
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Owen J Kelly | -- | 3376 | 2024-03-21 03:56:43 | | | |
| 2 | Lindsay Dong | -23 word(s) | 3353 | 2024-03-22 09:19:53 | | |
Video Upload Options
Medical (healthcare) deserts and food deserts, either separate or combined, exist in rural areas, globally. The physicians and other healthcare professionals who serve rural and other underserved populations, to some extent, also experience life in these areas. Dietary guidelines, from expert societies, for people with diabetes, have been helpful in guiding healthcare professionals through nutritional interventions. However, these guidelines are not designed for rural areas where healthcare resources are scarce, and access to the built environment for a healthy lifestyle and affordable healthy foods are not available.
1. Introduction
Although the definition of rural is not universal [1], in the US, more people are diagnosed with diabetes in rural compared to urban counties. Of note is that more than one-third of these non-metropolitan counties reside within the southeastern region, sometimes referred to as the diabetes belt. Furthermore, the majority of nonmetropolitan counties do not have a diabetes self-management education and support (DSMES) program [2]. Therefore, diabetes is an increased concern for rural communities because of the limited access to resources that promote a healthy life [3]. This is not a new or novel finding; people in rural communities have had the same main concerns regarding healthcare as they did a decade ago [4], suggesting few positive changes have occurred. In addition, the need for more rural physicians has been known for at least two decades [5].
2. Nutritional Guidelines under a Rural Lens
3. Dietary Intakes of People with Diabetes
4. Initiating Nutritional Therapy in Rural Environments
4.1. Estimating Energy Intake
4.2. Macronutrient Distribution
4.3. Protein and Fat
4.4. Carbohydrate
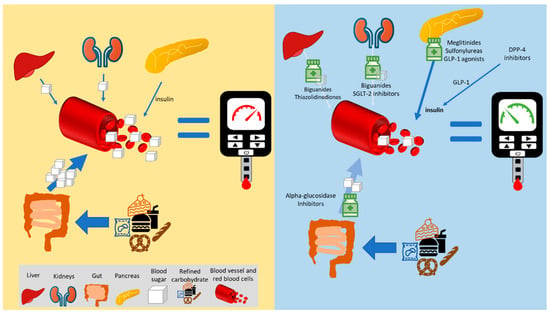
4.5. Water
4.6. Micronutrients
4.7. Non-Nutritive Sweeteners
4.8. Alcohol
4.9. Dietary Patterns/Quality
5. Cultural Preferences
6. Summary
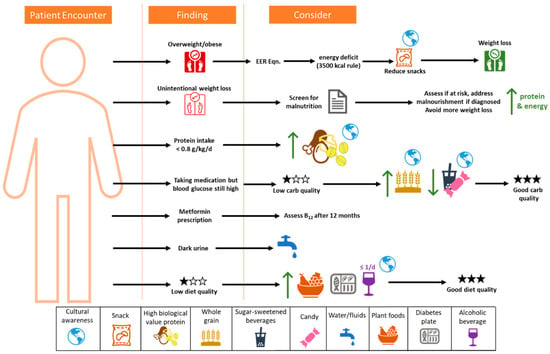
-
The process must be supportive and positive, and include cultural preferences.
-
Screen for malnutrition;
-
For weight loss, the 3500 kcal rule is a good starting point but has limitations.
-
Focus on improving diet quality, especially carbohydrate quality, to reduce micronutrient insufficiencies;
-
Limit sugar-sweetened beverages;
-
Focus on modifying snacks to help reduce energy intake, refined carbohydrates, and added sugars;
-
Increase plant foods and whole foods in the diet;
-
Decrease processed meat consumption, and reduce processed food where possible to reduce sodium;
-
Maintain good hydration;
-
Assess B12 status at least annually for those on metformin.
References
- Bennett, K.J.; Borders, T.F.; Holmes, G.M.; Kozhimannil, K.B.; Ziller, E. What Is Rural? Challenges And Implications Of Definitions That Inadequately Encompass Rural People And Places. Health Aff. 2019, 38, 1985–1992.
- Rutledge, S.A.; Masalovich, S.; Blacher, R.J.; Saunders, M.M. Diabetes Self-Management Education Programs in Nonmetropolitan Counties—United States, 2016. Surveill. Summ. 2017, 66, 1–6.
- O’Connor, A.; Wellenius, G. Rural–urban disparities in the prevalence of diabetes and coronary heart disease. Public Health 2012, 126, 813–820.
- Bolin, J.N.; Bellamy, G.R.; Ferdinand, A.O.; Vuong, A.M.; Kash, B.A.; Schulze, A.; Helduser, J.W. Rural Healthy People 2020: New Decade, Same Challenges. J. Rural Health 2015, 31, 326–333.
