave maps with the contoured CO2 and CH4 levels reveal the importance of cave morphology in complex subterranean systems which control the gaseous composition of cave air, particularly in terms of gas variations due to the occurrence of elevation changes, multiple entrances or presence of dead-end passages. In the case of CH4, its average concentration decreased drastically below 0.7 ppm from the connection of the intermediate gallery with the lower gallery and was practically null (<0.5 ppm) in the most distant sectors of the cave entrances (Figure 1c). This CH4 pattern results from a decreasing percentage of mixing with the exterior and, consequently, a more effective methanotrophic activity of bacterial origin.
Cuezva et al. [42] are developing seasonal campaigns for CH4 and CO2 daily fluxes with continuous monitoring by a closed chamber-based gas exchange system (LI-COR Automated Soil Gas Flux System), in conjunction with a compatible Gasmet Fourier Transform Infrared (FTIR) gas analyzer and combined with δ13C geochemical tracing by cavity ring-down spectroscopy (CRDS) to understand the underlying mechanisms in cave sediments. Moreover, an autonomous piece of equipment monitored the main microenvironmental parameters of the local subsurface-soil-atmosphere system. Preliminary results showed net CO2 emissions from cave sediments resulting from respiration by chemolithotrophic microorganisms. The results also revealed simultaneous net CH4 uptake from cave sediments on a daily scale, with no significant level of variations along the day (Figure 2). Anaerobic oxidation of CH4 coupled to nitrite reduction is produced by members of the phylum Rokubacteria. These bacteria have also been found in Pindal Cave [42] and in an Alpine cave [43].
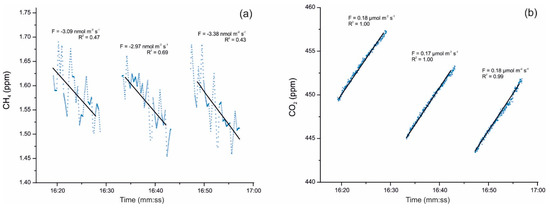
Figure 2. (a) Detail of CH4 uptake fluxes with an average of −3.15 nmol m−2 s−1 and (b) simultaneous CO2 emission fluxes uptake fluxes with an average of +0.17 µmol m−2 s−1, monitored on 17 December 2019 directly above sediments inside Pindal Cave (Asturias, Spain). The value of the diffusive flux (F) and the corresponding exponential adjustment (R2) of each measurement are indicated.
Other studies combining the depletion of CH4 with other GHGs (N2O and NO2) were carried out in Vapor Cave, Southeast Spain. This is a hypogenic cave formed by the upwelling of hydrothermal CO2-rich fluids in which anomalous concentrations of nitrogen oxides can be found [44]. The cave is characterized by a combination of rising warm air with large CO2 outgassing and highly diluted CH4 of endogenous origin. Additionally, extreme environmental conditions were observed, such as high air temperatures (38–43 °C) and 100% relative humidity, hypoxic conditions (17% O2), CO2 concentrations that exceed 1%, 222Rn activity with values above 50 kBq/m3, and a vertical thermal gradient of 3.2 °C/100 m [45]. These conditions, associated with the combined effects of tectonic activity and hydrothermalism, make this cave a remarkable site for the study of uncommon or extremophilic microbial communities. In Vapor Cave, the depletion of CH4 was quantified to account for more than 60% removal of the deep endogenous component of this gas [45].
Martin-Pozas et al. [44] collected different cave air and sediment samples from −2 to −80 m in Vapor Cave. The analyses were conducted by taking advantage of technological advances in high-precision field-deployable CRDS and FTIR spectrometers, which allowed to measure target tracer gases (NO2, N2O, CH4, and CO2) and δ13C of both carbon-GHGs in situ. The δ13CCO2 data (−4.5 to −7.5‰) suggested a mantle-rooted CO2 likely generated by the thermal decarbonation of underlying marine carbonates, combined with degassing from CO2-rich groundwater. CH4 molar fractions and their δD (−77 to −48‰) and δ13C values (−52 to −30‰) indicated that the CH4 reaching Vapor Cave is the remnant of a larger and deep-sourced CH4, which was likely generated by the microbial reduction in carbonates. This CH4 was affected by a postgenetic depletion during its migration through the cave environment as a component of the rising warm air.
