Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ursula K Braun | -- | 1704 | 2024-03-21 17:00:30 | | | |
| 2 | Camila Xu | Meta information modification | 1704 | 2024-03-22 02:45:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Braun, U.K.; Jackson, L.K.; Garcia, M.A.; Imam, S.N.; Braun, U.K. Opioid-Induced Constipation in Cancer Patients. Encyclopedia. Available online: https://encyclopedia.pub/entry/56454 (accessed on 08 February 2026).
Braun UK, Jackson LK, Garcia MA, Imam SN, Braun UK. Opioid-Induced Constipation in Cancer Patients. Encyclopedia. Available at: https://encyclopedia.pub/entry/56454. Accessed February 08, 2026.
Braun, Ursula K., Leanne K. Jackson, Mary A. Garcia, Syed N. Imam, Ursula K Braun. "Opioid-Induced Constipation in Cancer Patients" Encyclopedia, https://encyclopedia.pub/entry/56454 (accessed February 08, 2026).
Braun, U.K., Jackson, L.K., Garcia, M.A., Imam, S.N., & Braun, U.K. (2024, March 21). Opioid-Induced Constipation in Cancer Patients. In Encyclopedia. https://encyclopedia.pub/entry/56454
Braun, Ursula K., et al. "Opioid-Induced Constipation in Cancer Patients." Encyclopedia. Web. 21 March, 2024.
Copy Citation
Opioid-induced constipation (OIC) is a disabling symptom which 60–90 percent of cancer patients with chronic opioid use experience. Peripherally acting μ-opioid receptor antagonists (PAMORAs) are a class of medications aiming to reverse opioids’ adverse effects on the gut by interacting with opioid receptors in the gastrointestinal tract without significantly crossing the blood–brain barrier, and therefore they are not affecting the analgesic opioid effects in the central nervous system.
naldemedine
naloxegol
opioid-induced constipation
obstipation
cancer patients
1. Introduction
Opioid-induced constipation (OIC) is a disabling symptom which 60–90 percent of cancer patients with chronic opioid use experience [1][2][3][4]. Opioids bring about analgesia largely by binding to μ-receptors in the central nervous system, but they also bind to the μ-receptor in the myenteric plexus in the gastrointestinal tract, leading to the adverse side effect of constipation by decreasing intestinal motility, increasing fluid and electrolyte absorption in the small intestine and the colon, while also increasing the anal sphincter tone [1][2][3][4]. This can lead to more water absorption from the feces resulting in hard and dry stool. OIC has been defined by the Rome IV criteria as worsening symptoms of constipation when initiating, changing, or increasing opioid therapy, and it must include at least two of the following: fewer than three spontaneous bowel movements per week, straining during more than one-fourth of defecations, lumpy or hard stools in more than one-fourth of defecations, sensation of incomplete evacuation in more than one-fourth of defecations, or manual maneuvers to facilitate more than one-fourth of defecations (e.g., digital evacuation, support of the pelvic floor) [5][6].
Peripherally acting μ-opioid receptor antagonists (PAMORAs) are a class of medications aiming to reverse opioids’ adverse effects on the gut by interacting with opioid receptors in the gastrointestinal tract without significantly crossing the blood–brain barrier, and therefore they are not affecting the analgesic opioid effects in the central nervous system [7][8][9][10][11][12]. They are different from classic laxatives as, by their mechanism, they are targeted therapies for OIC. PAMORAs have been approved in the US by the Federal Drug Administration for OIC in patients with chronic non-cancer pain [13][14], and in Europe by the European Medicines Agency for use in patients with or without cancer [15][16]. In the US, naloxegol [12.5, 25 mg] was approved in September 2014, and naldemedine [0.1, 0.2 mg] was approved in March 2017 [13][14]. Patients with OIC can suffer greatly from reduced quality of life, as some may reduce their opioid dose in attempts to ease the OIC, leading to inadequate analgesia and a vicious circle without adequate relief of OIC. The American Gastroenterological Association published guidelines for the management of OIC [17], and other societies have published guidelines for the management of constipation in patients with cancer which specifically target OIC by including PAMORAs [18][19].
2. Mechanism of Action
PAMORAs are used in the treatment of opioid-induced constipation because they block and competitively prevent the binding of opioid agonists to μ-opioid receptors in the gastrointestinal tract [7]. PAMORAs act on gut motility, gut secretion and sphincter function [8]. Opioid agonists induce decreased cyclic adenosine monophosphate (cAMP) formation, and this effect is reversed by PAMORAs, leading to normalized chloride secretion. PAMORAs’ effect on gut motility leads to decreased transit time. This reduces the passive absorption of water from the stool, thus allowing for less dry and hard stools [9]. PAMORAs can also prevent sphincter of Oddi dysfunction and anal sphincter dysfunction caused by opioids, reducing straining and incomplete emptying.
