| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victoria Samanidou | + 2473 word(s) | 2473 | 2021-01-05 10:39:08 | | | |
| 2 | Victoria Samanidou | -25 word(s) | 2448 | 2021-01-07 10:42:46 | | | | |
| 3 | Rita Xu | -1032 word(s) | 1416 | 2021-01-08 04:21:56 | | |
Video Upload Options
Deep eutectic solvents (DESs), were introduced in 2001 as an alternative to ILs. These showed a stronger ecofriendly profile, with easier and cheaper production, while having similar properties. DESs contain large, asymmetrical ions that have low lattice energy and, thus, low melting points. They are often acquired by the complexation of a quaternary ammonium salt with a metal salt or hydrogen bond donor (HBD). The charge delocalization occurring through hydrogen bonding between, for instance a halide ion and the hydrogen-donor moiety, is responsible for the decrease in the melting point of the mixture, in relation to the melting points of the individual components. Since 2001, many scientists around the globe pursed the utilization of DESs and published a variety of studies.
The use of DESs in analytical microextraction techniques is on the rise, due to the many benefits they provide, such as lower cost and easier synthesis than ILs and an environmentally friendly profile, because of the low toxicity reported, although they need further investigation. To this day, the number of HBAs and HBDs is quite limited, so more studies ought to be carried out to present a plethora of DESs available for use. Moreover, DESs are not commercially available yet, substantially affecting and further limiting their usage for routine analyses in industrial or certified laboratories.
The extraordinary high relative recoveries, selectivity, low LODs and decent repeatability they offer, render them appropriate for the determination and quantification of lots of compounds in either simple or complex matrices. As seen, most applications regard liquid phase microextractions rather than solid phase microextractions, because of their liquid nature, as it is simpler to use them as supporting solid adsorbents. The fact that the sample preparation of complicated matrices is of high interest makes them ideal for the research.
Hopefully, DESs will be available for purchase in the foreseeable future and will replace organic solvents in some analytical methods commonly used nowadays, while more studies are carried out about their properties.
Our aim in this review will be towards the use of DESs in analytical extraction and microextraction techniques, while briefly presenting some frequently used DESs, their synthesis methods and their properties.
The ever-increasing use of deep eutectic solvents (DES) in microextraction techniques will be discussed, focusing on the reasons needed to replace conventional extraction techniques with greener approaches that follow the principles of green analytical chemistry.
1. Introduction
Sample preparation is considered to be the bottleneck of the whole analytical process, because it covers a plethora of operations that are essential to modify the sample, to make it amenable for chromatographic analysis, or to improve the analytical parameters, such as precision and accuracy. [1][2] Furthermore, it eliminates typical problems like possible interferences and low sensitivity. Samples can be considered as being made from two distinct parts, the analytes of interest and the matrix. The analytes of interest are the compounds to be determined, while the matrix is the rest of the sample, which not only does not necessitate analysis, but also may contain interfering substances. Sample preparation may focus on the analytes’ extraction, on the matrix transformation, or on both, in order to achieve dissolution, cleanup, preconcentration, or chemical modifications of the sample, so as to obtain better analytical results [3].
In recent years, more and more attention has been devoted to replacing conventional extraction techniques with the so-called ”green” extraction techniques. This started with the introduction of green analytical chemistry (GAC) and the 12 principles, formulated by P. Anastas in 1998, the main concern of which was to create environmentally friendly analytical techniques, especially extraction techniques [4]. Greener approaches to extraction techniques include more inexpensive, quicker, and environmentally safe practices in environmental, clinical, and food analysis [5]. The adverse environmental impact of analytical procedures has been reduced in three different ways: reduction of the volume of solvents required in sample preparation; reduction in the amount and the toxicity of solvents and reagents employed in the measurement step, especially with automation and miniaturization; and the development of different direct analytical techniques not requiring solvents or reagents [6]. Thus, newly developed extraction techniques, which have a greener approach, are on the rise. Moreover, microextraction techniques, for example solid phase microextraction (SPME) and liquid phase microextraction (LPME), which will be discussed below, are considered to be green extraction techniques, owing to the use of very small quantities of solvents of the extraction phase in relation to the volume of the sample or to the solvent volume used in classical approaches [7].
