
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mireya De la Garza Amaya | + 3665 word(s) | 3665 | 2020-12-16 07:21:15 | | | |
| 2 | Dean Liu | -1849 word(s) | 1816 | 2020-12-29 03:39:08 | | |
Video Upload Options
Lf is bacteriostatic and/or bactericidal, can stimulate cell proliferation and differentiation, facilitate iron absorption, improve neural development and cognition, promote bone growth, prevent cancer and exert anti-inflammatory and immunoregulatory effects.
1. Introduction
Except for some bacterial species, all life forms require iron to survive. This transition metal is toxic and has low solubility; thus, it is usually bound to proteins. Iron plays a key role in many cellular mechanisms; it is part of the prosthetic group of enzymes involved in respiration and DNA synthesis and is part of the active center of proteins devoted to oxygen and electron transport, such as hemoglobin (Hb) and cytochromes (Cy), respectively. In addition, iron is sequestered in proteins such as intracellular ferritin (Ft) for storage, extracellular transferrin (Tf) for iron transport to cells and lactoferrin (Lf) as a way to reduce iron availability for microorganisms. Iron homeostasis carried out by regulatory systems is necessary due to the reactive oxygen species (ROS) produced by the Fenton reaction, which damage lipids, proteins and DNA. This theme has been widely discussed elsewhere[1][2][3][4]. Iron metabolism disorders are health problems that affect populations worldwide, ranging from iron deficiency to iron overload. Thus, in addition to iron being an essential nutrient, the iron concentration inside the body must be perfectly regulated.
Since both the host and pathogenic invading microorganisms require iron for growth, a battle begins between the host and pathogens when pathogens enter the body and attempt to obtain iron for colonization and as a display of their virulence factors. Iron is practically absent in free form in bodily tissues and fluids due to an iron-withholding system that prevents iron toxicity and makes it unavailable to invaders; under physiological conditions, the iron-chelating proteins Tf and Lf maintain the concentration of free iron in fluids at approximately 10−18 M, which is several orders of magnitude below the ~10−6 M required for bacterial growth. Tf is mainly present in serum, lymph and cerebrospinal fluid, and Lf is present in exocrine secretions. As such, iron plays a fundamental role in host-pathogen interactions, and the coevolution of microbes and hosts has forced both to develop several iron acquisition/sequestration mechanisms[5]. Each microbial species has a predetermined iron concentration requirement for growth that varies from nanomolar to hundreds of micromolar or more and an iron electronic status requirement of ferric, ferrous or both. Unfortunately for the host, many species of parasites, bacteria and fungi can use diverse host iron-containing molecules to obtain iron. Host iron uptake by pathogens is considered a virulence mechanism[6][7][8][9][10][11][12].
Lf is normally 15% iron-saturated in humans; Lf could have higher iron saturation, depending on the diet and overall levels in some diseases. If this is the case, the resulting holo-Lf can be an iron source for specific pathogens for growth and colonization[13]; however, for a few pathogens, holo-Lf can be microbicidal. Apo-Lf (an iron-free molecule) can be microbiostatic due to its ability to capture ferric iron, preventing iron from being available to pathogens; this effect is reversible when iron is accessible. In addition, the microbicidal activity of apo-Lf occurs mostly via mechanisms that involve its interaction with the microbial surface; both properties of apo-Lf have been confirmed in different pathogens[14][15]. Nevertheless, the manner by which Lf and its derived peptides act to inhibit the mechanisms that pathogens have developed to cause disease has been less well examined. Therefore, the objective of this review was to evaluate the data that appeared in the literature about the effects of Lf and its derived peptides on pathogenic bacteria, protozoa, fungi and viruses and the ways in which Lf and Lfcins inhibit the mechanisms developed by these pathogens to cause disease.