3. Structure
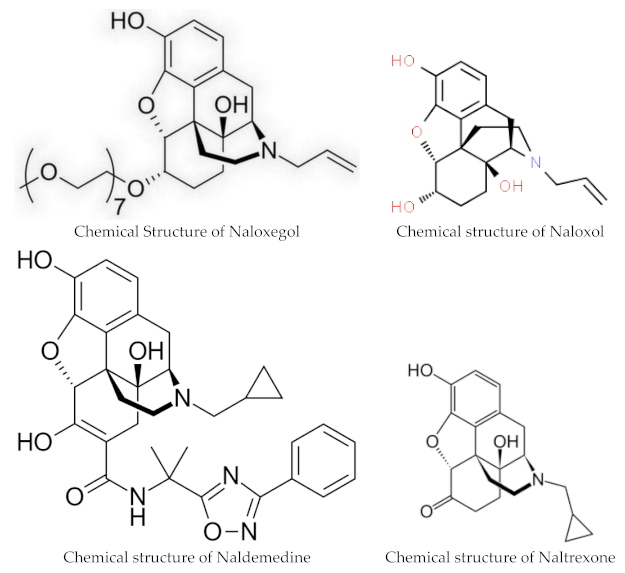
Naloxegol and naldemedine are structurally similar to morphine and other μ-opioid receptor agonists. They both have a pentacyclic structure with a benzene ring, tetrahydrofuran ring, two cyclohexane rings, and a piperidine ring. The phenolic ring and its 3-hydroxyl group play a central role in the analgesic effects of opioids, as removal of the OH group reduces analgesic activity significantly. Naldemedine, with a chemical formula of C32H34N4O6, is a peripherally acting μ-opioid receptor antagonist derived from naltrexone. It blocks opioid receptors of the μ, δ, and κ types in the gastrointestinal tract. Patents for naldemedine tosylate are expected to expire between 2026 and 2031. Unlike naltrexone, which can cross the blood–brain barrier and is used to treat opioid dependence, naldemedine has a large hydrophilic side chain and affinity to P-glycoprotein, resulting in minimal concentrations in the central nervous system. Due to its low abuse potential, the Drug Enforcement Administration removed naldemedine from Class II scheduling in September 2017 [20]. Naloxegol oxalate (chemical formula C34H53NO11) is another peripherally acting μ-opioid receptor antagonist (PAMORA) and a PEGylated derivative of naloxol, a derivative of naloxone (chemical formula C19H23NO4) [21]. It also does not cross the blood–brain barrier and is not a Class II schedule drug [22].
4. Pharmacokinetics
The oral bioavailability of Naldemedine ranges from 20% to 56%, with peak blood plasma levels achieved after 45 min on an empty stomach and 150 min when taken with a high-fat meal. The substance is highly bound to plasma proteins, primarily albumin, in the blood. The recommended dosage is 0.2 mg once daily with or without food [13][15]. Naldemedine is primarily metabolized by CYP3A to nor-naldemedine, and to a lesser extent by UDP-glucuronosyltransferase 1A3 to naldemedine 3-Glucuronide. Both metabolites are opioid receptor antagonists, but they are less potent than the original drug [23]. The drug is excreted in urine and feces, with an elimination half-life of 11 h. Patients with severe hepatic impairment should avoid naloxegol or naldemedine, although both drugs have been found to be safe and effective for those with mild to moderate hepatic impairments [24][25].
Naldemidine requires no adjustment for renal impairment [13][24][26]. For naloxegol, it is recommended that patients with a creatinine clearance <60 mL/min start with the lower naloxegol dose of 12.5 mg once daily and then, if tolerated, can increase the dose to 25 mg once daily [14][27].
Naloxegol clears mostly via hepatic metabolism (P450-CYP3A) with unknown actions of the metabolites. Naloxegol is excreted mostly in feces (and to some degree in urine), and its elimination half-life is 6–11 h [14]. Like naldemedine, when naloxegol is given with a fatty meal, absorption increases. Naloxegol is given as a once daily 12.5 or 25 mg tablet daily, and it should be taken on an empty stomach 1 h before or 2 h after the first meal of the day. Naloxegol may be crushed and can be given via nasogastric tube [14]. Maintenance laxatives should be discontinued prior to starting PAMORA therapy but may be resumed if OIC persists after 3 days of daily treatment. Decreased need for other laxatives may significantly reduce pill burden as, in many studies, PAMORAs alone were sufficient for relief of OIC.