CH4 concentrations and δ13CH4 varied with depth. At −80 m, higher concentrations were found but above −30 m depth lower CH4 concentrations were found and heavier δ13C values were found near the cave entrance. This was consistent with a methane oxidation mediated by microorganisms and in fact, next generation sequencing (NGS) analysis of sediments showed a relative abundance of Candidatus Methylomirabilis 4 to 5 times higher in the deepest sample (−80 m) with respect to −30 and −15 m. Candidatus Methylomirabilis oxyfera (Rokubacteria) is an anaerobic denitrifying methanotroph [46]. It must be noticed that Isobe et al. [47] found that members of the uncultivated candidate phylum Rokubacteria responded positively to elevate CO2 concentrations.
In a similar way, Cappelleti et al. [48] studied an area of agricultural soils in Italy with anomalously high temperatures (up to ≅ 50 °C) and found emissions of biogenic CO2 linked to CH4 oxidation at a depth of 0.7 m from the surface. A strong biological methane-oxidizing activity in these soils was found and an examination of the pmoA clone libraries revealed the large biodiversity of methanotrophs including Methylomonas, Methylococcus, Methylocystis, and Methylocaldum.
Regarding the nitrogen gases, Martin-Pozas et al. [44] stated that the analysis of the ecological functions and metabolism of the microbiota from cave sediments suggested that N2O is mainly produced in the deepest areas of Vapor Cave (below −15 m depth). In these areas, high CO2 concentrations and low O2 levels within the sediments determine a prevailing hypoxic and acidic environment that promotes the release of nitrite, nitric oxide, and hydroxylamine as products of the metabolism of ammonia-oxidizing archaea and nitrate reduction. In fact, at −15 m depth, the archaeal communities were dominated by the class Nitrososphaeria (69.0% of the total Archaea), with a majority of uncultured members and only two identified genera, Nitrososphaera and Nitrosotenuis. This is consistent with the abundant occurrence of these Archaea in deep sediments and better survival under conditions of low dissolved oxygen.
To summarize, considerable advances have been reached in recent years regarding processes of production, consumption, and storage of greenhouse gases (CO2, CH4 and NxOx) by cave microorganisms in subterranean vadose ecosystems. Recent and current research has shown that cave Actinobacteria are active agents in the fixation of CO2, capturing CO2 from air and forming calcium carbonate polymorphs [8]. In particular, direct CO2 flux measurements in areas heavily colonized by bacteria indicate that they were promoting the uptake of this gas. Subterranean environments act as sinks or net sources of soil-derived carbon dioxide (CO2) on annual and even daily scales, reaching up to ten times higher than the mean atmospheric CO2 content, which involves the exchange of large amounts of CO2(g) with the lower troposphere and its role as a depot and/or emitter. In a very recent in-situ experimental work (Pindal Cave, Spain) with a closed chamber-based gas exchange system—research in progress—we have verified negative CH4 fluxes (uptake) from microbial communities, simultaneously linked to positive CO2 fluxes (emission) directly related to microbial methanotrophy. The most recent data from direct measurements of gas exchange fluxes indicate that both gases are inextricably linked in these microbial-induced processes.
References
- Ford, D.C.; Williams, P.W. Karst Hydrogeology and Geomorphology; Wiley: Chichester, UK, 2007.
- Giardino, J.R.; Houser, C. (Eds.) Introduction to the critical zone. In Principles and Dynamics of the Critical Zone; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–13.
- Gold, T. The deep, hot biosphere. Proc. Natl. Acad. Sci. USA 1992, 89, 6045–6049, doi:10.1073/pnas.89.13.6045.
- Jones, A.A.; Bennett, P.C. Mineral ecology: Surface specific colonization and geochemical drivers of biofilm accumulation, composition, and phylogeny. Front. Microbiol. 2017, 8, 491, doi:10.3389/fmicb.2017.00491.
- Cuezva, S.; Sanchez-Moral, S.; Saiz-Jimenez, C.; Cañaveras, J.C. Microbial communities and associated mineral fabrics in Altamira Cave, Spain. Int. J. Speleol. 2009, 38, 83–92, doi:10.5038/1827-806x.38.1.9.
- Northup, D.E.; Lavoie, K.H. Geomicrobiology of caves: A review. Geomicrobiol. J. 2001, 18, 199–222, doi:10.1080/01490450152467750.