2. Lactoferrin: General Features
2.1. Human and Bovine Lactoferrin
Lactoferrin (lactotransferrin) is a multifunctional glycoprotein of the innate immune system of mammals that provides numerous benefits. In addition to being bacteriostatic and/or bactericidal, Lf can stimulate cell proliferation and differentiation, facilitate iron absorption, improve neural development and cognition, promote bone growth, exert anti-inflammatory and immunoregulatory effects and protect against cancer development and metastasis [16][17][18][19][20][21][22][23]. Lf can bind to its specific receptor on epithelial cells, become internalized, bind to the nucleus and act as a transcription factor, which would explain its multifunctionality[24][25]. Lactoferrin is a conserved protein among mammals, showing high amino acid sequence homology in different species (up to 99%), although each homolog has a unique glycosylation pattern that may be responsible for the heterogeneity of its biological properties[26]; for example, human Lf (hLf) and bovine Lf (bLf) share almost 69% of the primary sequence homology[27]. Bovine Lf is considered a nutraceutical protein[28], and it is sold as a food supplement at a relatively low cost.
Lactoferrin in milk is synthesized in the mammary gland by secretory epithelial cells, reaching concentrations as high as 5–8 mg/mL in human colostrum and 1–3.2 mg/mL in mature milk and accounting for 15–20% of total milk proteins [22][29]. Bovine milk contains a lower quantity of Lf (2 mg/mL in colostrum and 0.31–485 µg/mL in mature milk)[30][31]. Together with other proteins from the innate immune system present in milk, such as immunoglobulins and lysozyme, Lf contributes to immune defense in newborns, which indicates the importance of breastfeeding. Lf is also present in many fluids and exocrine secretions, such as tears, nasal exudate, saliva, bronchial mucus, gastrointestinal fluids, cervicovaginal mucus and seminal fluid, on the surfaces of the digestive, respiratory and reproductive systems that are commonly exposed to normal flora and pathogens[32][33].
Lactoferrin is also produced by the secondary granules of polymorphonuclear (PMN) leukocytes, which store this glycoprotein (3–15 μg/106 neutrophils) and release it at sites of infection[34][35]. Lf has been recognized as an acute-phase protein that increases in concentration during infections, causing hyposideremia of inflammation[36]. In plasma, Lf is derived from neutrophils, and its concentration is very low (0.4–2 µg/mL)[37]; however, in sepsis, the degranulation of activated neutrophils leads to the secretion of significant levels of Lf (~0.2 mg/mL) into the bloodstream[38]. Neutrophils also release Lf in feces, in which the concentration markedly increases during the inflammatory process in diseases such as inflammatory bowel disease, ulcerative colitis and Crohn′s disease[39].
The identification of Lf as a protein was made at almost the same time in human[40] and bovine[41] milk 60 years ago. It is a nonheme cationic monomeric glycoprotein with an approximate molecular weight (MW) of 80 kDa and a pI value of 8.5–9 (depending on the origin). The tertiary structure of Lf (Figure 1) consists of the two main N and C lobes, each of which contains two domains (N1:N2 and C1:C2). Both lobes are linked at the N1 and C1 domains by a three-turn α-chain[38][42][43]. Lf belongs to the transferrin family and shows the best affinity for iron among all members of the family. Each cleft between the N1:N2 and C1:C2 domains can bind one ferric ion (Fe3+) with a Kd = 10−23 M. Lf can bind up to two ferric ions derived from the diet or from ferric Tf (holo-Tf), each of which is associated with a carbonate ion (CO32−) and with the two domains of each lobe, which are completely closed over the bound ferric ion [38][44][45]. The iron-free Lf molecule is called apo-Lf; Lf with one ferric ion is called monoferric Lf, and that with two iron ions is known as holo-Lf[46]. The holo-Lf molecule is conformationally more rigid and resistant to denaturation and proteolysis than apo-Lf, but apo-Lf is generally more effective against microbes than holo-Lf[47][48][49][50][51]. Physiologically, Lf is mainly found in the apo form[37]. The tertiary structure of bovine ferric Lf can be found in the Protein Data Bank (https://www.rcsb.org/structure/1BLF).
