The lysyl oxidase (LOX) family members are secreted copper-dependent amine oxidases, comprised of five paralogues: LOX and LOX-like l-4 (LOXL1-4), which are characterized by catalytic activity contributing to the remodeling of the cross-linking of the structural extracellular matrix (ECM). ECM remodeling plays a key role in the angiogenesis surrounding tumors, whereby a corrupt tumor microenvironment (TME) takes shape. Primary liver cancer includes hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA), ranked as the seventh most common cancer globally, with limited therapeutic options for advanced stages. In recent years, a growing body of evidence has revealed the key roles of LOX family members in the pathogenesis of liver cancer and the shaping of TME, indicating their notable potential as therapeutic targets.
- liver cancer,hepatocellular carcinoma,cholangiocarcinoma,lysyl oxidase family members,tumor microenvironment
1. Introduction
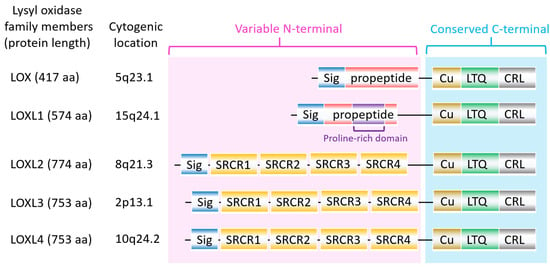
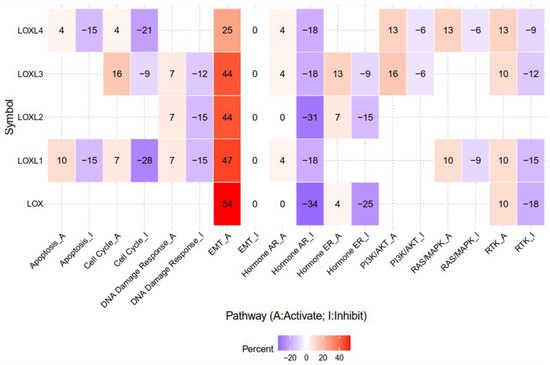
The lysyl oxidase (LOX) family members are secreted copper-dependent amine oxidases, comprised of five paralogues: LOX and LOX-like l-4 (LOXL1-4) [1]. As shown in Figure 1, LOX family members encoded by the human LOX/LOXLs genes are located at various chromosome sites, including 5q23.1, 15q24.1, 8p21.3, 2p13.1, and 10q24.2 [2,3]. These members structurally consist of a variable N-terminal domain and a highly conserved C-terminal domain (Figure 1). The conserved C- terminal consists of copper binding domain amino acid residues forming lysine tryosylquinone (LTQ), and a cytokine receptor-like (CRL) domain [4]. In the N-terminal domain, LOX and LOXL1 possess a propeptide sequence, whereas LOXL2–4 present four scavenger-receptor cysteine-rich (SRCR) domains in this region [5]. The matured active forms of LOX and LOXL1 are formed by a cleavage process executed by bone morphogenetic protein 1 (BMP-1), which is not a required program for LOXL2, LOXL3, or LOXL4 [6] (Figure 1). LOX family members are characterized by their catalytic activity contributing to structural integrity and increased tensile strength, acting to remodel the cross-linking of the structural extracellular matrix (ECM) of fibrotic organs such as the liver [7,8,9,10], as well as that of the cancer microenvironments [2,4]. A growing body of evidence indicates that the expression of LOX family members increases in invasive and metastatic cancers, and their elevated expression correlates with poor survival [11,12,13]. Their crucial role in tumor proliferation, epithelial–mesenchymal transition (EMT), migration, invasion, formation of pre-metastatic niches, and immunomodulation have been well documented [11,14,15,16,17]. Consistent with these reports, we note that a genomic big data-centric pathway activity analysis reveals their role in the activation of the EMT pathway in cancer (Figure 2).
Primary liver cancer is ranked as the seventh most common cancer globally, including hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA) [18]. HCC accounts for approximately 90%, while CCA and the combination of HCC and CCA account for 10% of liver cancers [19]. HCC features hepatocellular characteristics at the morphological and molecular levels, whereas CCA exhibits biliary epithelial cell properties. Of note, limited therapeutic options are currently available for the advanced stages of liver cancers. Furthermore, as estimated by the World Health Organization (WHO), more than one million patients are projected to die from liver cancer in the next decade [20]. Growing evidence supports the role of the tumor microenvironment (TME) in the development and progression of HCC. The TME is composed of cellular and non-cellular components. Cellular components include angiogenic endothelial cells, immune system cells, tumor-associated fibroblasts (TAF), and tumor-associated macrophage (TAM); while non-cellular components involve ECM, exosomes, soluble cytokines, and signaling molecules [21].