- Sánchez-Moral, S.; Portillo, M.D.C.; Janices, I.; Cuezva, S.; Cortés, Ángel, F.; Cañaveras, J.C.; Gonzalez, J.M. The role of microorganisms in the formation of calcitic moonmilk deposits and speleothems in Altamira Cave. Geomorphology 2012, 139–140, 285–292, doi:10.1016/j.geomorph.2011.10.030.
- Cuezva, S.; Fernandez-Cortes, A.; Porca, E.; Pašić, L.; Jurado, V.; Hernández, M.; Serrano-Ortiz, P.; Hermosin, B.; Cañaveras, J.C.; Sanchez-Moral, S.; et al. The biogeochemical role of Actinobacteria in Altamira Cave, Spain. FEMS Microbiol. Ecol. 2012, 81, 281–290, doi:10.1111/j.1574-6941.2012.01391.x.
- Jones, R.M.; Goordial, J.M.; Orcutt, B.N. Low energy subsurface environments as extraterrestrial analogs. Front. Microbiol. 2018, 9, 1605, doi:10.3389/fmicb.2018.01605.
- Lavoie, K.H.; Winter, A.S.; Read, K.J.H.; Hughes, E.M.; Spilde, M.N.; Northup, D.E. Comparison of bacterial communities from lava cave microbial mats to overlying surface soils from Lava Beds National Monument, USA. PLoS ONE 2017, 12, e0169339, doi:10.1371/journal.pone.0169339.
- Miller, A.Z.; Garcia‐Sanchez, A.M.; Martin‐Sanchez, P.M.; Pereira, M.F.C.; Spangenberg, J.E.; Jurado, V.; Dionísio, A.; Afonso, M.J.; Chaminé, H.I.I.S.; Hermosin, B.; et al. Origin of abundant moonmilk deposits in a subsurface granitic environment. Sedimentology 2018, 65, 1482–1503, doi:10.1111/sed.12431.
- Sasowsky, I.D.; Mylroie, J. Studies of Cave Sediments. Physical and Chemical Records of Paleoclimate; Springer: Dordrecht, The Netherlands, 2012.
- Ghosh, S.; Kuisiene, N.; Cheeptham, N. The cave microbiome as a source for drug discovery: Reality or pipe dream? Biochem. Pharmacol. 2017, 134, 18–34, doi:10.1016/j.bcp.2016.11.018.
- Engel, A.S. Microbial Life of Cave Systems; De Gruiter: Berlin, Germany, 2015.
- Moldovan, O.T.; Kovac, L.; Halse, S. Cave Ecology; Springer: Cham, Switzerland, 2018.
- Jones, B. Microbial activity in caves—A geological perspective. Geomicrobiol. J. 2001, 18, 345–357.
- Barton, H.A.; Northup, D.E. Geomicrobiology in cave environments: Past, current and future perspectives. J. Cave Karst Stud. 2007, 69, 163–178.
- Engel, A.S. Microbial diversity of cave ecosystems. In Geomicrobiology: Molecular and Environmental Perspectives; Barton, L.L., Mandl, M., Loy, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 219–238.
- Tomczyk-Żak, K.; Zielenkiewicz, U. Microbial diversity in caves. Geomicrobiol. J. 2016, 33, 20–38, doi:10.1080/01490451.2014.1003341.
- Jones, D.S.; Macalady, J.L. The snotty and the stringy: Energy for subsurface life in caves. In Their World: A Diversity of Microbial Environments; Hurst, C.J., Ed.; Springer: Cham, Switzerland, 2016; pp. 203–224.
- De Mandal, S.; Chatterjee, R.; Kumar, N.S. Dominant bacterial phyla in caves and their predicted functional roles in C and N cycle. BMC Microbiol. 2017, 17, 90, doi:10.1186/s12866-017-1002-x.
- Etiope, G.; Klusman, R.W. Geologic emissions of methane to the atmosphere. Chemosphere 2002, 49, 777–789, doi:10.1016/s0045-6535(02)00380-6.
- Etiope, G.; Lollar, B.S. Abiotic methane on earth. Rev. Geophys. 2013, 51, 276–299, doi:10.1002/rog.20011.
- Intergovernmental Panel on Climate Change. Carbon and other biogeochemical cycles. In Climate Change 2013: The Physical Science Basis; Cambridge Univ. Press: Cambridge, UK, 2014; pp. 465–570.