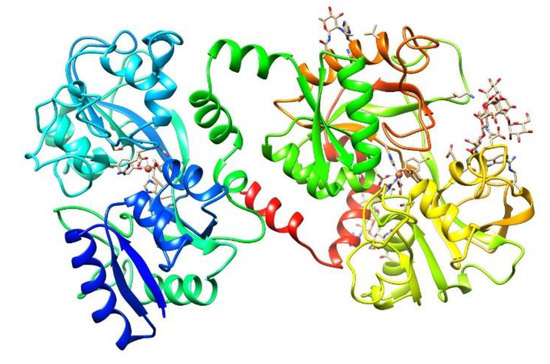
Figure 1. Structure of bovine diferric lactoferrin (holo-bLf). The three-dimensional structure of holo-bLf was determined by X-ray crystallography to investigate the factors that influence iron binding and release by transferrins. https://www.rcsb.org/structure/1BLF. Visualized with UCSF Chimera[52].
2.2. Lactoferrin-Derived Natural Peptides (Lactoferricins)
Lactoferricins (Lfcins) are peptides derived from the N-terminus of Lf produced by proteolysis with pepsin under acidic conditions; thus, they can be found naturally in the mammalian stomach and pass through the gastrointestinal tract. Lfcins have also been experimentally produced by acidic enzymatic hydrolysis and tested against different types of microbes[53]. The antimicrobial properties of Lfcins were described by Bellamy in 1992[54]. The Lf antimicrobial sequence was found to consist mainly of a loop of 18 amino acid residues formed by a disulfide bond between cysteine residues 20 and 37 of hLf or 19 and 36 of bLf. Interestingly, synthetic analogs of this region similarly exhibited potent antibacterial properties[55]. Lfcins are amphipathic molecules with a structure containing strongly hydrophobic and positively charged surfaces, which is a peculiar feature that they share with other antimicrobial peptides. Lfcins lack iron chelation activity; thus, they do not have a microbiostatic effect via the capture of iron. Two of the main Lfcins from bLf that were initially studied are LfcinB17–41, which forms a looped structure through an intramolecular disulfide bond, and lactoferrampin (Lfampin265–284)[56][57]. Lfcins not only retain the microbicidal activity of Lf against pathogens but, in most cases, are more potent than the parent protein. Lfcins, similar to Lf, show synergistic action with antibiotics and other drugs[58][59][60]. Lfcins also possess strong antitumor and immunological properties[61][62][63]. Thus, human and bovine Lfcins, similar to the lactoferrins (Lfs) from which they originate, are involved in a broad range of host defense functions.
Interestingly, the antimicrobial, antifungal, antitumor and antiviral properties of 25 residues in LfcinB have been associated with the Trp/Arg-rich portion of the peptide, while the anti-inflammatory and immunomodulatory properties have been related to a positively charged region of the molecule; it has been suggested that this region, similar to alpha- and beta-defensins, may act as a chemokine. In addition, it has been suggested that Lfcins can spontaneously translocate across the bacterial cell membrane in a similar way to Arg-rich peptides such as penetratins[64]. As penetratins can spontaneously cross the nuclear envelope, it is also suggested that nucleic acids may be a potential target of Lfcins[61]. The Lfcin and Lfampin peptides have shown antibacterial, antiparasitic, antifungal, antiviral and anti-inflammatory activities[65]. Several Lfcin structures can be found in the Protein Data Bank (https://www.rcsb.org/structure/1LFC).