- Rosenblatt, R.A.; Hart, L.G. Physicians and rural America. West. J. Med. 2000, 173, 348–351.
- Rosik, P.; Stępniak, M.; Wiśniewski, R. Delineation of health care deserts using accessibility measures: The case of Poland. Eur. Plan. Stud. 2020, 29, 1151–1173.
- Lucas-Gabrielli, V.; Chevillard, G. Medical deserts” and accessibility to care: What are we talking about? Med. Sci. 2018, 34, 599–603.
- Delk, J.A.; Singleton, B.A.; Al-Dahir, S.; Kirchain, W.; Bailey-Wheeler, J. The effect of food access on type 2 diabetes control in patients of a New Orleans, Louisiana, clinic. J. Am. Pharm. Assoc. 2022, 62, 1675–1679.
- Berkowitz, S.A.; Karter, A.J.; Corbie-Smith, G.; Seligman, H.K.; Ackroyd, S.A.; Barnard, L.S.; Atlas, S.J.; Wexler, D.J. Food Insecurity, Food “Deserts,” and Glycemic Control in Patients With Diabetes: A Longitudinal Analysis. Diabetes Care 2018, 41, 1188–1195.
- Look, A.R.G.; Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154.
- Feng, W.; Page, E.T.; Cash, S.B. Dollar Stores and Food Access for Rural Households in the United States, 2008–2020. Am. J. Public Health 2023, 113, 331–336.
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117.
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754.
- Dao, M.C.; Subar, A.F.; Warthon-Medina, M.; Cade, J.E.; Burrows, T.; Golley, R.K.; Forouhi, N.G.; Pearce, M.; Holmes, B.A. Dietary assessment toolkits: An overview. Public Health Nutr. 2019, 22, 404–418.
- Heitman, K.; Thomas, S.E.; Kelly, O.; Fanelli, S.M.; Krok-Schoen, J.L.; Luo, M.; Taylor, C.A. Snacks contribute considerably to total dietary intakes among adults stratified by glycemia in the United States. PLoS Glob. Public Health 2023, 3, e0000802.
- Fanelli, S.M.; Kelly, O.J.; Krok-Schoen, J.L.; Taylor, C.A. Low Protein Intakes and Poor Diet Quality Associate with Functional Limitations in US Adults with Diabetes: A 2005–2016 NHANES Analysis. Nutrients 2021, 13, 2582.
- Kelly, O.; Krok-Schoen, J.L.; Luo, M.; Taylor, C.A. Evaluation of Dietary Intakes of Macronutrients in Adults with Different A1C Levels. Diabetes 2018, 67, 763-P.
- Fanelli, S.; Kelly, O.; Luo, M.; Krok-Schoen, J.; Taylor, C. Differences in Micronutrient Intakes by Levels of Glycemic Control in US Adults. J. Acad. Nutr. Diet. 2018, 118, A133.
- Kelly, O.; Fanelli, S.M.; Krok-Schoen, J.L.; Taylor, C.A. 1577-P: Dietary Intake Trends Associated with Breakfast Skipping in U.S. Adults by Diabetes Status. Diabetes 2019, 68, 1577-P.
- National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Energy; The National Academies Press: Washington, DC, USA, 2023; p. 460.
- Hall, K.D.; Sacks, G.; Chandramohan, D.; Chow, C.C.; Wang, Y.C.; Gortmaker, S.L.; Swinburn, B.A. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011, 378, 826–837.
- Thomas, D.M.; Gonzalez, M.C.; Pereira, A.Z.; Redman, L.M.; Heymsfield, S.B. Time to correctly predict the amount of weight loss with dieting. J. Acad. Nutr. Diet. 2014, 114, 857–861.
- U.S. Department of Agriculture. MyPlate Plan. Available online: https://www.myplate.gov/myplate-plan (accessed on 1 January 2024).
- Fleming, S.; Arensberg, M.B.; Kerr, K.; Blancato, R. The Opportunity for Quality Malnutrition Care to Improve Rural Health Outcomes and Health Equity for Older Americans. OBM Geriatr. 2023, 07, 227.
- Saunders, J.; Smith, T. Malnutrition: Causes and consequences. Clin. Med. 2010, 10, 624–627.
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S68–S96.
- Medicine, I.O. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; p. 1358.
- Guzman, J.R.; Lyra, R.; Aguilar-Salinas, C.A.; Cavalcanti, S.; Escano, F.; Tambasia, M.; Duarte, E.; Group, A.C. Treatment of type 2 diabetes in Latin America: A consensus statement by the medical associations of 17 Latin American countries. Latin American Diabetes Association. Rev. Panam. Salud Publica 2010, 28, 463–471.
