
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hung-Yu Lin | + 2764 word(s) | 2764 | 2020-12-31 08:01:44 | | | |
| 2 | Vivi Li | + 436 word(s) | 3200 | 2021-01-07 05:13:14 | | |
Video Upload Options
The lysyl oxidase (LOX) family members are secreted copper-dependent amine oxidases, comprised of five paralogues: LOX and LOX-like l-4 (LOXL1-4), which are characterized by catalytic activity contributing to the remodeling of the cross-linking of the structural extracellular matrix (ECM). ECM remodeling plays a key role in the angiogenesis surrounding tumors, whereby a corrupt tumor microenvironment (TME) takes shape. Primary liver cancer includes hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA), ranked as the seventh most common cancer globally, with limited therapeutic options for advanced stages. In recent years, a growing body of evidence has revealed the key roles of LOX family members in the pathogenesis of liver cancer and the shaping of TME, indicating their notable potential as therapeutic targets.
1. Introduction
The lysyl oxidase (LOX) family members are secreted copper-dependent amine oxidases, comprised of five paralogues: LOX and LOX-like l-4 (LOXL1-4) [1]. As shown in Figure 1, LOX family members encoded by the human LOX/LOXLs genes are located at various chromosome sites, including 5q23.1, 15q24.1, 8p21.3, 2p13.1, and 10q24.2 [2][3]. These members structurally consist of a variable N-terminal domain and a highly conserved C-terminal domain (Figure 1). The conserved C- terminal consists of copper binding domain amino acid residues forming lysine tryosylquinone (LTQ), and a cytokine receptor-like (CRL) domain [4]. In the N-terminal domain, LOX and LOXL1 possess a propeptide sequence, whereas LOXL2–4 present four scavenger-receptor cysteine-rich (SRCR) domains in this region [5]. The matured active forms of LOX and LOXL1 are formed by a cleavage process executed by bone morphogenetic protein 1 (BMP-1), which is not a required program for LOXL2, LOXL3, or LOXL4 [6] (Figure 1). LOX family members are characterized by their catalytic activity contributing to structural integrity and increased tensile strength, acting to remodel the cross-linking of the structural extracellular matrix (ECM) of fibrotic organs such as the liver [7][8][9][10], as well as that of the cancer microenvironments [2][4]. A growing body of evidence indicates that the expression of LOX family members increases in invasive and metastatic cancers, and their elevated expression correlates with poor survival [11][12][13]. Their crucial role in tumor proliferation, epithelial–mesenchymal transition (EMT), migration, invasion, formation of pre-metastatic niches, and immunomodulation have been well documented [11][14][15][16][17]. Consistent with these reports, we note that a genomic big data-centric pathway activity analysis reveals their role in the activation of the EMT pathway in cancer (Figure 2).
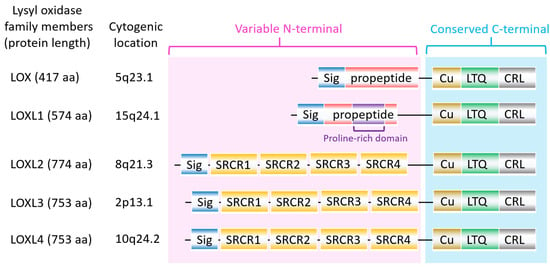
Figure 1. The structure of lysyl oxidase (LOX) family members. LOX family members encoded by the human LOX/LOXLs genes are located at various chromosome sites, including 5q23.1, 15q24.1, 8p21.3, 2p13.1, and 10q24.2. These members consist of a variable N-terminal domain and a highly conserved C-terminal domain. Sig, signal peptide (Sig); copper binding domain (Cu); lysyl-tyrosyl-quinone (LTQ) co-factor; scavenger receptor cysteine-rich (SRCR) domain; cytokine receptor-like (CRL) domain.
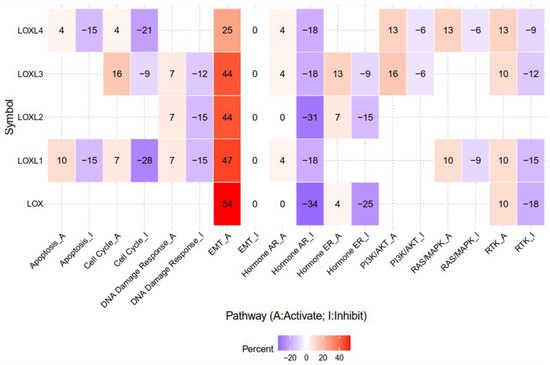
Figure 2. The epithelial–mesenchymal transition (EMT) pathway is activated by LOX, LOXL1, LOXL2, LOXL3, and LOXL4 in genomic big data-centric pathway analysis. Heatmap data demonstrated that LOX, LOXL1, LOXL2, LOXL3, and LOXL4 have activating/inhibiting (red/blue) functions on each cancer-related pathway. Note that LOX, LOXL1, LOXL2, LOXL3, and LOXL4 account for 54%, 47%, 44%, 44%, and 25% of cancers in the EMT-activating pathway, respectively. The pathway activity module was assessed with the GSCALite web server. High-throughput antibody-based technique reverse phase protein array (RPPA) was conduct to determine the expression of The Cancer Genome Atlas (TCGA) samples of at least 5 cancer types. Known cancer-related pathways are included: TSC/mTOR, RTK, RAS/MAPK, PI3K/AKT, hormone ER, hormone AR, EMT, DNA damage response, cell cycle, apoptosis.
Primary liver cancer is ranked as the seventh most common cancer globally, including hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA) [18]. HCC accounts for approximately 90%, while CCA and the combination of HCC and CCA account for 10% of liver cancers [19]. HCC features hepatocellular characteristics at the morphological and molecular levels, whereas CCA exhibits biliary epithelial cell properties. Of note, limited therapeutic options are currently available for the advanced stages of liver cancers. Furthermore, as estimated by the World Health Organization (WHO), more than one million patients are projected to die from liver cancer in the next decade [20]. Growing evidence supports the role of the tumor microenvironment (TME) in the development and progression of HCC. The TME is composed of cellular and non-cellular components. Cellular components include angiogenic endothelial cells, immune system cells, tumor-associated fibroblasts (TAF), and tumor-associated macrophage (TAM); while non-cellular components involve ECM, exosomes, soluble cytokines, and signaling molecules [21].