2.3. Lactoferrin-Derived Synthetic Peptides
Various analogs of human and bovine Lfcins have been synthetized and tested against microorganisms and cancer cell lines. One synthetic peptide corresponding to the loop region of hLfcin (HLT1, 16 residues) and another peptide corresponding to the charged portion (HLT2, 11 residues) were prepared and assayed against pathogenic strains of Escherichia coli serotype O111, a species in which apo-Lf alone does not have an effect; the synthetic peptides exhibited potent bactericidal effects[66]. In 2009, the synthetic peptides LfcinB17–30 and Lfampin (Lfampin265–284) and a fusion peptide of both, Lfchimera, were designed and assayed against multidrug-resistant bacteria. The chimeric peptide was less sensitive to ionic strength and showed much stronger bactericidal effects than its constituent peptides; in addition, this chimera showed a strongly enhanced interaction with negatively charged model membranes [67][68]. Since then, other researchers have found similar results by using these peptides against diverse pathogens[69][70][71].
References
- Frazer, D.M.; Anderson, G.J. The regulation of iron transport. BioFactors 2014, 40, 206–214.
- Guo, S.; Frazer, D.M.; Anderson, G.J. Iron homeostasis: Transport, metabolism, and regulation. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 276–281.
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. In Proceedings of the American. J. Clin. Nutr. 2017, 106, 1559S–1566S.
- Ramm, G.A.; Ruddell, R.G. Hepatotoxicity of iron overload: Mechanisms of iron-induced hepatic fibrogenesis. Semin. Liver Dis. 2005, 25, 433–449.
- Sheldon, J.R.; Laakso, H.A.; Heinrichs, D.E. Iron acquisition strategies of bacterial pathogens. Microbiol. Spectr. 2016, 4, 43–85.
- Cruz-Castañeda, A.; Olivares-Trejo, J.J. Ehhmbp45 is a novel hemoglobin-binding protein identified in Entamoeba histolytica. FEBS Lett. 2008, 582, 2806–2810.
- Benz, C.; Lo, W.; Fathallah, N.; Connor-Guscott, A.; Benns, H.J.; Urbaniak, M.D. Dynamic regulation of the Trypanosoma brucei transferrin receptor in response to iron starvation is mediated via the 3′UTR. PLoS ONE 2018, 13, e0206332.
- Peterson, K.M.; Alderete, J.F. Iron uptake and increased intracellular enzyme activity follow host lactoferrin binding by Trichomonas vaginalis receptors. J. Exp. Med. 1984, 160, 398–410.
- Reyes-López, M.; Serrano-Luna, J.D.J.; Negrete-Abascal, E.; León-Sicairos, N.; Guerrero-Barrera, A.L.; De la Garza, M. Entamoeba histolytica: Transferrin binding proteins. Exp. Parasitol. 2001, 99, 132–140.
- López-Soto, F.; González-Robles, A.; Salazar-Villatoro, L.; León-Sicairos, N.; Piña-Vázquez, C.; Salazar, E.P.; de la Garza, M. Entamoeba histolytica uses ferritin as an iron source and internalises this protein by means of clathrin-coated vesicles. Int. J. Parasitol. 2009, 39, 417–426.
- Ortíz-Estrada, G.; Calderón-Salinas, V.; Shibayama-Salas, M.; León-Sicairos, N.; de La Garza, M. Binding and endocytosis of bovine hololactoferrin by the parasite Entamoeba histolytica. Biomed. Res. Int. 2015, 2015, 375836.
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut. Res. 2016, 23, 3984–3999.
- Morgenthau, A.; Pogoutse, A.; Adamiak, P.; Moraes, T.F.; Schryvers, A.B. Bacterial receptors for host transferrin and lactoferrin: Molecular mechanisms and role in host-microbe interactions. Future Microbiol. 2013, 8, 1575–1785.
- Mach, J.; Sutak, R. Iron in parasitic protists—From uptake to storage and where we can interfere. Metallomics 2020, 12, 1335–1347.
- Castro, S.L.; Samaniego-Barron, L.; Serrano-Rubio, L.E.; Olvera, I.C.; Avalos-Gomez, C.; de la Garza, M. Lactoferrin: A powerful antimicrobial protein present in milk. Adv. Dairy Res. 2017, 05, 1–10.