The development and application of sustainable solvents has been a hot topic in different scientific and technological areas [8][9][10][11][12][13][14][15]. In this regard, remarkable advances towards the replacement of volatile organic solvents have been achieved by a group of organic salts with melting points below 100 °C, generally referred to as ionic liquids (ILs) [16]. Nevertheless, the problems with their biodegradability, toxicity, stability, and the expensive synthesis make them less than perfect solvents [17]. Therefore, deep eutectic solvents (DESs), were introduced in 2001 as an alternative to ILs. These showed a stronger ecofriendly profile, with easier and cheaper production, while having similar properties [18]. DESs contain large, asymmetrical ions that have low lattice energy and, thus, low melting points. They are often acquired by the complexation of a quaternary ammonium salt with a metal salt or hydrogen bond donor (HBD). The charge delocalization occurring through hydrogen bonding between, for instance a halide ion and the hydrogen-donor moiety, is responsible for the decrease in the melting point of the mixture, in relation to the melting points of the individual components [19][20]. Since 2001, many scientists around the globe pursed the utilization of DESs and published a variety of studies [21][22][23][24][25][26][27][28][29][30][31].
2. Synthesis and Properties of Deep Eutectic Solvents
2.1. Synthesis of Deep Eutectic Solvents
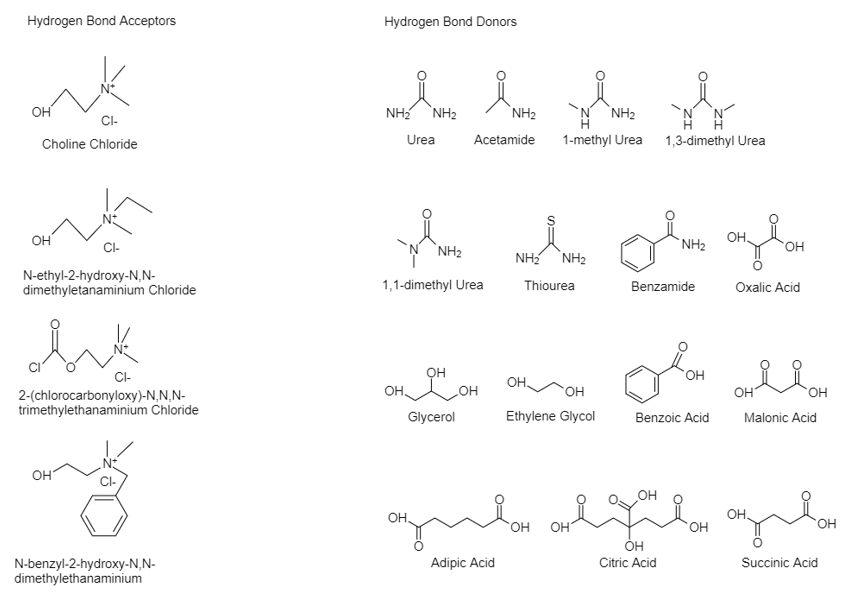
One of the approaches used to synthesize DESs is by mixing and heating two or more salts to form a homogenous solution. Typically, ambient temperature molten salts have been formed by mixing quaternary ammonium salts, a hydrogen bond acceptor (HBA) with metal salts [32][33]. Hence, four main different types of DES occur: I. quaternary salt with a metal chloride, II. quaternary salt with a hydrated metal chloride, III. quaternary salt (HBA) with an HBD compound and IV. metal chloride with an HBD. The respective general formulas of the four types are shown at Table 1. The first two types are used to synthesize hydrophilic DESs, whilst the other two for hydrophobic DESs [20][34]. Due to the stability in the aquatic solutions which type III and IV provide, they are often utilized in many papers, with choline chloride (ChCl) being the most used HBA. In Figure 1, some common HBA and HBD are shown.