5. Interactions
Even though PAMORAs in the US were approved for treatment of OIC in adults with noncancer pain, they have also been approved in other countries for both cancer and non-cancer pain [15][16], and are often prescribed off-label for OIC in cancer patients in the US. A very low risk of opioid withdrawal exists, so patients starting PAMORAs should be monitored for withdrawal symptoms such as hyperhidrosis, rhinorrhea, anxiety, and chills, though this was not commonly observed in clinical real-world practice. Opioid antagonists such as naloxone and naltrexone should not be used in conjunction with PAMORAs because of the potential increased risk of withdrawal. Both Naldemidine and naloxegol are no longer considered Schedule II controlled substances [20][22].
Naldemedine undergoes primary metabolism by the liver enzyme CYP3A4. Inhibitors of this enzyme can elevate naldemedine levels in the body, potentially leading to more side effects (see Table 1). Drugs like itraconazole, ketoconazole, clarithromycin, and grapefruit juice are examples of such inhibitors. In contrast, substances such as rifampicin and St John’s wort, which induce CYP3A4 activity, can significantly decrease naldemedine concentrations.
Table 1. Inhibitors and inducers of CYP3A4 which potentially increase or decrease naldemedine concentrations.
| Strong Inhibitors of CYP3A4: Increase Naldemedine Concentration | Inducers of CYP3A4: Decrease Naldemedine Concentration |
|---|---|
| Itraconazole | Rifampine |
| Ketoconaxole | St. John’s wort |
| Clarithromycin | |
| Grapefruit juice | |
| Moderate Inhibitors of CYP3A4: Diltiazem Erythromycin Verapamil |
Potent inhibitors of the P-glycoprotein pump, like ciclosporin, have the potential to elevate naldemedine blood levels.
6. Contraindications
Both naloxego and naldemidine are contraindicated in patients with gastrointestinal obstruction or patients with hypersensitivity to the medication. Naloxegol should be avoided with strong CYP3A4 inhibitors like clarithromycin and ketoconazole, as they can raise Naloxegol levels and increase the risk of side effects. If taking moderate CYP3A4 inhibitors, such as diltiazem, erythromycin, or verapamil, the dosage of Naloxegol needs to be reduced. Grapefruit and grapefruit juice may also increase Naloxegol levels. Rifampin, a CYP3A4 inducer, may reduce the effectiveness of Naloxegol.
7. Side Effects
The most common side effects of PAMORAs are diarrhea, abdominal pain, nausea, flatulence, vomiting and headache [7]. As pure opioid antagonists, Naloxegol and Naldemedine have no potential for abuse. During subgroup analysis of the COMPOSE trials I–III, no increase in adverse events (45.9%) for patients aged ≥65 years (N = 344) were found for naldemedine 0.2 mg compared to the overall group (47.1%) or compared to the placebo (51.6%), nor was there a difference in proportion of responders between older adults compared to the overall group [28]. Other subgroup analyses also found no increase in adverse events for naldemedine users with renal impairments [26] or in patients with hepatobiliary impairments from pancreatic cancer [29]. Moderate and strong CYP3A4 inhibitors and P-glycoprotein inhibitors may increase naldemedine concentrations; therefore, monitoring for adverse reactions is recommended in patients taking these medications. PAMORAs can improve quality of life, are generally safe and well tolerated, and offer a good response without reducing opioid-mediated analgesia.
8. Clinical Trials
Naldemedine was approved based on the results of the Japanese-led COMPOSE trials, which were phase three clinical studies in adult outpatients with chronic non-cancer pain and opioid-induced constipation. COMPOSE-I and COMPOSE-II were 12-week double-blind multi-country randomized controlled trials comparing 0.2 mg oral once daily naldemedine with a placebo between 2013–2015 [30]. Responders had to have at least three spontaneous bowel movements per week, with an increase of one spontaneous bowel movement for nine of the twelve weeks; the proportion of responders were significantly higher in the naldemedine group in both trials. COMPOSE-III tested the long term safety of naldemedine in patients with non-cancer chronic pain over 52 weeks, finding a statistically significant increase in weekly bowel movements without any evidence of opioid withdrawal symptoms [31].
