Medical (healthcare) deserts and food deserts, either separate or combined, exist in rural areas, globally. The physicians and other healthcare professionals who serve rural and other underserved populations, to some extent, also experience life in these areas. Dietary guidelines, from expert societies, for people with diabetes, have been helpful in guiding healthcare professionals through nutritional interventions. However, these guidelines are not designed for rural areas where healthcare resources are scarce, and access to the built environment for a healthy lifestyle and affordable healthy foods are not available.
- nutrition guidelines
- underserved
- rural
- diabetes
1. Introduction
Although the definition of rural is not universal [1], in the US, more people are diagnosed with diabetes in rural compared to urban counties. Of note is that more than one-third of these non-metropolitan counties reside within the southeastern region, sometimes referred to as the diabetes belt. Furthermore, the majority of nonmetropolitan counties do not have a diabetes self-management education and support (DSMES) program [2]. Therefore, diabetes is an increased concern for rural communities because of the limited access to resources that promote a healthy life [3]. This is not a new or novel finding; people in rural communities have had the same main concerns regarding healthcare as they did a decade ago [4], suggesting few positive changes have occurred. In addition, the need for more rural physicians has been known for at least two decades [5].
2. Nutritional Guidelines under a Rural Lens
3. Dietary Intakes of People with Diabetes
Before providing some practical nutritional considerations for people with diabetes, it is best to have some idea of what they are consuming. The National Health and Nutrition Examination Survey (NHANES) has dietary intake estimates, based on 24 h dietary recall, for people with diabetes. The limitations and strengths of dietary recall are widely publicized [33,34][15][16]; however, NHANES data are used to develop the Dietary Guidelines for Americans. While these are estimates based off one day’s food intake, and are mostly based on an urban population, they could act as a reference point for people in rural areas. Overall, people with diabetes were observed as not achieving a good diet quality score (based on the United States Department of Agriculture’s Healthy Eating Index (HEI) score. In addition, compared to individuals without diabetes, people with prediabetes or diabetes, seem to have lower caloric (up to 264 kcal), protein, carbohydrate and fat intakes, of up to approximately 264 kcal. Furthermore, dinner was the largest meal and contained the highest amount of carbohydrate, while snacks contained the most added sugars [35][17]. For people with diabetes, mean intakes of thiamin, niacin, vitamin B6, vitamin C, magnesium, and potassium were significantly lower compared to those in people without diabetes [36][18]. Snacks contributed approximately 20% of the daily energy intake for people with prediabetes or diabetes, which was significantly less than that for those without diabetes [33][15]. People with diabetes who skipped breakfast compensated with snacks, and actually consumed more energy, carbohydrate, and added sugars from snacks compared to those who ate breakfast [37][19].4. Initiating Nutritional Therapy in Rural Environments
4.1. Estimating Energy Intake
Estimating energy intake is most important when the objective is weight loss. New estimated energy requirement (EER) equations from the National Academies of Sciences, Engineering, and Medicine are available [39][20]; they are based on age, sex, physical activity level, and pregnancy/lactation. EER equations can be incorporated into digital records and can be calculated in the physician’s office. The ADA guidelines recommend a target weight loss of 5% to achieve a clinical benefit, with optimal benefits seen with 15% weight loss for patients with diabetes. For people with prediabetes, weight loss should be 7–10% of the body weight to help slow the progression to diabetes [14][13]. Body weight optimization is fundamental to diabetes management. The ‘3500 kcal rule’ is the typical method utilized to reduce body weight. It assumes 3500 kcal = 1 lb (0.454 kg) of body weight, so a deficit of 3500 kcal over one week (500 kcal/d) should result in a loss of 1 lb of body weight. The 3500 kcal rule has its limitations [40,41][21][22] because weight loss is not linear, and it probably overestimates the rate of weight loss due to the complex physiological and metabolic factors associated with energy homeostasis. Nevertheless, it does provide a starting point to help facilitate weight