Table 1. The four types of deep eutectic solvents.
|
Type |
Formula |
Terms |
|
|
I |
Cat+X− + zMClx |
M = Zn, Sn, Al, Ga, Fe, In |
|
|
II |
Cat+X− + zMClx · yH2O |
M = Co, Cu, Ni, Fe, Cr |
|
|
III |
Cat+X− + zRZ |
Z = OH, COOH, CONH2 |
|
|
IV |
MClx + RZ = MClx−1+ · RZ + MClx+1 − |
M = Zn, Al and Z = OH, CONH2 |
|
Cat+ = any phosphonium, ammonium or sulfonium cation, X− = a Lewis base, generally a halide anion, MClx = metal chloride, RZ = organic compound.
2.2. Properties of Deep Eutectic Solvents
Various interactions (such as anion exchange, weak non-covalent interactions, π-π and/or hydrogen bonding) take place, amongst an HBD and an HBA in various combinations and molar ratios that contribute to some of the physicochemical properties discussed below [35]. Those properties are viscosity, density, conductivity, acidity, surface tension, volatility, and melting or freezing point. There is also an intensive study explaining the effect of hydrogen bonding proton transfer mechanism on the physical and chemical properties [36]. Other properties taken into account before selecting the optimal DES are biodegradability, toxicity, and thermal stability [37]. Moreover, both ILs and DESs can exhibit different physicochemical properties as binary mixtures, depending on the selected co-solvent, such as water or organic solvents. The interactions that may occur from the solute–solvent combination are defined as solvatochromic properties, which can further aid in the comprehension of chemical reactions through preferential solvation (PS) [38]. A useful tool to investigate the PS parameters and interactions, like hydrogen bond between molecules and ions, is the molecular dynamics (MD) simulation, which can be used as completion and confirmation of experimental results. For instance, Aryafard et. al. conducted an extensive investigation for three different DESs and their binary mixtures with polyethylene glycol (PEG 400) as co-solvent, through MD simulations analysis, while showing that PEG 400 can make a strong hydrogen bond with DESs [39].
Figure 1. Structures of some halide salts (HBAs) and hydrogen bond donors (HBDs) used in the formation of deep eutectic solvents.
References
- Moldoveanu, S.; David, V. The Role of Sample Preparation. Modern Sample Preparation for Chromatography; Elsevier: Amsterdam, The Netherlands, 2015; pp. 33–49, doi:10.1016/b978-0-444-54319-6.00002-5.
- Wen, Y. Recent advances in solid-phase extraction techniques with nanomaterials. In Handbook of Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 57–73.
- Moldoveanu, C.S.; David. V. Preparatory Information. Chromatogr. Libr. 2020, 65, 3–111, doi:10.1016/s0301-4770(02)80002-1.
- Armenta, S.; Garrigues, S.; Esteve-Turrillas, F.A.; De La Guardia, M. Green extraction techniques in green analytical chemistry. TrAC Trends Anal. Chem. 2019, 116, 248–253, doi:10.1016/j.trac.2019.03.016.
- Armenta, S.; Garrigues, S.; De La Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. TrAC Trends Anal. Chem. 2015, 71, 2–8, doi:10.1016/j.trac.2014.12.011.
- Armenta, S.; Garrigues, S.; De La Guardia, M. Green Analytical Chemistry. TrAC Trends Anal. Chem. 2008, 27, 497–511, doi:10.1016/j.trac.2008.05.003.
- Lord, H.; Pawliszyn, J. Microextraction of drugs. Chromatogr. A 2000, 902, 17–63, doi:10.1016/s0021-9673(00)00836-0.
- Sanderson, K. Chemistry: It’s not easy being green. Nature 2011, 469, 18–20, doi:10.1038/469018a.