While there is ample literature on the use of PAMORAs in patients with non-cancer pain, recruiting seriously ill patients with cancer into clinical research trials outside of cancer-directed treatment trials is difficult due to the patients’ short life expectancy, impaired functional status, and high symptom burden [32][33].
References
- Rang, H.P.; Dale, M.M.; Ritter, J.M. Analgesic drugs. Pharmacology 1999, 13, 579–603.
- Kurz, A.; Sessler, D.I. Opioid-induced bowel dysfunction: Pathophysiology and potential new therapies. Drugs 2003, 63, 649–671.
- Sykes, N.P. The relationship between opioid use and laxative use in terminally ill cancer patients. Palliat. Med. 1998, 12, 375–382.
- Bell, T.J.; Panchal, S.J.; Miaskowski, C.; Bolge, S.C.; Milanova, T.; Williamson, R. The prevalence, severity, and impact of opioid-induced bowel dysfunction: Results of a US and European Patient Survey (PROBE 1). Pain Med. 2009, 10, 35–42.
- Drossman, D.A.; Hasler, W.L. Rome IV-Functional GI disorders: Disorders of gut-brain interaction. Gastroenterology 2016, 150, 1257–1261.
- Schmulson, M.J.; Drossman, D.A. What is New in Rome IV. J. Neurogastroenterol. Motil. 2017, 23, 151–163.
- Streicher, J.M.; Bilsky, E.J. Peripherally Acting μ-Opioid Receptor Antagonists for the Treatment of Opioid-Related Side Effects: Mechanism of Action and Clinical Implications. J. Pharm. Pract. 2017, 31, 658–669.
- Brock, C.; Olesen, S.S.; Olesen, A.E.; Frøkjaer, J.B.; Andresen, T.; Drewes, A.M. Opioid Induced Bowel Dysfunction. Drugs 2012, 72, 1847–1865.
- Floettmann, E.; Bui, K.; Sostek, M.; Payza, K.; Eldon, M. Pharmacologic Profile of Naloxegol, a Peripherally Acting µ-Opioid Receptor Antagonist, for the Treatment of Opioid-Induced Constipation. J. Pharmacol. Exp. Ther. 2017, 361, 280–291.
- Hu, K.; Bridgeman, M.B. Naldemedine (Symproic) for the Treatment of Opioid-Induced Constipation. Pharm. Ther. 2018, 43, 601–627.
- Garnock-Jones, K.P. Naloxegol: A review of its use in patients with opioid-induced constipation. Drugs 2015, 75, 419–425.
- Essmat, N.; Karádi, D.Á.; Zádor, F.; Király, K.; Fürst, S.; Al-Khrasani, M. Insights into the Current and Possible Future Use of Opioid Antagonists in Relation to Opioid-Induced Constipation and Dysbiosis. Molecules 2023, 28, 7766.
- FDA. Symproic (Naldemedine) Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208854Orig1s000TOC.cfm (accessed on 28 November 2023).
- FDA. Movantik (Naloxegol) Prescribing Information Highlights (PDF). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204760s000lbl.pdf (accessed on 28 November 2023).
- European Medicines Agency (EMA). “Rizmoic: EPAR—Product Information” (PDF). 2019. Available online: https://www.ema.europa.eu/en/documents/product-information/rizmoic-epar-product-information_en.pdf (accessed on 7 February 2024).
- European Medicines Agency (EMA). “Moventig”: EPAR—Product Information (PDF). 2014. Available online: https://www.ema.europa.eu/en/documents/product-information/moventig-epar-product-information_en.pdf (accessed on 31 January 2024).
- Crockett, S.D.; Greer, K.B.; Heidelbaugh, J.J.; Falck-Ytter, Y.; Hanson, B.J.; Sultan, S.; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on the Medical Management of Opioid-Induced Constipation. Gastroenterology 2019, 156, 218–226.
- Larkin, P.J.; Cherny, N.I.; La Carpia, D.; Guglielmo, M.; Ostgathe, C.; Scotté, F.; Ripamonti, C.I. ESMO Guidelines Committee. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. S4), iv111–iv125.
- Farmer, A.D.; Drewes, A.M.; Chiarioni, G.; De Giorgio, R.; O’Brien, T.; Morlion, B.; Tack, J. Pathophysiology and management of opioid-induced constipation: European expert consensus statement. United Eur. Gastroenterol. J. 2019, 7, 7–20, Erratum in: United Eur. Gastroenterol. J. 2019, 7, 178.