loss and requires close monitoring. An alternative solution is to utilize the MyPlate Plan [42][23] to estimate energy requirements, which can be easily performed during an office visit, with the aid of office staff. The patient could also complete this at home. This will provide two energy levels: (1) to help achieve a healthy weight and, (2) to maintain body weight. It must be noted that these numbers are estimates and will need to be modified over time, based on the rate of weight gain or loss, and patient perceptions. The key point is that weight management is a journey that requires considerable commitment from both the physician and the patient. Before leaving the topic of energy intake, malnutrition must be addressed. For older adults, malnutrition (undernutrition) is more prevalent in rural areas [43][24], which can complicate nutritional therapy. Malnutrition can easily be screened for and assessed using various tools. Nutritional assessment is more involved and leads to a medical diagnosis of malnutrition. It is more common to utilize malnutrition screening and assessment in hospitals and long-term care facilities; however, for the rural physician, regular malnutrition screening could improve patient health outcomes. Malnutrition can have profound effects on homeostasis, organ function, and mood [44][25]. Screening for malnutrition is rapid, inexpensive and is predominately based on recent unintentional weight loss. Two options for screening are the Malnutrition Universal Screening Tool (MUST) for adults, and the Malnutrition Screening Tool (MST). These instruments are readily available online and consist of three questions each. Answers to these questions are scored, determining if the individual is at risk of malnutrition or not. Those at risk should be then assessed to make a medical diagnosis. Assessments can be subjective and require practice. The two widely utilized recommended instruments for assessment are the Mini Nutrition Assessment® (MNA; Nestlé Nutrition Institute) and the Subjective Global Assessment (SGA). The MNA has more questions compared to the SGA (18 versus 7); however, both should take less than 15 min to complete. These are readily available online and can be utilized in rural settings.4.2. Macronutrient Distribution
While “there is no ideal macronutrient pattern for people with diabetes” [45][26], NHANES data show that people with prediabetes and diabetes have approximately the same macronutrient distribution as that of people with a healthy A1C; 15% of total energy is from protein, 50% of total energy is from carbohydrates; and 35% of total energy is from fat (12% is from saturated fat) [35][17]. This distribution pattern is relatively close to the U.S. Acceptable Macronutrient Distribution Ranges (AMDR) [46][27]. Although AMDRs are technically designed for healthy people, they provide a useful frame of reference (for the most part, most patients, with or without diabetes, will be within the AMDR). Some expert bodies have determined evidence-based macronutrient distribution ranges for people with diabetes. The Asociación Latinoamericana de Diabetes (ALAD; Latin American Diabetes Association) brought together medical associations from 17 countries to produce a consensus statement regarding the treatment of type 2 diabetes [47][28].4.3. Protein and Fat
When considering protein for rural patients, achieving the RDA could be the minimum goal [34][16]. Per ADA guidelines [14][13], lean fresh meats (the fat in red meat is high in stearic acid, an 18-carbon saturated fatty acid, which is associated with cardiovascular disease [52][29]), and a mixture of protein sources—plants and animal proteins are recommended. Soy protein and animal proteins, including dairy, seafood and eggs, are all of high biological value. Eggs may be more readily available in rural areas; however, overconsuming eggs should be limited for those with diabetes [53][30]. Consuming more egg whites, than whole eggs, could be encouraged, to increase good-quality protein intake, as the cholesterol is in the yolk.4.4. Carbohydrate
The most important macronutrient in relation to diabetes is carbohydrate, with and emphasis on carbohydrate quality [34][16]. This includes making more whole grain choices and limiting refined carbohydrates, especially from ultraprocessed foods and snacks. Nutritional intervention is a key component of diabetes management, along with medications and other lifestyle changes. One key point is that people with diabetes need reminding that glycemic medications perform specific functions to address hyperglycemia. However, poor- quality dietary carbohydrate will still raise blood sugar, independent of most medications. The primary exception are α-glucosidase inhibitors, which can reduce the amount of carbohydrate digested (not 100% effectively), although simple sugars (i.e., monosaccharides such as glucose and fructose) will still become absorbed; [57][31], see Figure 21.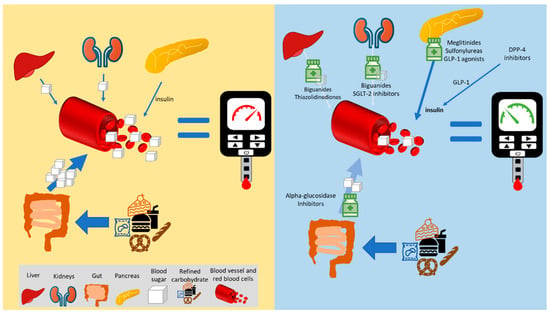