Mounting evidence in recent years has revealed the key roles of LOX family members in the pathogenesis of liver cancer. Their ECM-remodeling and secretable nature permits the shaping of TME in both the primary organ and distal metastatic sites. More importantly, their potential as therapeutic targets is notably emerging. This expeditious progress prompted us to summarize the prognostic significance of such research, and to review the novel biological roles of LOX family members in tumor cells and the TME of liver cancer. Furthermore, we highlight recent insights into their mechanisms and potential for target therapy approaches.
2. Role of LOX in Liver Cancer
2.1. Prognostic Value and Biological Role of LOX in HCC
LOX mRNA encodes pre-pro-LOX protein which is subsequently transformed into inactive pro-LOX protein in the cytoplasm. The pro-LOX protein is then cleaved by BMP-1, resulting in an active LOX protein and LOX peptide (LOX-PP). Elevated expressions of the LOX level have been noted in HCC tissue compared to that of normal tissue [22][23][24], and is associated with poor overall survival (OS) and disease-free survival [22][24], indicating a significant prognostic value for HCC (Table 1). Knockdown of LOX in HCC cells has been reported to suppress proliferation, migration, and invasion, and reduce vascular endothelial growth factor (VEGF) through p38 mitogen-activated protein kinase (MAPK) signaling [22]. Meanwhile, overexpression of LOX in tumor initiating cells (TICs)-enriched HCC enhances tube formation of endothelial cells through secreted VEGF, wherein the stimulated angiogenesis can be blocked by LOX inhibitor β-aminopropionitrile [23]. LOX has been shown to mediate hypoxia-induced cancer metastasis [25]. The LOX expression in HCC cells is upregulated under hypoxia in a hypoxia inducible factor (HIF-1α)-dependent manner [26]. Specifically, hypoxia response elements (HREs) in the LOX gene promoter have been identified [27][28]. In addition, transactivator protein X (HBx), a viral oncoprotein encoded by hepatitis B virus (HBV), activates the HIF-1α/LOX signaling pathway to enhance cross-link collagen in the extracellular matrix (ECM), leading to HCC growth and metastasis [29]. Huang et al. demonstrated that the food components pterostilbene and curcumin suppress migration and invasion induced by long-term ethanol exposure through inhibiting LOX [30]. These results collectively indicate an oncogenic role of LOX in HCC, while demonstrating an anti-tumor effect of LOX-PP. Furthermore, Zheng et al. reported that HCC tissues express a decreased level of LOX-PP as compared to that of normal tissue [31]. Adenovirus-delivered overexpression of LOX-PP in HCC cells enhances apoptosis and represses proliferation, migration, and invasion via the mitogen-activated protein kinase (MAPK) pathway [31].
Table 1. Clinical relevance of LOX family members in liver cancer.
| LOX Family Member | Patients | Expression Level in Tumor | Clinical Relevance | PMID |
|---|---|---|---|---|
| LOX | HCC | Up | Prognostic marker for high recurrence rate and poor OS | 30919528 |
| HCC | Up | Prognostic marker for poor OS and DFS | 26048020 | |
| LOXL2 | HCC | Up | Prognostic marker for poor OS | 28449718 |
| HCC | Up | Prognostic marker for poor OS and DFS | 29620290 | |
| HCC | Up | Prognostic marker for poor OS and RFS | 29938458 | |
| HCC | Up | Prognostic marker for poor OS and DFS | 30506621 | |
| CCA | Up | Prognostic marker for poor OS and DFS | 31322171 | |
| CCA | Up | Prognostic marker for poor OS and DFS | 27363654 | |
| HCC | Up | Serum LOXL2 as an excellent differential marker | 25048396 | |
| LOXL4 | HCC | Up | Prognostic marker for poor OS | 33068461 |
| HCC | Up | High LOXL4 indicates poor OS and DFS | 30704479 | |
| HCC | Down | High LOXL4 indicates high recurrence rate and poor OS | 26097573 |
CCA, cholangiocarcinoma; DFS, disease-free survival; HCC, hepatocellular carcinoma; OS, overall survival; RFS, recurrence-free survival.
Taken together, these studies indicate that the upregulation of the LOX level is a predictive sign for HCC. It must be noted however, that the precise role of LOX in CCA remains unclear and thus requires further study.
2.2. LOX and Angiogenesis
ECM remodeling is a key aspect involved in the angiogenesis surrounding tumors. A stiff ECM supports angiogenesis with abnormal vasculature, leading to a corrupt TME which promotes tumor dissemination [32]. As an ECM remodeling enzyme, the relevance of LOX in promoting angiogenesis to reshape the TME of HCC has recently been identified by Yang et al. They demonstrated that TICs express higher levels of LOX than the corresponding control cells, and that the LOX expression level correlates positively with VEGFA and VEGFC. LOX secreted by TICs-enriched HCC promotes the tube formation of endothelial cells through the upregulation of VEGF [23]. Overexpression of LOX enhances the extent of angiogenesis, whereas LOX inhibitor β-aminopropionitrile (BAPN) reverses the effect [23]. In addition, LOX inhibitor potentiates the anti-angiogenesis effect of the clinical drug Sorafenib. Zhu et al. also observed a positive correlation between LOX and VEGF in HCC cells, demonstrating that the knockdown of LOX suppresses HCC proliferation, migration, and invasion. With regard to the functional mechanism, LOX acts to mediate TGF-β-induced p38 AMPK-VEGF signaling pathway activity [22]. Interestingly, LOX-PP processed from pro-LOX protein appears to play a tumor-suppressive role on HCC cells. Zheng et al. showed that adenovirus-delivered LOX-PP overexpression in HCC cells hinders cell cycle progression cellular motility, as well as angiogenic activators MMP-2 and MMP-9 [31]. Overall, these reports support the positive role of LOX in angiogenesis. Nevertheless, the exact mechanism underlying LOX-PP perturbation of EC proliferation and vessel formation requires further study.