- De Mejia, E.G.; Dia, V.P. The role of nutraceutical proteins and peptides in apoptosis, angiogenesis, and metastasis of cancer cells. Cancer Metast. Rev. 2010, 29, 511–528.
- Actor, J.; Hwang, S.-A.; Kruzel, M. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973.
- Tsuda, H.; Fukamachi, K.; Xu, J.; Sekine, K.; Ohkubo, S.; Takasuka, N.; Iigo, M. Prevention of carcinogenesis and cancer metastasis by bovine lactoferrin. Proc. Japn. Acad. Ser. B Phys. Biol. Sci. 2006, 82, 208–215.
- Rodrigues, L.; Teixeira, J.; Schmitt, F.; Paulsson, M.; Månsson, H.L. Lactoferrin and cancer disease prevention. Crit. Rev. Food Sci. Nutr. 2009, 49, 203–217.
- Chen, Y.; Zheng, Z.; Zhu, X.; Shi, Y.; Tian, D.; Zhao, F.; Liu, N.; Hüppi, P.S.; Troy, F.A.; Wang, B. Lactoferrin promotes early neurodevelopment and cognition in postnatal piglets by upregulating the BDNF signaling pathway and polysialylation. Mol. Neurobiol. 2015, 52, 256–269.
- Cornish, J. Lactoferrin promotes bone growth. BioMetals 2004, 17, 331–335.
- Lönnerdal, B. Nutritional roles of lactoferrin. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 293–297.
- Ward, P.P.; Paz, E.; Conneely, O.M. Multifunctional roles of lactoferrin: A critical overview. Cell. Mol. Life Sci. 2005, 62, 2540–2548.
- Liao, Y.; Jiang, R.; Lönnerdal, B. Biochemical and molecular impacts of lactoferrin on small intestinal growth and development during early life. Biochem. Cell Biol. 2012, 90, 476–484.
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Mammalian lactoferrin receptors: Structure and function. Cell. Mol. Life Sci. 2005, 62, 2560–2575.
- Karav, S.; German, J.B.; Rouquié, C.; Le Parc, A.; Barile, D. Studying lactoferrin N-glycosylation. Int. J. Mol. Sci. 2017, 18, 870.
- Le Provost, F.; Nocart, M.; Guerin, G.; Martin, P. Characterization of the goat lactoferrin cDNA: Assignment of the relevant locus to bovine U12 synteny group. Biochem. Biophys. Res. Commun. 1994, 203, 1324–1332.
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596.
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Hematologica 1995, 80, 2552–2567.
- Cheng, J.B.; Wang, J.Q.; Bu, D.P.; Liu, G.L.; Zhang, C.G.; Wei, H.Y.; Zhou, L.Y.; Wang, J.Z. Factors affecting the lactoferrin concentration in bovine milk. J. Dairy Sci. 2008, 91, 970–976.
- Tsuji, S.; Hirata, Y.; Mukai, F.; Ohtagaki, S. Comparison of lactoferrin content in colostrum between different cattle breeds. J. Dairy Sci. 1990, 73, 125–128.
- Inoue, M.; Yamada, J.; Kitamura, N.; Shimazaki, K.I.; Andrén, A.; Yamashita, T. Immunohistochemical localization of lactoferrin in bovine exocrine glands. Tissue Cell 1993, 25, 791–797.
- Weinberg, E.D. Human lactoferrin: A novel therapeutic with broad spectrum potential. J. Pharm. Pharmacol. 2001, 53, 1303–1310.
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis. Int. J. Mol. Sci. 2017, 18, 1985.
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin: Balancing ups and downs of inflammation due to microbial infections. Int. J. Mol. Sci. 2017, 18, 501.
- Van Snick, J.L.; Masson, P.L.; Heremans, J.F. The involvement of lactoferrin in the hyposideremia of acute inflammation. J. Exp. Med. 1974, 140, 1068–1084.
- Bennett, R.M.; Kokocinski, T. Lactoferrin content of peripheral blood cells. Br. J. Haematol. 1978, 39, 509–521.