- Ávalos, M.; Babiano, R.; Cintas, P.; Jiménez, J.L.; Palacios, J.C. Greener Media in Chemical Synthesis and Processing. Chem. Int. Ed. 2006, 45, 3904–3908, doi:10.1002/anie.200504285.
- Clark, J.H. Chemistry goes green. Chem. 2009, 1, 12, doi: 10.1038/nchem.146.
- Ranke, J.; Stolte, S.; Störmann, R.; Aming, A.J.; Jastorff, B. Design of Sustainable Chemical Products The Example of Ionic Liquids. Rev. 2007, 107, 2183–2206, doi:10.1021/cr050942s.
- Kunz, W.; Maurer, E.; Klein, R.; Touraud, D.; Rengstl, D.; Harrar, A.; Dengler, S.; Zech, O. Low Toxic Ionic Liquids, Liquid Catanionics, and Ionic Liquid Microemulsions. Dispers. Sci. Technol. 2011, 32, 1694–1699, doi:10.1080/01932691.2011.616109.
- Rogers, R.D.; Seddon, K.R. CHEMISTRY: Ionic Liquids—Solvents of the Future. Science 2003, 302, 792–793, doi:10.1126/science.1090313.
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as ‘green’ solvents for extractions. Chem. Technol. Biotechnol. 2005, 80, 1089–1096, doi:10.1002/jctb.1333.
- Li, Z.; Pei, Y.; Wang, H.; Fan, J.; Wang, J. Ionic liquid-based aqueous two-phase systems and their applications in green separation processes. TrAC Trends Anal. Chem. 2010, 29, 1336–1346, doi:10.1016/j.trac.2010.07.014.
- Pena-Pereira, F.; Namieśnik, J. Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes. ChemSusChem 2014, 7, 1784–1800, doi:10.1002/cssc.201301192.
- Romero, A.; Santos, A.; Tojo, J.; Rodríguez, A. Toxicity and biodegradability of imidazolium ionic liquids. Hazard. Mater. 2008, 151, 268–273, doi:10.1016/j.jhazmat.2007.10.079.
- Kissoudi, M.; Samanidou, V. Recent Advances in Applications of Ionic Liquids in Miniaturized Microextraction Techniques. Molecules 2018, 23, 1437, doi:10.3390/molecules23061437.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Commun. 2001, 1, 2010–2011, doi:10.1039/b106357j.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Rev. 2014, 114, 11060–11082, doi:10.1021/cr300162p.
- Shishov, A.; Pochivalov, A.; Nugbienyo, L.; Andruch, V.; Bulatov, A. Deep eutectic solvents are not only effective extractants. TrAC Trends Anal. Chem. 2020, 129, 115956, doi:10.1016/j.trac.2020.115956.
- Kabeer, M.; Hakami, Y.; Asif, M.; Alrefaei, T.; Sajid, M. Modern solutions in magnetic analytical extractions of metals: A review. TrAC Trends Anal. Chem. 2020, 130, 115987, doi:10.1016/j.trac.2020.115987.
- Xu, K.; Wang, Y.; Huang, Y.; Li, N.; Wen, Q. A green deep eutectic solvent-based aqueous two-phase system for protein extracting. Chim. Acta 2014, 864, 9–20, doi:10.1016/j.aca.2015.01.026.
- Yao, X.-H.; Zhang, D.-Y.; Duan, M.-H.; Cui, Q.; Xu, W.-J.; Luo, M.; Li, C.-Y.; Zu, Y.-G.; Fu, Y.-J. Preparation and determination of phenolic compounds from Pyrola incarnata Fisch. with a green polyols based-deep eutectic solvent. Purif. Technol. 2015, 149, 116–123, doi:10.1016/j.seppur.2015.03.037.
- Wang, M.; Wang, J.; Zhang, Y.; Xia, Q.; Bi, W.; Yang, X.; Chen, D.D.Y. Fast environment-friendly ball mill-assisted deep eutectic solvent-based extraction of natural products. Chromatogr. A 2016, 1443, 262–266, doi:10.1016/j.chroma.2016.03.061.