3. Therapeutic Potential of Targeting Approaches on LOX Family Members
To date, a couple of drugs targeting LOX family members are in the early stage of clinical trials, including those focused on pancreatic and colorectal adenocarcinoma [33][34]. However, most clinical trials engaged in LOX family member-targeting drugs for liver cancer are still lacking, based on information issued by ClinicalTrials.gov (https://clinicaltrials.gov/ct2/home). To further explore the potential applications of approaches targeting the LOX family members, we conducted a survey of drugs and approaches reported in preclinical and clinical settings (Table 2).
Table 2. Inhibitors targeting LOX family members in preclinical models.
| Agents | Biological Property | Targets of Action | Disease Model | PMID |
|---|---|---|---|---|
| BAPN | Small-molecule inhibitor | (-) LOX, LOXL1-4 | HCC | 30720077 |
| (-) LOXL2 | HCC | 29620290 | ||
| (-) LOX | Liver metastasis of GC | 31678002 | ||
| (-) LOX, LOXL1-4 | Liver fibrosis | 26700732 | ||
| GW4869 | N-SMase inhibitor | Exosome-mediated transfer of LOXL4 | HCC | 30704479 |
| pterostilbene/curcumin analogues | Stilbene/curcuminoids compounds | (-) LOX | HCC | 23560895 |
| AB0023 | mAb | (-) LOXL2 | Liver fibrosis | 28073888 |
| (-) LOXL2 | Liver fibrosis | 20818376 | ||
| LOXL2-IN-1 hydrochloride | Small-molecule inhibitor | (-) LOXL2 | HCC | 32323822 |
| PXS-5153A | Small-molecule inhibitor | (-) LOXL2/3 | Liver fibrosis | 30536539 |
| 5-aza-CR | DNA methylation Inhibitor | (+) LOXL4 | HCC | 30728460 |
| CCT365623 | Small-molecule inhibitor | (-) LOX | Lung metastasis of BC | 31070916 |
| AMTz-21b | Small-molecule inhibitor | (-) LOX, LOXL2 | Lung metastasis of BC | 31430136 |
| Salidroside | Glucoside of tyrosol | (-) LOX, LOXL1-4 | Lung metastasis of PC | 31162697 |
| escin Ia | Subclass of SFAC | (-) LOXL2 | Lung metastasis of BC | 27008697 |
| ammonium tetrathiomolybdate | Copper chelator | (-) LOX | Bone invasion of HNSCC | 29328370 |
| miR-26a, miR-29a | Non-coding RNAs | (-) LOXL2 | HCC | 25048396 |
| miR-26a/b, miR-29a/b/c, miR-218 | Non-coding RNAs | (-) LOXL2 | HNSCC | 26490187 |
| miR-26a/b, miR-29a/b/c, miR-218 | Non-coding RNAs | (-) LOXL2 | PC | 27278788 |
| miR-26a/b | Non-coding RNAs | (-) LOXL2 | RCC | 26983694 |
| miR-142 | Non-coding RNAs | (-) LOX | BC | 32415208 |
| miR-30a | Non-coding RNAs | (-) LOX | ATC | 25488748 |
| miR-29a/b/c | Non-coding RNAs | (-) LOXL2 | LSCC | 26676674 |
| miR-29a | Non-coding RNAs | (-) LOXL2 | NSCLC | 27488440 |
| miR-30b | Non-coding RNAs | (-) LOX | GCCL | 31093946 |
| miR-135a | Non-coding RNAs | (-) LOXL4 | NSCLC | 30993701 |
| miR-504 | Non-coding RNAs | (-) LOXL2 | NSCLC | 29156517 |
(-) inhibit; (+) activate; 5-aza-CR, 5-azacytidine; ATC, anaplastic thyroid cancer; BAPN, beta-aminopropionitrile; BC, breast cancer; GC, gastric cancer; GCCL, giant-cell carcinoma of the lung; HNSCC, head and neck squamous cell carcinoma; LSCC, lung squamous cell carcinoma; NSCLC, non-small cell lung cancer; PC, prostate cancer; RCC, renal cell carcinoma; SFAC, saponin fraction of Aesculus chinensis Bunge fruits.
β-aminopropionitrile (BAPN), an irreversible inhibitor of catalytic activity of LOX and LOX1-4 [78,79,80,81,82], has been shown to exert a suppressive effect on metastatic colonization of circulating breast cancer cells [35], hypoxia-induced invasion of cervical cancer cells [36], and the angiogenic capacity of HUVEC [37]. Despite these reports underscoring BAPN’s role in combating tumors, its dual action on tumor promotion and suppression in a context-dependent manner in prostate cancer [38] should be taken into consideration. BAPN has been reported to hamper the TME that constitutes cross-talk between cancer-associated fibroblast-gastric cancer, leading to attenuated liver metastasis [39]. With regard to HCC, BAPN has been shown to block HCC-promoted proliferation and tube formation of endothelial cells in vitro and suppress angiogenesis and tumor growth in vivo [23]. Ninoyama et al. showed that BAPN inhibits LOXL2 to impede the ability of migration and invasion of HCC cells [40]. In addition, Liu et al. demonstrated the ameliorative effect of BAPN on liver fibrosis induced by CCl4 [41]. GW4869, a N-SMase inhibitor that blocks exosome generation [42][43][44][45], has been used to block intercellular exosome-LOXL4 transfer and reduce the cell migratory ability of HCC cells [46]. DNA demethylation small molecule 5-aza-CR exhibits a suppressive effect on tumor growth and cell proliferation by triggering the LOXL4-p53 signaling pathway to activate the expression of pro-apoptotic genes, p53 inducible gene 3 (PIG3) and Bcl-2-associated X protein (BAX) [47]. Thus, in addition to synthetic compounds, LOX-inhibiting phytochemicals may be potential candidates for the treatment of liver cancer. To illustrate, Huang et al. reported that pterostilbene/curcumin analogues exert a LOX-inhibiting effect to attenuate the migration and invasion of HCC [30].