4.5. Water
4.6. Micronutrients
The ADA guidelines do not support the use of supplementary vitamins and minerals, specifically to help control blood glucose or improve CVD risk factors [14][13]. The default target intakes for the micronutrients are the Dietary Reference Intakes; all the tables are freely available here [73][42]. Patients with diabetes on metformin should be monitored for B12 deficiency at least annually [14][13]. Epidemiological studies suggest that lower potassium, magnesium, and zinc status are associated with uncontrolled diabetes [74][43], and may be worth monitoring. Chromium may not help with blood sugar management [14][13], although some studies do support it use [74][43]. However, it is important to know what supplements the patient is taking. Maintaining a non-judgmental approach to micronutrient supplements and herbals will help gain patient trust and facilitate the open sharing of information. The major reason to ask a patient to stop taking a supplement is if it contraindicated for any aspect of their medical treatment (e.g., nutrient–drug interactions). Overall, as there is not strong evidence to support the use of certain micronutrients and herbs/spices specifically for glycemic control, the patient’s money may be best spent on other means to control glycemia.4.7. Non-Nutritive Sweeteners
The original goal of non-nutritive sweeteners was to reduce calories and avoid blood sugar spikes. Regardless, it is logical to assume that the use of non-nutritive sweeteners does not break the sweet habit [75][44]. While low-/non-caloric sweeteners do reduce the overall energy intake of a patient, they may not improve glycemic outcomes [76,77][45][46] as they may disrupt the patient’s insulin sensitivity [78][47]. Diet sodas that contain aspartame have been associated with higher A1C levels and higher risks of metabolic syndrome [79][48]. This could be that individual’s pair higher-calorie and carbohydrate foods with low-calorie/zero-calorie beverages.4.8. Alcohol
Moderate alcohol consumption is recommended for people with diabetes, and equates to approximately one drink per day. One drink is defined as a 12 oz of beer, a 5 oz glass of wine, or 1.5 oz of distilled spirits (approx. 15 g alcohol) [14][13]. However, one additional consideration is the carbohydrate/sugar content of different alcoholic drinks; that of beers is the highest and that of distilled spirits is the lowest; low-carb beers are available. It is also important to educate people with diabetes about the signs, symptoms, and self-management of delayed hypoglycemia after drinking alcohol, especially when using insulin or insulin secretagogues.4.9. Dietary Patterns/Quality
The ADA guidelines state that a variety of eating patterns are suitable for people with diabetes [14][13]. Improving diet quality means increasing the consumption of plant foods and whole foods, in addition to reducing processed food intake. There is considerable flexibility for the rural physician within the ADA guidelines to modify patients’ dietary patterns. The core concept is to improve carbohydrate quality (more whole grains and less refined grains) to help improve glycemia. Rural physicians must assess what foods the patient has access to and suggest an eating pattern that works best with their situation [21][8]. Most people are aware of the Mediterranean diet and a low-fat diet; however, one dietary pattern that is receiving more attention is the very-low-carbohydrate diet. This is typically referred to as the ketogenic diet, and it is defined as maintaining a state of nutritional ketogenesis (a blood ketone level between 0.5 and 4.0 mM), by limiting the daily intake of carbohydrates to 20–50 g/d and that of protein to 1.5 g/kg of body weight, with the remainder of energy coming from fats [83][49]. While this diet is appropriate for some patients with diabetes (uncontrolled diabetes or requiring medication reduction), it requires time and resources to remain compliant, including monitoring blood and/or urinary ketones.5. Cultural Preferences