- Baker, E.N.; Baker, H.M. A structural framework for understanding the multifunctional character of lactoferrin. Biochimie 2009, 91, 3–10.
- Sipponen, T.; Savilahti, E.; Kolho, K.L.; Nuutinen, H.; Turunen, U.; Färkkilä, M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: Correlation with Crohn’s disease activity index and endoscopic findings. Inflamm. Bowel Dis. 2008, 14, 40–46.
- Groves, M.L. The Isolation of a red protein from milk. J. Am. Chem. Soc. 1960, 82, 3345–3350.
- Montreuil, J.; Tonnelat, J.; Mullet, S. Preparation and properties of the lactosiderophilin (lactotransferrine) of human milk. Biochim. Biophys. Acta 1960, 45, 413–421.
- Moguilevsky, N.; Retegui, L.A.; Masson, P.L. Comparison of human lactoferrins from milk and neutrophilic leucocytes. Relative molecular mass, isoelectric point, iron-binding properties and uptake by the liver. Biochem. J. 1985, 229, 353–359.
- Steijns, J.M.; Van Hooijdonk, A.C.M. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br. J. Nutr. 2000, 84, 11–17.
- Masson, P.L.; Heremans, J.F. Metal-combining properties of human lactoferrin (Red Milk Protein): 1. The involvement of bicarbonate in the reaction. Eur. J. Biochem. 1968, 6, 579–584.
- Testa, U. Proteins of Iron Metabolism; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780849386763.
- Baker, H.M.; Baker, E.N. Lactoferrin and iron: Structural and dynamic aspects of binding and release. BioMetals 2004, 17, 209–216.
- Lönnerdal, B.; Iyer, S. Lactoferrin: Molecular structure and biological function. Annu. Rev. Nutr. 1995, 15, 93–110.
- Jenssen, H.; Hancock, R.E.W. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29.
- Mirza, S.; Benjamin, W.H.; Coan, P.A.; Hwang, S.A.; Winslett, A.K.; Yother, J.; Hollingshead, S.K.; Fujihashi, K.; Briles, D.E. The effects of differences in pspA alleles and capsular types on the resistance of Streptococcus pneumoniae to killing by apolactoferrin. Microb. Pathog. 2016, 99, 209–219.
- Arnold, R.R.; Russell, J.E.; Champion, W.J.; Brewer, M.; Gauthier, J.J. Bactericidal activity of human lactoferrin: Differentiation from the stasis of iron deprivation. Infect. Immun. 1982, 35, 792–799.
- Salamah, A.A.; al-Obaidi, A.S. Effect of some physical and chemical factors on the bactericidal activity of human lactoferrin and transferrin against Yersinia pseudotuberculosis. New Microbiol. Off. J. Ital. Soc. Med. Odontoiatr. Clin. Microbiol. 1995, 18, 275–281.
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612.
- Lönnerdal, B. Human milk: Bioactive proteins/peptides and functional properties. Nestle Nutr. Inst. Workshop Ser. 2016, 86, 97–107.
- Bellamy, W.; Takase, M.; Wakabayashi, H.; Kawase, K.; Tomita, M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 1992, 73, 472–479.
- Bellamy, W.; Takase, M.; Yamauchi, K.; Wakabayashi, H.; Kawase, K.; Tomita, M. Identification of the bactericidal domain of lactoferrin. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. 1992, 1121, 130–136.
- Elass-Rochard, E.; Roseanu, A.; Legrand, D.; Trif, M.; Salmon, V.; Motas, C.; Montreuil, J.; Spik, G. Lactoferrin-lipopolysaccharide interaction: Involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli 055B5 lipopolysaccharide. Biochem. J. 1995, 312, 839–845.
- Van Der Kraan, M.I.A.; Groenink, J.; Nazmi, K.; Veerman, E.C.I.; Bolscher, J.G.M.; Nieuw Amerongen, A.V. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 2004, 25, 177–183.