The LOXL2-neutrolazing monoclonal antibody AB0023 exerts the inhibitory effect of LOXL2 by targeting its SRCR domain [48]; whereby, AB0023 has been shown to effectively alleviate liver fibrosis in mouse models induced by CCl4 [49], by thioacetamide (TAA), and using Mdr2−/− plus 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) [8]. Interestingly, Simtuzumab (AB0024/GS-6624) is the humanized version of AB0023, which has been tested in phase II clinical trials directed at colorectal adenocarcinoma, pancreatic adenocarcinoma, and primary sclerosing cholangitis [33][34][50]. Furthermore, PXS-5153A, a dual LOXL2/LOXL3 inhibitor developed by Schilter et al., has been shown to ameliorate liver fibrosis in a CCl4 model and in a streptozotocin plus high fat diet-induced steatohepatitis model [51]. The first selective inhibitor for LOXL2, LOXL2-IN-1 hydrochloride [52], has recently been identified to act to suppress Snail, HIF-1α, and VEGF, which are promotion factors in HCC invasion and angiogenesis [53].
A number of LOX-targeting drugs which have recently been reported to feature anti-cancer efficacy are likely to serve as candidates in the treatment of liver cancer. Springer et al. demonstrated that the LOX inhibitor CCT365623, bearing an aminomethylenethiophene (AMT) scaffold, has exhibited anti-metastasis efficacy in a LOX-driven spontaneous breast cancer model [54]. The team soon after suggested a series of 2-aminomethylene-5-sulfonylthiazole (AMTz) as dual inhibitors of LOX and LOXL2 [55]. One of the AMTz-bearing inhibitors, AMTz-21b, effectively suppressed tumor growth in their spontaneous breast cancer mouse model [55]. Meanwhile, dextran sulfate (DS) acts to down-regulate the expression of LOX to suppress invasive and migratory behaviors in gastric cancer cells [56]. The combination of DS and BAPN exerts a superior effect compared to DS or BAPN alone [56]. In addition, salidroside, a phenylpropanoid glycoside isolated from Rhodiola rosea L, down-regulates the mRNA expression level of LOX, LOXL1-4, and HIF-1α in a dose-dependent manner in pancreatic cancer cells [57]; thereby, the invasive activity of cancer cells, xenograft tumor growth, and distal metastasis can be hindered by treatment with salidroside [57]. Further studies have indicated that Escin Ia, belonging to a subclass of the saponin fraction of Aesculus chinensis Bunge fruits (SFAC), acts towards the down-regulation of LOXL2 and the inhibition of the metastatic behavior of triple-negative breast cancer in vitro and in vivo [58]. Escin Ia perturbs the EMT program, as evidenced by enhanced E-cadherin and curbed Vimentin and alpha smooth muscle actin (α-SMA), as well as transcription factors Snail, Slug, Zeb1, Zeb2, and Twist [58]. Meanwhile, ammonium tetrathiomolybdate (TM), acting as copper chelator, has been shown to suppress LOX activity, cell proliferation, and the bone destruction behavior of head and neck squamous cell carcinoma (HNSCC) [59]. Finally, Hutchinson et al. indicated that (2chloropyridin-4-yl) methanamine 20 is the most potent compound for LOXL2, amid two series of novel LOXL2 enzyme inhibitors, benzylamines substituted with electron withdrawing groups at the para-position and 2-substituted pyridine-4-ylmethanamines [52].
MiRs are approximately 22 nucleotides in length, short non-coding RNAs, functioning in a pathway-centric manner by targeting multiple genes, and are potential therapeutic targets for liver cancer [60]. In recent years, there has been growing evidence elucidating the pathway-centric manner of microRNAs (miRs) on the modulation of the LOX family members in carcinogenesis. More specifically, Wong et al. found that miR-29a-3p and miR-26a-5p bind to the 3′untranslated region (3′UTR) of LOXL2 mRNA, leading to suppression of LOXL2 expression, which is essential for the promotion of TME and the formation of a pre-metastatic niche in HCC [61]. In addition, Seki’s team have demonstrated that a set of miRs, miR-26a/b, miR-29a/b/c, and miR-218, significantly inhibit metastasis by down-regulating LOXL2 mRNA in HNSCC [62]; demonstrating similar findings in prostate cancer [63]. In renal cell carcinoma (RCC), miR-26a/b overexpression exhibits inhibitory efficacy on cancer cell proliferation, migration, and invasion through the direct binding of 3′UTR of LOXL2 mRNA [64]. Saatci et al. identified that miR-142-3p exerts an inhibitory role on LOX expression for overcoming chemoresistance in triple-negative breast cancer [65]. In anaplastic thyroid cancer (ATC), Boufraqech et al. reported that miR-30a interacts with the 3′UTR of LOX to mediate anti-tumor efficacy, as evidenced by suppressing cell invasion and migration, EMT markers expression, LOX expression, and metastatic capacity [66]. In lung squamous cell carcinoma (LSCC), miR-29a/b/c restricts cell migration and invasion by binding to the 3′UTR of LOXL2 and prevents its transcription [67]. Kamikawaji et al. identified that miR-29a exerts an anti-aggressive effect on lung cancer cells and an anti-proliferation effect in lung fibroblasts by directly binding to LOXL2 [68]. In non-small cell lung cancer (NSCLC), miR-504 has been reported to function as a tumor-suppressing factor by directly targeting the 3′UTR of LOXL2 [69]. Furthermore, Duan et al. found that miR-30b reduces LOX expression by directly interacting with the 3′UTR of LOX in lung cancer cells [70]. Several lines of study have revealed the tumor-promoting role of particular miRs. To illustrate, miR-135a-5p presents a tumor-promoting role as evidenced by in vitro and in vivo studies directly targeting LOXL4 [71]. Additionally, miR-210 has been found to promote lung cancer cell proliferation, colony formation, migration, and invasion via targeting LOXL4 [72].