Cultural preferences are a vital component when providing dietary advice. Cultural preferences are not directly addressed in the ADA guidelines [14][13]; however, they come into play when creating an individualized diet. For example, African American diets often have starchy vegetables (white and sweet potatoes) as a staple food [84][50]. Starchy vegetables should be limited as they contribute to higher postprandial blood glucose [14][13]. In this case, a good starting point may be to reduce the serving size. Substitutes include pinto, kidney, or navy beans. Patients could also be encouraged to embrace the diversity of traditional vegetables in their diets, such as green leafy vegetables such as collard, mustard, or spinach greens [84][50], if available. Understanding the value of traditional foods in an individual’s life is a core component of culturally competent nutritional recommendations, which can play a huge role in patient compliance and overall improved health. Recommending foods outside of the patients’ cultural preferences has been shown to lead to cultural disconnection, which in turn can lead to decreased adherence to recommended diet plans, and the possible loss of social support [85][51].6. Summary
Nutritional guidelines for prediabetes and diabetes from expert societies are excellent resources; however, their utility may diminish in medical/food deserts. This represents a large gap in rural patient management as well as the gap in the literature related to dietary habits, dietary behaviors, and cultural preferences of people with diabetes in rural America. Table 1 summarizes the barriers to fully implementing nutritional guidelines, including DSMES, DPP, and intensive lifestyle interventions that have been shown to be effective. In rural areas, these programs are either not available or are cost-prohibitive to create locally. However, because of the complexity of the nutritional therapy required, certain groups are at a higher risk for complications in rural areas due to food and/or healthcare insecurity; these are those with eating disorders and other conditions (diabetes-related gastroparesis and chronic kidney disease). To encourage rural physicians and other rural healthcare professionals to utilize dietary management in diabetes, some form of Medicare/Medicaid reimbursement for nutritional therapy could be developed (as an alternative to MNT, which is legally defined). In addition, less expensive software licenses for dietary assessment solutions may alleviate the cost barrier. There is a great opportunity to create specific DSMES and DPP toolkits for rural physicians, which could be easier to implement (e.g., online modules and patient workbooks). Telemedicine remains an option for rural areas; however, it may need to be modified from its current form to fit the rural environment. A crucial aspect of successful dietary intervention is meeting the patient where they are. Cultural preferences must be included, as well as knowledge of the local foods available. For rural physicians initiating dietary therapy, this may be more important than any other factors. The following bullet points and figure (Figure 42) summarize the information provided here to help supplement the ADA guidelines: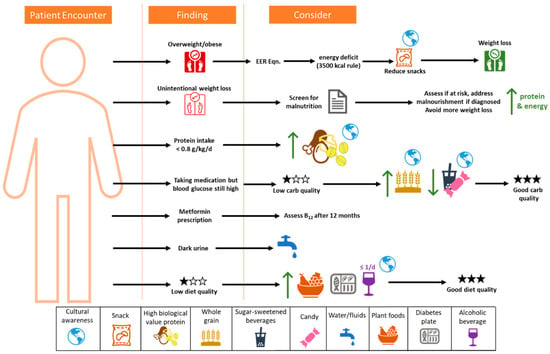
-
The process must be supportive and positive, and include cultural preferences.
-
Screen for malnutrition;
-
For weight loss, the 3500 kcal rule is a good starting point but has limitations.
-
Focus on improving diet quality, especially carbohydrate quality, to reduce micronutrient insufficiencies;
-
Limit sugar-sweetened beverages;
-
Focus on modifying snacks to help reduce energy intake, refined carbohydrates, and added sugars;
-
Increase plant foods and whole foods in the diet;
-
Decrease processed meat consumption, and reduce processed food where possible to reduce sodium;
-
Maintain good hydration;
-
Assess B12 status at least annually for those on metformin.
References
- Bennett, K.J.; Borders, T.F.; Holmes, G.M.; Kozhimannil, K.B.; Ziller, E. What Is Rural? Challenges And Implications Of Definitions That Inadequately Encompass Rural People And Places. Health Aff. 2019, 38, 1985–1992.
- Rutledge, S.A.; Masalovich, S.; Blacher, R.J.; Saunders, M.M. Diabetes Self-Management Education Programs in Nonmetropolitan Counties—United States, 2016. Surveill. Summ. 2017, 66, 1–6.
- O’Connor, A.; Wellenius, G. Rural–urban disparities in the prevalence of diabetes and coronary heart disease. Public Health 2012, 126, 813–820.
- Bolin, J.N.; Bellamy, G.R.; Ferdinand, A.O.; Vuong, A.M.; Kash, B.A.; Schulze, A.; Helduser, J.W. Rural Healthy People 2020: New Decade, Same Challenges. J. Rural Health 2015, 31, 326–333.
- Rosenblatt, R.A.; Hart, L.G. Physicians and rural America. West. J. Med. 2000, 173, 348–351.
- Rosik, P.; Stępniak, M.; Wiśniewski, R. Delineation of health care deserts using accessibility measures: The case of Poland. Eur. Plan. Stud. 2020, 29, 1151–1173.
- Lucas-Gabrielli, V.; Chevillard, G. Medical deserts” and accessibility to care: What are we talking about? Med. Sci. 2018, 34, 599–603.