- Chen, P.W.; Ho, S.P.; Shyu, C.L.; Mao, F.C. Effects of bovine lactoferrin hydrolysate on the in vitro antimicrobial susceptibility of Escherichia coli strains isolated from baby pigs. Am. J. Vet. Res. 2004, 65, 131–137.
- Oo, T.Z.; Cole, N.; Garthwaite, L.; Willcox, M.D.P.; Zhu, H. Evaluation of synergistic activity of bovine lactoferricin with antibiotics in corneal infection. J. Antimicrob. Chemother. 2010, 65, 1243–1251.
- León-Sicairos, N.; Reyes-López, M.; Ordaz-Pichardo, C.; de la Garza, M. Microbicidal action of lactoferrin and lactoferricin and their synergistic effect with metronidazole in Entamoeba histolytica. Biochem. Cell Biol. 2006, 84, 327–336.
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598.
- Freiburghaus, C.; Janicke, B.; Lindmark-Månsson, H.; Oredsson, S.M.; Paulsson, M.A. Lactoferricin treatment decreases the rate of cell proliferation of a human colon cancer cell line. J. Dairy Sci. 2009, 92, 2477–2484.
- Mader, J.S.; Salsman, J.; Conrad, D.M.; Hoskin, D.W. Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol. Cancer Ther. 2005, 4, 612–624.
- Vogel, H.J.; Schibli, D.J.; Jing, W.; Lohmeier-Vogel, E.M.; Epand, R.F.; Epand, R.M. Towards a structure-function analysis of bovine lactoferricin and related tryptophan- and arginine-containing peptides. Biochem. Cell Biol. 2002, 80, 49–63.
- Yin, C.; Wong, J.H.; Ng, T.B. Recent studies on the antimicrobial peptides lactoferricin and lactoferrampin. Curr. Mol. Med. 2014, 14, 1139–1154.
- Odell, E.W.; Sarra, R.; Foxworthy, M.; Chapple, D.S.; Evans, R.W. Antibacterial activity of peptides homologous to a loop region in human lactoferrin. FEBS Lett. 1996, 382, 175–178.
- Bolscher, J.G.M.; Adão, R.; Nazmi, K.; van den Keybus, P.A.M.; van ’t Hof, W.; Nieuw Amerongen, A.V.; Bastos, M.; Veerman, E.C.I. Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength than its constituent lactoferricin and lactoferrampin peptides. Biochimie 2009, 91, 123–132.
- Xu, G.; Xiong, W.; Hu, Q.; Zuo, P.; Shao, B.; Lan, F.; Lu, X.; Xu, Y.; Xiong, S. Lactoferrin-derived peptides and lactoferricin chimera inhibit virulence factor production and biofilm formation in Pseudomonas aeruginosa. J. Appl. Microbiol. 2010, 109, 1311–1318.
- Bolscher, J.; Nazmi, K.; Van Marle, J.; Van ’T Hof, W.; Veerman, E. Chimerization of lactoferricin and lactoferrampin peptides strongly potentiates the killing activity against Candida albicans. Biochem. Cell Biol. 2012, 90, 378–388.
- Acosta-Smith, E.; Viveros-Jiménez, K.; Canizalez-Román, A.; Reyes-Lopez, M.; Bolscher, J.G.M.; Nazmi, K.; Flores-Villaseñor, H.; Alapizco-Castro, G.; de la Garza, M.; Martínez-Garcia, J.J.; et al. Bovine lactoferrin and lactoferrin-derived peptides inhibit the growth of Vibrio cholerae and other Vibrio species. Front. Microbiol. 2018, 8, 2633.
- López-Soto, F.; León-Sicairos, N.; Nazmi, K.; Bolscher, J.G.; De La Garza, M. Microbicidal effect of the lactoferrin peptides Lactoferricin17-30, Lactoferrampin265-284, and Lactoferrin chimera on the parasite Entamoeba histolytica. BioMetals 2010, 23, 563–568.