4. Future Perspective
The LOX family members are responsible for remodeling the cross-linking of structural ECM. There is mounting clinical evidence indicating their significance in predicting prognosis and diagnosis, and their roles in promoting cancer cell proliferation, invasiveness, and shaping the TME of liver cancer (Figure 3), particularly LOX, LOXL2, and LOXL4. As the majority of current studies focus on HCC, insight into the mechanisms underlying LOX family members in CCA requires further investigation. Furthermore, the roles of LOXL1 and LOXL3 in the pathogenesis of liver cancer remain unclear, which also necessitates further study. It is important to note that drugs developed to target LOX family members have been effective at inhibiting the progression of HCC in preclinical models, and have shown efficacy in clinical trials of other cancer types. Investigations into miRs-dictated mechanisms for the activity of LOX family members could further shed light on the molecular activity of TME and pave the way to prospective clinical therapeutic approaches. To summarize, LOX family members represent attractive therapeutic targets for the treatment of liver cancer.
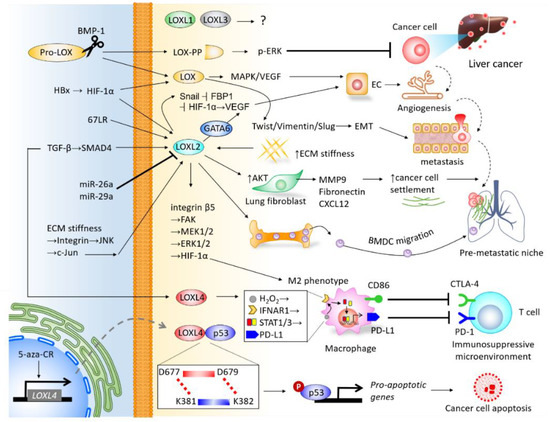
Figure 3. Integrative network depicting the biological roles of LOX family members in the tumor microenvironment (TME) of liver cancer.
References
- Barker, H.E.; Cox, T.R.; Erler, J.T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 2012, 12, 540–552.
- Ye, M.; Song, Y.; Pan, S.; Chu, M.; Wang, Z.-W.; Zhu, X. Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol. Ther. 2020, 215, 107633.
- López, B.; González, A.; Hermida, N.; Valencia, F.; De Teresa, E.; Díez, J. Role of lysyl oxidase in myocardial fibrosis: From basic science to clinical aspects. Am. J. Physiol. Circ. Physiol. 2010, 299, H1–H9.
- Wang, T.-H.; Hsia, S.-M.; Shieh, T.-M. Lysyl Oxidase and the Tumor Microenvironment. Int. J. Mol. Sci. 2016, 18, 62.
- Xiao, Q.; Ge, G. Lysyl Oxidase, Extracellular Matrix Remodeling and Cancer Metastasis. Cancer Microenviron. 2012, 5, 261–273.
- Borel, A.; Eichenberger, D.; Farjanel, J.; Kessler, E.; Gleyzal, C.; Hulmes, D.J.S.; Sommer, P.; Font, B. Lysyl Oxidase-like Protein from Bovine Aorta. Isolation and maturation to an active form by bone morphogenetic protein-1. J. Biol. Chem. 2001, 276, 48944–48949.
- Zhao, W.; Yang, A.; Chen, W.; Wang, P.; Liu, T.; Cong, M.; Xu, A.; Yan, X.; Jia, J.; You, H. Inhibition of lysyl oxidase-like 1 (LOXL1) expression arrests liver fibrosis progression in cirrhosis by reducing elastin crosslinking. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 1129–1137.
- Ikenaga, N.; Peng, Z.-W.; Vaid, K.A.; Liu, S.B.; Yoshida, S.; Sverdlov, D.Y.; Mikels-Vigdal, A.; Smith, V.; Schuppan, D.; Popov, Y.V. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut 2017, 66, 1697–1708.
- Dongiovanni, P.; Meroni, M.; Baselli, G.A.; Bassani, G.A.; Rametta, R.; Pietrelli, A.; Maggioni, M.; Facciotti, F.; Trunzo, V.; Badiali, S.; et al. Insulin resistance promotes Lysyl Oxidase Like 2 induction and fibrosis accumulation in non-alcoholic fatty liver disease. Clin. Sci. 2017, 131, 1301–1315.
- Aumiller, V.; Strobel, B.; Romeike, M.; Schuler, M.; Stierstorfer, B.E.; Kreuz, S. Comparative analysis of lysyl oxidase (like) family members in pulmonary fibrosis. Sci. Rep. 2017, 7, 1–13.
- Miller, B.W.; Morton, J.P.; Pinese, M.; Saturno, G.; Jamieson, N.B.; McGhee, E.; Timpson, P.; Leach, J.; McGarry, L.; Shanks, E.; et al. Targeting the LOX / hypoxia axis reverses many of the features that make pancreatic cancer deadly: Inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol. Med. 2015, 7, 1063–1076.
- Wilgus, M.-L.; Borczuk, A.C.; Stoopler, M.; Ginsburg, M.; Gorenstein, L.; Sonett, J.R.; Powell, C.A. Lysyl oxidase: A lung adenocarcinoma biomarker of invasion and survival. Cancer 2010, 117, 2186–2191.
- Erler, J.T.; Bennewith, K.L.; Nicolau, M.; Dornhöfer, N.; Kong, C.; Le, Q.-T.; Chi, J.-T.A.; Jeffrey, S.S.; Giaccia, A.J. Lysyl oxidase is essential for hypoxia-induced metastasis. Nat. Cell Biol. 2006, 440, 1222–1226.
- Tenti, P.; Vannucci, L. Lysyl oxidases: Linking structures and immunity in the tumor microenvironment. Cancer Immunol. Immunother. 2019, 69, 223–235.