- Werner, J.; Grześkowiak, T.; Zgoła-Grześkowiak, A.; Stanisz, E. Recent trends in microextraction techniques used in determination of arsenic species. TrAC Trends Anal. Chem. 2018, 105, 121–136, doi:10.1016/j.trac.2018.05.006.
- Moghadam, A.G.; Rajabi, M.; Asghari, A. Efficient and relatively safe emulsification microextraction using a deep eutectic solvent for influential enrichment of trace main anti-depressant drugs from complicated samples. Chromatogr. B 2018, 1072, 50–59, doi:10.1016/j.jchromb.2017.09.042.
- Kanberoglu, G.S.; Yilmaz, E.; Soylak, M. Application of deep eutectic solvent in ultrasound-assisted emulsification microextraction of quercetin from some fruits and vegetables. Mol. Liq. 2019, 279, 571–577, doi:10.1016/j.molliq.2019.01.130.
- Li, L.; Liu, Y.; Wang, Z.; Yang, L.; Liu, H. Development and applications of deep eutectic solvent derived functional materials in chromatographic separation. Sep. Sci. 2020, doi:10.1002/jssc.202000523.
- Doğan, B.; Elik, A.; Altunay, N. Determination of paracetamol in synthetic urea and pharmaceutical samples by shaker-assisted deep eutectic solvent microextraction and spectrophotometry. J. 2020, 154, 104645, doi:10.1016/j.microc.2020.104645.
- Othman, Z.A.A.L.; Habila, M.A.; Yilmaz, E.; Alabdullkarem, E.A.; Soylak, M. A novel deep eutectic solvent microextraction procedure for enrichment, separation and atomic absorption spectrometric determination of palladium at ultra-trace levels in environmental samples. Measurement 2020, 153, 107394, doi:10.1016/j.measurement.2019.107394.
- Abbott, A.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. Am. Chem. Soc. 2004, 126, 9142–9147, doi:10.1021/ja048266j.
- Sereshti, H.; Jamshidi, F.; Nouri, N.; Nodeh, H.R. Hyphenated dispersive solid‐ and liquid‐phase microextraction technique based on a hydrophobic deep eutectic solvent: Application for trace analysis of pesticides in fruit juices. Sci. Food Agric. 2020, 100, 2534–2543, doi:10.1002/jsfa.10279.
- Abbott, A.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Commun. 2003, 70–71, doi:10.1039/b210714g.
- Chen, J.; Liu, M.; Wang, Q.; Du, H.; Zhang, L. Deep Eutectic Solvent-Based Microwave-Assisted Method for Extraction of Hydrophilic and Hydrophobic Components from Radix Salviae miltiorrhizae. Molecules 2016, 21, 1383, doi:10.3390/molecules21101383.
- Doi, H.; Song, X.; Minofar, B.; Kanzaki, R.; Takamuku, T.; Umebayashi, Y. A New Proton Conductive Liquid with No Ions: Pseudo-Protic Ionic Liquids. A Eur. J. 2013, 19, 11522–11526, doi:10.1002/chem.201302228.
- Aryafard, M.; Karimi, A.; Harifi-Mood, A.R.; Minofar, B. Molecular Dynamics Simulations, Solvatochromic Parameters, and Preferential Solvation in Aqueous Solutions of Ethaline, Ethylene Glycol, and Choline Chloride. Chem. Eng. Data 2020, 65, 4556–4566, doi:10.1021/acs.jced.0c00381.
- Aryafard, M.; Abbasi, M.; Řeha, D.; Harifi-Mood, A.R.; Minofar, B. Experimental and theoretical investigation of solvatochromic properties and ion solvation structure in DESs of reline, glyceline, ethaline and their mixtures with PEG 400. Mol. Liq. 2019, 284, 59–67, doi:10.1016/j.molliq.2019.03.149.
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425, doi:10.1021/sc500439w.