- Schedules of Controlled Substances: Removal Of Naldemedine from Control (PDF). Federal Register. Available online: https://www.govinfo.gov/content/pkg/FR-2017-07-12/pdf/2017-14482.pdf (accessed on 28 November 2023).
- Bui, K.; Zhou, D.; Xu, H.; Floettmann, E.; Al-Huniti, N. Clinical Pharmacokinetics and Pharmacodynamics of Naloxegol, a Peripherally Acting µ-Opioid Receptor Antagonist. Clin. Pharmacokinet. 2017, 56, 573–582.
- Federal Register. Available online: https://www.federalregister.gov/documents/2015/01/23/2015-01172/schedules-of-controlled-substances-removal-of-naloxegol-from-control (accessed on 28 November 2023).
- Kanemasa, T.; Koike, K.; Arai, T.; Ono, H.; Horita, N.; Chiba, H.; Nakamura, A.; Morioka, Y.; Kihara, T.; Hasegawa, M. Pharmacologic effects of naldemedine, a peripherally acting μ-opioid receptor antagonist, in in vitro and in vivo models of opioid-induced constipation. Neurogastroenterol. Motil 2019, 31, e13563.
- Fukumura, K.; Yamada, T.; Yokota, T.; Kawasaki, A. The Influence of Renal or Hepatic Impairment on the Pharmacokinetics, Safety, and Tolerability of Naldemedine. Clin. Pharmacol. Drug Dev. 2020, 9, 162–174.
- Bui, K.; She, F.; Sostek, M. The effects of mild or moderate hepatic impairment on the pharmacokinetics, safety, and tolerability of naloxegol. J. Clin. Pharmacol. 2014, 54, 1368–1374.
- Webster, L.R.; Hale, M.E.; Yamada, T.; Wild, J.E. A Renal Impairment Subgroup Analysis of the Safety and Efficacy of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Chronic Non-Cancer Pain Receiving Opioid Therapy. J. Pain Res. 2020, 13, 605–612.
- Bui, K.; She, F.; Sostek, M. The effects of renal impairment on the pharmacokinetics, safety, and tolerability of naloxegol. J. Clin. Pharmacol. 2014, 54, 1375–1382.
- Wild, J.; Webster, L.; Yamada, T.; Hale, M. Safety and Efficacy of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients with Chronic Non-Cancer Pain Receiving Opioid Therapy: A Subgroup Analysis of Patients ≥ 65 Years of Age. Drugs Aging 2020, 37, 271–279.
- Kamiya, T.; Imai, H.; Fujita, Y.; Hiruta, E.; Masuno, T.; Yamazaki, S.; Tanaka, H.; Sandoh, M.; Takei, S.; Arai, K.; et al. A Retrospective Study of the Efficacy and Safety of Naldemedine for Treatment of Opioid-Induced Constipation in Patients with Hepatobiliary Pancreatic Cancer. Medicina 2023, 59, 492.
- Hale, M.; Wild, J.; Reddy, J.; Yamada, T.; Arjona Ferreira, J.C. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): Two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol. Hepatol. 2017, 2, 555–564.
- Camilleri, M.; Hale, M.; Morlion, B.; Tack, J.; Webster, L.; Wild, J. Naldemedine Improves Patient-Reported Outcomes of Opioid-Induced Constipation in Patients with Chronic Non-Cancer Pain in the COMPOSE Phase 3 Studies. J. Pain Res. 2021, 14, 2179–2189.
- von Roenn, J.H.; Tack, J.; Barker, P.N.; Lowe, E.S.; Fleischmann, C.; Sostek, M. Challenges in patient recruitment during KODIAC-06, a randomized, placebo-controlled, double-blind, multicenter, phase 3 trial of naloxegol in patients with neoplasia and opioid-induced constipation (OIC). In Multinational Association of Supportive Care in Cancer, Proceedings of the MASCC/ISOO International Symposium on Supportive Care in Cancer, Berlin, Germany, 27–29 June 2013; John Wiley & Sons: Hoboken, NJ, USA, 2013.
- Bull, J.; Bonsignore, L.; Massie, L.; Riggs, A.; Knotkova, H.; Wellman, C.; Portenoy, R. Challenges in Recruiting Patients to a Controlled Feasibility Study of a Drug for Opioid-Induced Constipation: Lessons from the Population With Advanced Cancer. J. Pain Symptom Manag. 2019, 57, e5–e8.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
506
Revisions:
2 times
(View History)
Update Date:
22 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