- Ramsden, C.E.; Zamora, D.; Majchrzak-Hong, S.; Faurot, K.R.; Broste, S.K.; Frantz, R.P.; Davis, J.M.; Ringel, A.; Suchindran, C.M.; Hibbeln, J.R. Re-evaluation of the traditional diet-heart hypothesis: Analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ 2016, 353, i1246.
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; American Heart Association Nutrition Committee of the Council; et al. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory From the American Heart Association. Circulation 2020, 141, e39–e53.
- Derosa, G.; Maffioli, P. alpha-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci. 2012, 8, 899–906.
- Rehani, P.R.; Iftikhar, H.; Nakajima, M.; Tanaka, T.; Jabbar, Z.; Rehani, R.N. Safety and Mode of Action of Diabetes Medications in comparison with 5-Aminolevulinic Acid (5-ALA). J. Diabetes Res. 2019, 2019, 4267357.
- Hershon, K.S.; Hirsch, B.R.; Odugbesan, O. Importance of Postprandial Glucose in Relation to A1C and Cardiovascular Disease. Clin. Diabetes 2019, 37, 250–259.
- Armstrong, L.E.; Barquera, S.; Duhamel, J.F.; Hardinsyah, R.; Haslam, D.; Lafontan, M. Recommendations for healthier hydration: Addressing the public health issues of obesity and type 2 diabetes. Clin. Obes. 2012, 2, 115–124.
- Janbozorgi, N.; Allipour, R.; Djafarian, K.; Shab-Bidar, S.; Badeli, M.; Safabakhsh, M. Water intake and risk of type 2 diabetes: A systematic review and meta-analysis of observational studies. Diabetes Metab. Syndr. 2021, 15, 102156.
- Vanhaecke, T.; Perrier, E.T.; Melander, O. A Journey through the Early Evidence Linking Hydration to Metabolic Health. Ann. Nutr. Metab. 2020, 76 (Suppl. S1), 4–9.
- Johnson, E.C.; Bardis, C.N.; Jansen, L.T.; Adams, J.D.; Kirkland, T.W.; Kavouras, S.A. Reduced water intake deteriorates glucose regulation in patients with type 2 diabetes. Nutr. Res. 2017, 43, 25–32.
- Wolfsdorf, J.; Glaser, N.; Sperling, M.A.; American Diabetes, A. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care 2006, 29, 1150–1159.
- Gosmanov, A.R.; Gosmanova, E.O.; Dillard-Cannon, E. Management of adult diabetic ketoacidosis. Diabetes Metab. Syndr. Obes. 2014, 7, 255–264.
- Medicine, I.O. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; The National Academies Press: Washington, DC, USA, 2005; p. 638.
- Begg, D.P. Disturbances of thirst and fluid balance associated with aging. Physiol. Behav. 2017, 178, 28–34.
- National Institutes of Health. Nutrient Recommendations and Databases. Available online: https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx (accessed on 30 December 2023).
- Chehade, J.M.; Sheikh-Ali, M.; Mooradian, A.D. The Role of Micronutrients in Managing Diabetes. Diabetes Spectr. 2009, 22, 214–218.
- Shearer, J.; Swithers, S.E. Artificial sweeteners and metabolic dysregulation: Lessons learned from agriculture and the laboratory. Rev. Endocr. Metab. Disord. 2016, 17, 179–186.
- Tey, S.L.; Salleh, N.B.; Henry, J.; Forde, C.G. Effects of aspartame-, monk fruit-, stevia- and sucrose-sweetened beverages on postprandial glucose, insulin and energy intake. Int. J. Obes. 2017, 41, 450–457.
- Mejia, E.; Pearlman, M. Natural Alternative Sweeteners and Diabetes Management. Curr. Diabetes Rep. 2019, 19, 142.
- Purohit, V.; Mishra, S. The truth about artificial sweeteners—Are they good for diabetics? Indian Heart J. 2018, 70, 197–199.
- Nettleton, J.A.; Lutsey, P.L.; Wang, Y.; Lima, J.A.; Michos, E.D.; Jacobs, D.R., Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009, 32, 688–694.
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am. J. Clin. Nutr. 2022, 116, 640–652.
- Kulkarni, K.D. Food, Culture, and Diabetes in the United States. Clin. Diabetes 2004, 22, 190–192.
- Woodside, J.; Young, I.S.; McKinley, M.C. Culturally adapting the Mediterranean Diet pattern—A way of promoting more ‘sustainable’ dietary change? Br. J. Nutr. 2022, 128, 693–703.