- Tan, H.Y.; Wang, N.; Zhang, C.; Chan, Y.T.; Yuen, M.F.; Feng, Y. LOXL4 Fosters an Immunosuppressive Microenvironment During Hepatocarcinogenesis. Hepatology 2020.
- Kasashima, H.; Yashiro, M.; Kinoshita, H.; Fukuoka, T.; Morisaki, T.; Masuda, G.; Sakurai, K.; Kubo, N.; Ohira, M.; Hirakawa, K. Lysyl oxidase is associated with the epithelial–mesenchymal transition of gastric cancer cells in hypoxia. Gastric Cancer 2016, 19, 431–442.
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell 2009, 139, 891–906.
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768.
- Nault, J.; Bioulac–Sage, P.; Zucman–Rossi, J. Hepatocellular Benign Tumors—From Molecular Classification to Personalized Clinical Care. Gastroenterology 2013, 144, 888–902.
- World Health Organization. Projections of Mortality and Causes of Death, 2016 to 2060. Available online: http://www.who.int/healthinfo/global_burden_disease/projections/en/ (accessed on 4 November 2020).
- Tahmasebi-Birgani, M.; Carloni, V. Tumor Microenvironment, a Paradigm in Hepatocellular Carcinoma Progression and Therapy. Int. J. Mol. Sci. 2017, 18, 405.
- Zhu, J.; Huang, S.; Wu, G.; Huang, C.; Li, X.; Chen, Z.; Zhao, L.; Zhao, Y. Lysyl Oxidase Is Predictive of Unfavorable Outcomes and Essential for Regulation of Vascular Endothelial Growth Factor in Hepatocellular Carcinoma. Dig. Dis. Sci. 2015, 60, 3019–3031.
- Yang, M.; Liu, J.; Wang, F.; Tian, Z.; Ma, B.; Li, Z.; Wang, B.; Zhao, W. Lysyl oxidase assists tumor-initiating cells to enhance angiogenesis in hepatocellular carcinoma. Int. J. Oncol. 2019, 54, 1398–1408.
- Umezaki, N.; Nakagawa, S.; Yamashita, Y.; Kitano, Y.; Arima, K.; Miyata, T.; Hiyoshi, Y.; Okabe, H.; Nitta, H.; Hayashi, H.; et al. Lysyl oxidase induces epithelial-mesenchymal transition and predicts intrahepatic metastasis of hepatocellular carcinoma. Cancer Sci. 2019, 110, 2033–2043.
- Erler, J.T.; Giaccia, A.J. Lysyl Oxidase Mediates Hypoxic Control of Metastasis: Figure 1. Cancer Res. 2006, 66, 10238–10241.
- Triantafyllou, E.-A.; Mylonis, I.; Simos, G.; Paraskeva, E. Hypoxia Induces Pro-Fibrotic and Fibrosis Marker Genes in Hepatocellular Carcinoma Cells Independently of Inflammatory Stimulation and the NF-κΒ Pathway. Hypoxia 2019, 7, 87–91.
- Wang, V.; Davis, D.A.; Yarchoan, R. Identification of functional hypoxia inducible factor response elements in the human lysyl oxidase gene promoter. Biochem. Biophys. Res. Commun. 2017, 490, 480–485.
- Wang, V.; Davis, D.A.; Haque, M.; Huang, L.E.; Yarchoan, R. Differential Gene Up-Regulation by Hypoxia-Inducible Factor-1α and Hypoxia-Inducible Factor-2α in HEK293T Cells. Cancer Res. 2005, 65, 3299–3306.
- Tse, A.P.-W.; Sze, K.M.-F.; Shea, Q.T.-K.; Chiu, E.Y.-T.; Tsang, F.H.-C.; Chiu, D.K.-C.; Zhang, M.S.; Lee, D.; Xu, I.M.-J.; Chan, C.Y.-K.; et al. Hepatitis transactivator protein X promotes extracellular matrix modification through HIF/LOX pathway in liver cancer. Oncogenesis 2018, 7, 44.
- Huang, C.-S.; Ho, C.-T.; Tu, S.-H.; Pan, M.-H.; Chang, H.-W.; Wu, C.-H.; Ho, Y.-S.; Chang, C.-H.; Chuang, C.-H. Long-Term Ethanol Exposure-Induced Hepatocellular Carcinoma Cell Migration and Invasion through Lysyl Oxidase Activation Are Attenuated by Combined Treatment with Pterostilbene and Curcumin Analogues. J. Agric. Food Chem. 2013, 61, 4326–4335.
- Zheng, Y.; Wang, X.; Wang, H.; Yan, W.; Zhang, Q.; Chang, X. Expression of the lysyl oxidase propeptide in hepatocellular carcinoma and its clinical relevance. Oncol. Rep. 2014, 31, 1669–1676.
- Chakraborty, S.; Njah, K.; Hong, W. Agrin Mediates Angiogenesis in the Tumor Microenvironment. Trends Cancer 2020, 6, 81–85.
- Hecht, J.R.; Benson, A.B.; Vyushkov, D.; Yang, Y.; Bendell, J.; Verma, U. A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second-Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma. Oncologist 2017, 22, 243.
- Benson, A.B.; Wainberg, Z.A.; Hecht, J.R.; Vyushkov, D.; Dong, H.; Bendell, J.; Kudrik, F. A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab or Placebo in Combination with Gemcitabine for the First-Line Treatment of Pancreatic Adenocarcinoma. Oncologist 2017, 22, 241.
- Bondareva, A.; Downey, C.M.; Ayres, F.; Liu, W.; Boyd, S.K.; Hallgrímsson, B.; Jirik, F.R. The Lysyl Oxidase Inhibitor, β-Aminopropionitrile, Diminishes the Metastatic Colonization Potential of Circulating Breast Cancer Cells. PLoS ONE 2009, 4, e5620.
- Yang, X.; Li, S.; Li, W.; Chen, J.; Xiao, X.; Wang, Y.; Yan, G.; Chen, L. Inactivation of lysyl oxidase by β-aminopropionitrile inhibits hypoxia-induced invasion and migration of cervical cancer cells. Oncol. Rep. 2012, 29, 541–548.