- Delk, J.A.; Singleton, B.A.; Al-Dahir, S.; Kirchain, W.; Bailey-Wheeler, J. The effect of food access on type 2 diabetes control in patients of a New Orleans, Louisiana, clinic. J. Am. Pharm. Assoc. 2022, 62, 1675–1679.
- Berkowitz, S.A.; Karter, A.J.; Corbie-Smith, G.; Seligman, H.K.; Ackroyd, S.A.; Barnard, L.S.; Atlas, S.J.; Wexler, D.J. Food Insecurity, Food “Deserts,” and Glycemic Control in Patients With Diabetes: A Longitudinal Analysis. Diabetes Care 2018, 41, 1188–1195.
- Look, A.R.G.; Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154.
- Feng, W.; Page, E.T.; Cash, S.B. Dollar Stores and Food Access for Rural Households in the United States, 2008–2020. Am. J. Public Health 2023, 113, 331–336.
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117.
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754.
- Dao, M.C.; Subar, A.F.; Warthon-Medina, M.; Cade, J.E.; Burrows, T.; Golley, R.K.; Forouhi, N.G.; Pearce, M.; Holmes, B.A. Dietary assessment toolkits: An overview. Public Health Nutr. 2019, 22, 404–418.
- Heitman, K.; Thomas, S.E.; Kelly, O.; Fanelli, S.M.; Krok-Schoen, J.L.; Luo, M.; Taylor, C.A. Snacks contribute considerably to total dietary intakes among adults stratified by glycemia in the United States. PLoS Glob. Public Health 2023, 3, e0000802.
- Fanelli, S.M.; Kelly, O.J.; Krok-Schoen, J.L.; Taylor, C.A. Low Protein Intakes and Poor Diet Quality Associate with Functional Limitations in US Adults with Diabetes: A 2005–2016 NHANES Analysis. Nutrients 2021, 13, 2582.
- Kelly, O.; Krok-Schoen, J.L.; Luo, M.; Taylor, C.A. Evaluation of Dietary Intakes of Macronutrients in Adults with Different A1C Levels. Diabetes 2018, 67, 763-P.
- Fanelli, S.; Kelly, O.; Luo, M.; Krok-Schoen, J.; Taylor, C. Differences in Micronutrient Intakes by Levels of Glycemic Control in US Adults. J. Acad. Nutr. Diet. 2018, 118, A133.
- Kelly, O.; Fanelli, S.M.; Krok-Schoen, J.L.; Taylor, C.A. 1577-P: Dietary Intake Trends Associated with Breakfast Skipping in U.S. Adults by Diabetes Status. Diabetes 2019, 68, 1577-P.
- National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Energy; The National Academies Press: Washington, DC, USA, 2023; p. 460.
- Hall, K.D.; Sacks, G.; Chandramohan, D.; Chow, C.C.; Wang, Y.C.; Gortmaker, S.L.; Swinburn, B.A. Quantification of the effect of energy imbalance on bodyweight. Lancet 2011, 378, 826–837.
- Thomas, D.M.; Gonzalez, M.C.; Pereira, A.Z.; Redman, L.M.; Heymsfield, S.B. Time to correctly predict the amount of weight loss with dieting. J. Acad. Nutr. Diet. 2014, 114, 857–861.
- U.S. Department of Agriculture. MyPlate Plan. Available online: https://www.myplate.gov/myplate-plan (accessed on 1 January 2024).
- Fleming, S.; Arensberg, M.B.; Kerr, K.; Blancato, R. The Opportunity for Quality Malnutrition Care to Improve Rural Health Outcomes and Health Equity for Older Americans. OBM Geriatr. 2023, 07, 227.
- Saunders, J.; Smith, T. Malnutrition: Causes and consequences. Clin. Med. 2010, 10, 624–627.
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S68–S96.
- Medicine, I.O. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; p. 1358.
- Guzman, J.R.; Lyra, R.; Aguilar-Salinas, C.A.; Cavalcanti, S.; Escano, F.; Tambasia, M.; Duarte, E.; Group, A.C. Treatment of type 2 diabetes in Latin America: A consensus statement by the medical associations of 17 Latin American countries. Latin American Diabetes Association. Rev. Panam. Salud Publica 2010, 28, 463–471.