- Hall, E.K.; Bernhardt, E.S.; Bier, R.L.; Bradford, M.A.; Boot, C.M.; Cotner, J.B.; Del Giorgio, P.A.; Evans, S.E.; Graham, E.B.; Jones, S.E.; et al. Understanding how microbiomes influence the systems they inhabit. Nat. Microbiol. 2018, 3, 977–982, doi:10.1038/s41564-018-0201-z.
- Op den Camp, H.J.M.; Islam, T.; Stott, M.B.; Harhangi, H.R.; Hynes, A.; Schouten, S.; Jetten, M.S.M.; Birkeland, N.-K.; Pol, A.; Dunfield, P.F. Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia. Environ. Microbiol. Rep. 2009, 1, 293–306, doi:10.1111/j.1758-2229.2009.00022.x.
- Butterfield, C.N.; Li, Z.; Andeer, P.F.; Spaulding, S.; Thomas, B.C.; Singh, A.; Hettich, R.L.; Suttle, K.B.; Probst, A.J.; Tringe, S.G.; et al. Proteogenomic analyses indicate bacterial methylotrophy and archaeal heterotrophy are prevalent below the grass root zone. PeerJ 2016, 4, e2687, doi:10.7717/peerj.2687.
- Hutchens, E.; Radajewski, S.; Dumont, M.G.; McDonald, I.R.; Murrell, J.C. Analysis of methanotrophic bacteria in Movile Cave by stable isotope probing. Environ. Microbiol. 2004, 6, 111–120, doi:10.1046/j.1462-2920.2003.00543.x.
- Chen, Y.; Wu, L.; Boden, R.; Hillebrand, A.; Kumaresan, D.; Moussard, H.; Baciu, M.; Lu, Y.; Murrell, J.C. Life without light: Microbial diversity and evidence of sulfur- and ammonium-based chemolithotrophy in Movile Cave. ISME J. 2009, 3, 1093–1104, doi:10.1038/ismej.2009.57.
- Kumaresan, D.; Wischer, D.; Stephenson, J.; Hillebrand-Voiculescu, A.; Murrell, J.C. Microbiology of Movile Cave—A chemolithoautotrophic ecosystem. Geomicrobiol. J. 2014, 31, 186–193, doi:10.1080/01490451.2013.839764.
- Kumaresan, D.; Stephenson, J.; Doxey, A.C.; Bandukwala, H.; Brooks, E.; Hillebrand-Voiculescu, A.; Whiteley, A.S.; Murrell, J.C. Aerobic proteobacterial methylotrophs in Movile Cave: Genomic and metagenomic analyses. Microbiome 2018, 6, 1–10, doi:10.1186/s40168-017-0383-2.
- Saiz-Jimenez, C. The microbiology of show caves, mines tunnels and tombs: Implications for management and conservation. In Microbial Life of Cave Systems; Engel, A.S., Ed.; DeGruiter: Berlin, Germany, 2015; pp. 231–261.
- Gonzalez-Pimentel, J.L.; Miller, A.Z.; Jurado, V.; Laiz, L.; Pereira, M.F.C.; Saiz-Jimenez, C. Yellow coloured mats from lava tubes of La Palma (Canary Islands, Spain) are dominated by metabolically active Actinobacteria. Sci. Rep. 2018, 8, 1944, doi:10.1038/s41598-018-20393-2.
- Fernandez-Cortes, A.; Cuezva, S.; Alvarez-Gallego, M.; Garcia-Anton, E.; Pla, C.; Benavente, D.; Jurado, V.; Saiz-Jimenez, C.; Sanchez-Moral, S. Subterranean atmospheres may act as daily methane sinks. Nat. Commun. 2015, 6, 7003, doi:10.1038/ncomms8003.
- McDonald, I.R.; Murrell, J.C. The particulate methane monooxygenase gene pmoA and its use as a functional gene probe for methanotrophs. FEMS Microbiol. Lett. 1997, 156, 205–210, doi:10.1016/s0378-1097(97)00425-4.
- Tveit, A.T.; Hestnes, A.G.; Robinson, S.L.; Schintlmeister, A.; Dedysh, S.N.; Jehmlich, N.; Von Bergen, M.; Herbold, C.; Wagner, M.; Richter, A.; et al. Widespread soil bacterium that oxidizes atmospheric methane. Proc. Natl. Acad. Sci. USA 2019, 116, 8515–8524, doi:10.1073/pnas.1817812116.