- Shi, L.; Zhang, N.; Liu, H.; Zhao, L.; Liu, J.; Wan, J.; Wu, W.; Lei, H.; Liu, R.; Han, M. Lysyl oxidase inhibition via β-aminoproprionitrile hampers human umbilical vein endothelial cell angiogenesis and migration in vitro. Mol. Med. Rep. 2018, 17, 5029–5036.
- Nilsson, M.; Adamo, H.; Bergh, A.; Bergström, S.H. Inhibition of Lysyl Oxidase and Lysyl Oxidase-Like Enzymes Has Tumour-Promoting and Tumour-Suppressing Roles in Experimental Prostate Cancer. Sci. Rep. 2016, 6, 19608.
- Li, Q.; Zhu, C.-C.; Ni, B.; Zhang, Z.-Z.; Jiang, S.-H.; Hu, L.-P.; Wang, X.; Zhang, X.-X.; Huang, P.-Q.; Yang, Q.; et al. Lysyl oxidase promotes liver metastasis of gastric cancer via facilitating the reciprocal interactions between tumor cells and cancer associated fibroblasts. EBioMedicine 2019, 49, 157–171.
- Ninomiya, G.; Yamada, S.; Hayashi, M.; Takeda, S.; Suenaga, M.; Takami, H.; Kanda, M.; Iwata, N.; Niwa, Y.; Tanaka, C.; et al. Significance of Lysyl oxidase-like 2 gene expression on the epithelial-mesenchymal status of hepatocellular carcinoma. Oncol. Rep. 2018, 39, 2664–2672.
- Liu, S.B.; Ikenaga, N.; Peng, Z.; Sverdlov, D.Y.; Greenstein, A.; Smith, V.; Schuppan, D.; Popov, Y.V. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2015, 30, 1599–1609.
- Wang, X.; Huang, W.; Liu, G.; Cai, W.; Millard, R.W.; Wang, Y.; Chang, J.; Peng, T.; Fan, G.-C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J. Mol. Cell. Cardiol. 2014, 74, 139–150.
- Li, J.; Liu, K.; Liu, Y.; Xu, Y.; Zhang, F.; Yang, H.; Liu, J.; Pan, T.; Chen, J.; Wu, M.; et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat. Immunol. 2013, 14, 793–803.
- Kulshreshtha, A.; Ahmad, T.; Agrawal, A.; Ghosh, B. Proinflammatory role of epithelial cell–derived exosomes in allergic airway inflammation. J. Allergy Clin. Immunol. 2013, 131, 1194–1203.e14.
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of MicroRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452.
- Li, R.; Wang, Y.; Zhang, X.; Feng, M.; Ma, J.; Li, J.; Yang, X.; Fang, F.; Xia, Q.; Zhang, Z.; et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol. Cancer 2019, 18, 1–19.
- Shao, J.; Lu, J.; Zhu, W.; Yu, H.; Jing, X.; Wang, Y.; Wang, X.; Wang, X. Derepression of LOXL4 inhibits liver cancer growth by reactivating compromised p53. Cell Death Differ. 2019, 26, 2237–2252.
- Rodriguez, H.M.; Vaysberg, M.; Mikels, A.; McCauley, S.; Velayo, A.C.; Garcia, C.; Smith, V. Modulation of Lysyl Oxidase-like 2 Enzymatic Activity by an Allosteric Antibody Inhibitor. J. Biol. Chem. 2010, 285, 20964–20974.
- Barry-Hamilton, V.; Spangler, R.; Marshall, D.; McCauley, S.A.; Rodriguez, H.M.; Oyasu, M.; Mikels, A.; Vaysberg, M.; Ghermazien, H.; Wai, C.; et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 2010, 16, 1009–1017.
- Muir, A.J.; Levy, C.; Janssen, H.L.; Montano-Loza, A.J.; Shiffman, M.L.; Caldwell, S.; Luketic, V.; Ding, D.; Jia, C.; McColgan, B.J.; et al. Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results With Insights on the Natural History of the Disease. Hepatology 2019, 69, 684–698.
- Schilter, H.C.; Findlay, A.D.; Perryman, L.; Yow, T.T.; Moses, J.; Zahoor, A.; Turner, C.I.; Deodhar, M.; Foot, J.S.; Zhou, W.; et al. The lysyl oxidase like 2/3 enzymatic inhibitor, PXS-5153A, reduces crosslinks and ameliorates fibrosis. J. Cell. Mol. Med. 2018, 23, 1759–1770.
- Hutchinson, J.H.; Rowbottom, M.W.; Lonergan, D.; Darlington, J.; Prodanovich, P.; King, C.D.; Evans, J.F.; Bain, G. Small Molecule Lysyl Oxidase-like 2 (LOXL2) Inhibitors: The Identification of an Inhibitor Selective for LOXL2 over LOX. ACS Med. Chem. Lett. 2017, 8, 423–427.
- Fan, Z.; Zheng, W.; Li, H.; Wu, W.; Liu, X.; Sun, Z.; Hu, H.; Du, L.; Jia, Q.; Liu, Q. LOXL2 upregulates hypoxia-inducible factor-1α signaling through Snail-FBP1 axis in hepatocellular carcinoma cells. Oncol. Rep. 2020, 43, 1641–1649.
- Leung, L.M.H.; Niculescu-Duvaz, D.; Smithen, D.; Lopes, F.; Callens, C.; McLeary, R.; Saturno, G.; Davies, L.; Aljarah, M.; Brown, M.; et al. Anti-metastatic Inhibitors of Lysyl Oxidase (LOX): Design and Structure-Activity Relationships. J. Med. Chem. 2019, 62, 5863–5884.