- Ramsden, C.E.; Zamora, D.; Majchrzak-Hong, S.; Faurot, K.R.; Broste, S.K.; Frantz, R.P.; Davis, J.M.; Ringel, A.; Suchindran, C.M.; Hibbeln, J.R. Re-evaluation of the traditional diet-heart hypothesis: Analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ 2016, 353, i1246.
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; American Heart Association Nutrition Committee of the Council; et al. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory From the American Heart Association. Circulation 2020, 141, e39–e53.
- Derosa, G.; Maffioli, P. alpha-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci. 2012, 8, 899–906.
- Rehani, P.R.; Iftikhar, H.; Nakajima, M.; Tanaka, T.; Jabbar, Z.; Rehani, R.N. Safety and Mode of Action of Diabetes Medications in comparison with 5-Aminolevulinic Acid (5-ALA). J. Diabetes Res. 2019, 2019, 4267357.
- Hershon, K.S.; Hirsch, B.R.; Odugbesan, O. Importance of Postprandial Glucose in Relation to A1C and Cardiovascular Disease. Clin. Diabetes 2019, 37, 250–259.
- Armstrong, L.E.; Barquera, S.; Duhamel, J.F.; Hardinsyah, R.; Haslam, D.; Lafontan, M. Recommendations for healthier hydration: Addressing the public health issues of obesity and type 2 diabetes. Clin. Obes. 2012, 2, 115–124.
- Janbozorgi, N.; Allipour, R.; Djafarian, K.; Shab-Bidar, S.; Badeli, M.; Safabakhsh, M. Water intake and risk of type 2 diabetes: A systematic review and meta-analysis of observational studies. Diabetes Metab. Syndr. 2021, 15, 102156.
- Vanhaecke, T.; Perrier, E.T.; Melander, O. A Journey through the Early Evidence Linking Hydration to Metabolic Health. Ann. Nutr. Metab. 2020, 76 (Suppl. S1), 4–9.
- Johnson, E.C.; Bardis, C.N.; Jansen, L.T.; Adams, J.D.; Kirkland, T.W.; Kavouras, S.A. Reduced water intake deteriorates glucose regulation in patients with type 2 diabetes. Nutr. Res. 2017, 43, 25–32.
- Wolfsdorf, J.; Glaser, N.; Sperling, M.A.; American Diabetes, A. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care 2006, 29, 1150–1159.
- Gosmanov, A.R.; Gosmanova, E.O.; Dillard-Cannon, E. Management of adult diabetic ketoacidosis. Diabetes Metab. Syndr. Obes. 2014, 7, 255–264.
- Medicine, I.O. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; The National Academies Press: Washington, DC, USA, 2005; p. 638.
- Begg, D.P. Disturbances of thirst and fluid balance associated with aging. Physiol. Behav. 2017, 178, 28–34.
- National Institutes of Health. Nutrient Recommendations and Databases. Available online: https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx (accessed on 30 December 2023).
- Chehade, J.M.; Sheikh-Ali, M.; Mooradian, A.D. The Role of Micronutrients in Managing Diabetes. Diabetes Spectr. 2009, 22, 214–218.
- Shearer, J.; Swithers, S.E. Artificial sweeteners and metabolic dysregulation: Lessons learned from agriculture and the laboratory. Rev. Endocr. Metab. Disord. 2016, 17, 179–186.
- Tey, S.L.; Salleh, N.B.; Henry, J.; Forde, C.G. Effects of aspartame-, monk fruit-, stevia- and sucrose-sweetened beverages on postprandial glucose, insulin and energy intake. Int. J. Obes. 2017, 41, 450–457.
- Mejia, E.; Pearlman, M. Natural Alternative Sweeteners and Diabetes Management. Curr. Diabetes Rep. 2019, 19, 142.
- Purohit, V.; Mishra, S. The truth about artificial sweeteners—Are they good for diabetics? Indian Heart J. 2018, 70, 197–199.
- Nettleton, J.A.; Lutsey, P.L.; Wang, Y.; Lima, J.A.; Michos, E.D.; Jacobs, D.R., Jr. Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009, 32, 688–694.
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am. J. Clin. Nutr. 2022, 116, 640–652.
- Kulkarni, K.D. Food, Culture, and Diabetes in the United States. Clin. Diabetes 2004, 22, 190–192.
- Woodside, J.; Young, I.S.; McKinley, M.C. Culturally adapting the Mediterranean Diet pattern—A way of promoting more ‘sustainable’ dietary change? Br. J. Nutr. 2022, 128, 693–703.