- Schimmelmann, A.; Cortés, Ángel, F.; Cuezva, S.; Streil, T.; Lennon, J.T. Radiolysis via radioactivity is not responsible for rapid methane oxidation in subterranean air. PLoS ONE 2018, 13, e0206506, doi:10.1371/journal.pone.0206506.
- Webster, K.D.; Drobniak, A.; Etiope, G.; Mastalerz, M.; Sauer, P.E.; Schimmelmann, A. Subterranean karst environments as a global sink for atmospheric methane. Earth Planet. Sci. Lett. 2018, 485, 9–18, doi:10.1016/j.epsl.2017.12.025.
- Lennon, J.T.; Nguyễn-Thùy, D.; Phạm, T.M.; Drobniak, A.; Tạ, P.H.; Phạm, N.Ð.; Streil, T.; Webster, K.D.; Schimmelmann, A. Microbial contributions to subterranean methane sinks. Geobiology 2016, 15, 254–258, doi:10.1111/gbi.12214.
- Waring, C.L.; Hankin, S.I.; Griffith, D.W.T.; Kertesz, M.A.; Kobylski, V.; Wilson, N.L.; Coleman, N.V.; Kettlewell, G.; Zlot, R.; Bosse, M.; et al. Seasonal total methane depletion in limestone caves. Sci. Rep. 2017, 7, 1–12, doi:10.1038/s41598-017-07769-6.
- Ojeda, L.; Vadillo-Pérez, I.; Etiope, G.; Benavente, J.; Liñán, C.; Del Rosal, Y.; Tapia-Paniagua, S.; Moriñigo, M. Ángel; Carrasco, F. Methane sources and sinks in karst systems: The Nerja cave and its vadose environment (Spain). Geochim. Cosmochim. Acta 2019, 259, 302–315, doi:10.1016/j.gca.2019.06.011.
- Cuezva, S.; Martin-Pozas, T.; Fernandez-Cortes, A.; Cañaveras, J.C.; Janssens, I.; Sanchez-Moral, S. On the role of cave-soil in the carbon cycle. A first approach. EGU 2020, Abstract 21793, doi:10.5194/egusphere-egu2020-21793.
- Jurado, V.; Gonzalez-Pimentel, J.L.; Miller, A.Z.; Hermosin, B.; D’Angeli, I.M.; Tognini, P.; De Waele, J.; Saiz-Jimenez, C. Microbial communities in vermiculation deposits from an Alpine cave. Front Earth Sci. 2020, in press.
- Martin-Pozas, T.; Sánchez-Moral, S.; Cuezva, S.; Jurado, V.; Saiz-Jimenez, C.; López, R.P.; Carrey, R.; Otero, N.; Giesemann, A.; Well, R.; et al. Biologically mediated release of endogenous N2O and NO2 gases in a hydrothermal, hypoxic subterranean environment. Sci. Total Environ. 2020, 747, 141218, doi:10.1016/j.scitotenv.2020.141218.
- Fernandez-Cortes, A.; Perez-Lopez, R.; Cuezva, S.; Calaforra, J.M.; Cañaveras, J.C.; Sanchez-Moral, S. Geochemical fingerprinting of rising deep endogenous gases in an active hypogenic karst system. Geofluids 2018, 2018, 1–19, doi:10.1155/2018/4934520.
- He, Z.; Cai, C.; Wang, J.; Xu, X.; Zheng, P.; Jetten, M.S.M.; Hu, B. A novel denitrifying methanotroph of the NC10 phylum and its microcolony. Sci. Rep. 2016, 6, srep32241, doi:10.1038/srep32241.
- Isobe, K.; Bouskill, N.J.; Brodie, E.L.; Sudderth, E.A.; Martiny, J.B.H. Phylogenetic conservation of soil bacterial responses to simulated global changes. Philos. Trans. R. Soc. B 2020, 375, 20190242, doi:10.1098/rstb.2019.0242.
- Cappelletti, M.; Ghezzi, D.; Zannoni, D.; Capaccioni, B.; Fedi, S. Diversity of methane-oxidizing bacteria in soils from “Hot Lands of Medolla” (Italy) featured by anomalous high-temperatures and biogenic CO2 emission. Microbes Environ. 2016, 31, 369–377, doi:10.1264/jsme2.me16087.