- Smithen, D.A.; Leung, L.M.H.; Challinor, M.; Lawrence, R.; Tang, H.; Niculescu-Duvaz, D.; Pearce, S.P.; McLeary, R.; Lopes, F.; Aljarah, M.; et al. 2-Aminomethylene-5-sulfonylthiazole Inhibitors of Lysyl Oxidase (LOX) and LOXL2 Show Significant Efficacy in Delaying Tumor Growth. J. Med. Chem. 2019, 63, 2308–2324.
- Xu, Y.; Wang, X.; Huang, Y.; Ma, Y.; Jin, X.; Wang, H.; Wang, J. Inhibition of lysyl oxidase expression by dextran sulfate affects invasion and migration of gastric cancer cells. Int. J. Mol. Med. 2018, 42, 2737–2749.
- Chen, X.; Kou, Y.; Lu, Y.; Pu, Y. Salidroside ameliorated hypoxia-induced tumorigenesis of BxPC-3 cells via downregulating hypoxia-inducible factor (HIF)-1α and LOXL2. J. Cell. Biochem. 2019, 121, 165–173.
- Wang, Y.; Xu, X.; Zhao, P.; Tong, B.; Wei, Z.; Dai, Y. Escin Ia suppresses the metastasis of triple-negative breast cancer by inhibiting epithelial-mesenchymal transition via down-regulating LOXL2 expression. Oncotarget 2016, 7, 23684–23699.
- Morisawa, A.; Okui, T.; Shimo, T.; Ibaragi, S.; Okusha, Y.; Ono, M.; Nguyen, T.T.H.; Hassan, N.M.M.; Sasaki, A. Ammonium tetrathiomolybdate enhances the antitumor effects of cetuximab via the suppression of osteoclastogenesis in head and neck squamous carcinoma. Int. J. Oncol. 2018, 52, 989–999.
- Lou, W.; Liu, J.; Gao, Y.; Zhong, G.; Ding, B.; Xu, L.; Fan, W. MicroRNA regulation of liver cancer stem cells. Am. J. Cancer Res. 2018, 8, 1126–1141.
- Wong, C.C.-L.; Tse, A.P.-W.; Huang, Y.-P.; Zhu, Y.-T.; Chiu, D.K.-C.; Lai, R.K.-H.; Au, S.L.-K.; Kai, A.K.-L.; Lee, J.M.-F.; Wei, L.L.; et al. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology 2014, 60, 1645–1658.
- Fukumoto, I.; Kikkawa, N.; Matsushita, R.; Kato, M.; Kurozumi, A.; Nishikawa, R.; Goto, Y.; Koshizuka, K.; Hanazawa, T.; Enokida, H.; et al. Tumor-suppressive microRNAs (miR-26a/b, miR-29a/b/c and miR-218) concertedly suppressed metastasis-promoting LOXL2 in head and neck squamous cell carcinoma. J. Hum. Genet. 2016, 61, 109–118.
- Kato, M.; Kurozumi, A.; Goto, Y.; Matsushita, R.; Okato, A.; Nishikawa, R.; Fukumoto, I.; Koshizuka, K.; Ichikawa, T.; Seki, N. Regulation of metastasis-promoting LOXL2 gene expression by antitumor microRNAs in prostate cancer. J. Hum. Genet. 2016, 62, 123–132.
- Kurozumi, A.; Kato, M.; Goto, Y.; Matsushita, R.; Nishikawa, R.; Okato, A.; Fukumoto, I.; Ichikawa, T.; Seki, N. Regulation of the collagen cross-linking enzymes LOXL2 and PLOD2 by tumor-suppressive microRNA-26a/b in renal cell carcinoma. Int. J. Oncol. 2016, 48, 1837–1846.
- Saatci, O.; Kaymak, A.; Raza, U.; Ersan, P.G.; Akbulut, O.; Banister, C.E.; Sikirzhytski, V.; Tokat, U.M.; Aykut, G.; Ansari, S.A.; et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat. Commun. 2020, 11, 1–17.
- Boufraqech, M.; Nilubol, N.; Zhang, L.; Gara, S.K.; Sadowski, S.M.; Mehta, A.; He, M.; Davis, S.; Dreiling, J.; Copland, J.A.; et al. miR30a Inhibits LOX Expression and Anaplastic Thyroid Cancer Progression. Cancer Res. 2015, 75, 367–377.
- Mizuno, K.; Seki, N.; Mataki, H.; Matsushita, R.; Kamikawaji, K.; Kumamoto, T.; Takagi, K.; Goto, Y.; Nishikawa, R.; Kato, M.; et al. Tumor-suppressive microRNA-29 family inhibits cancer cell migration and invasion directly targeting LOXL2 in lung squamous cell carcinoma. Int. J. Oncol. 2016, 48, 450–460.
- Kamikawaji, K.; Seki, N.; Watanabe, M.; Mataki, H.; Kumamoto, T.; Takagi, K.; Mizuno, K.; Inoue, H. Regulation of LOXL2 and SERPINH1 by antitumor microRNA-29a in lung cancer with idiopathic pulmonary fibrosis. J. Hum. Genet. 2016, 61, 985–993.
- Ye, M.-F.; Zhang, J.-G.; Guo, T.-X.; Pan, X.-J. MiR-504 inhibits cell proliferation and invasion by targeting LOXL2 in non small cell lung cancer. Biomed. Pharmacother. 2018, 97, 1289–1295.
- Duan, Z.; Li, L.; Li, Y. Involvement of miR-30b in kynurenine-mediated lysyl oxidase expression. J. Physiol. Biochem. 2019, 75, 135–142.
- Zhang, Y.; Jiang, W.; Yang, J.; Huang, J.; Kang, G.; Hu, H.; Xie, S. Downregulation of lysyl oxidase-like 4 LOXL4 by miR-135a-5p promotes lung cancer progression in vitro and in vivo. J. Cell. Physiol. 2019, 234, 18679–18687.
- Xie, S.; Liu, G.; Huang, J.; Hu, H.; Jiang, W. miR-210 promotes lung adenocarcinoma proliferation, migration, and invasion by targeting lysyl oxidase-like 4. J. Cell. Physiol. 2019, 234, 14050–14057.