Mounting evidence in recent years has revealed the key roles of LOX family members in the pathogenesis of liver cancer. Their ECM-remodeling and secretable nature permits the shaping of TME in both the primary organ and distal metastatic sites. More importantly, their potential as therapeutic targets is notably emerging. This expeditious progress prompted us to summarize the prognostic significance of such research, and to review the novel biological roles of LOX family members in tumor cells and the TME of liver cancer. Furthermore, we highlight recent insights into their mechanisms and potential for target therapy approaches.
2. Role of LOX in Liver Cancer
2.1. Prognostic Value and Biological Role of LOX in HCC
2.2. LOX and Angiogenesis
ECM remodeling is a key aspect involved in the angiogenesis surrounding tumors. A stiff ECM supports angiogenesis with abnormal vasculature, leading to a corrupt TME which promotes tumor dissemination [32]. As an ECM remodeling enzyme, the relevance of LOX in promoting angiogenesis to reshape the TME of HCC has recently been identified by Yang et al. They demonstrated that TICs express higher levels of LOX than the corresponding control cells, and that the LOX expression level correlates positively with VEGFA and VEGFC. LOX secreted by TICs-enriched HCC promotes the tube formation of endothelial cells through the upregulation of VEGF [23]. Overexpression of LOX enhances the extent of angiogenesis, whereas LOX inhibitor β-aminopropionitrile (BAPN) reverses the effect [23]. In addition, LOX inhibitor potentiates the anti-angiogenesis effect of the clinical drug Sorafenib. Zhu et al. also observed a positive correlation between LOX and VEGF in HCC cells, demonstrating that the knockdown of LOX suppresses HCC proliferation, migration, and invasion. With regard to the functional mechanism, LOX acts to mediate TGF-β-induced p38 AMPK-VEGF signaling pathway activity [22]. Interestingly, LOX-PP processed from pro-LOX protein appears to play a tumor-suppressive role on HCC cells. Zheng et al. showed that adenovirus-delivered LOX-PP overexpression in HCC cells hinders cell cycle progression cellular motility, as well as angiogenic activators MMP-2 and MMP-9 [31]. Overall, these reports support the positive role of LOX in angiogenesis. Nevertheless, the exact mechanism underlying LOX-PP perturbation of EC proliferation and vessel formation requires further study.
3. Therapeutic Potential of Targeting Approaches on LOX Family Members
4. Future Perspective
The LOX family members are responsible for remodeling the cross-linking of structural ECM. There is mounting clinical evidence indicating their significance in predicting prognosis and diagnosis, and their roles in promoting cancer cell proliferation, invasiveness, and shaping the TME of liver cancer (Figure 4), particularly LOX, LOXL2, and LOXL4. As the majority of current studies focus on HCC, insight into the mechanisms underlying LOX family members in CCA requires further investigation. Furthermore, the roles of LOXL1 and LOXL3 in the pathogenesis of liver cancer remain unclear, which also necessitates further study. It is important to note that drugs developed to target LOX family members have been effective at inhibiting the progression of HCC in preclinical models, and have shown efficacy in clinical trials of other cancer types. Investigations into miRs-dictated mechanisms for the activity of LOX family members could further shed light on the molecular activity of TME and pave the way to prospective clinical therapeutic approaches. To summarize, LOX family members represent attractive therapeutic targets for the treatment of liver cancer.
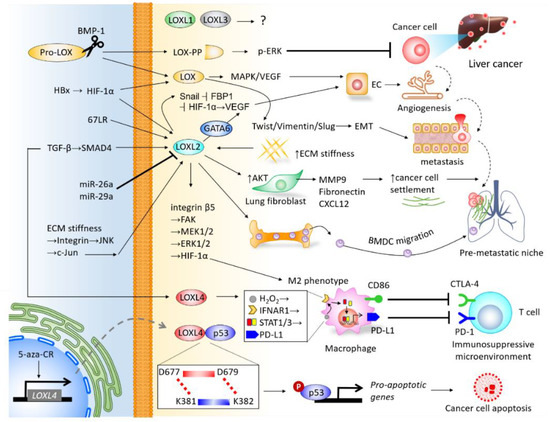
Figure 4. Integrative network depicting the biological roles of LOX family members in the tumor microenvironment (TME) of liver cancer.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21249751
