
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | francesca maria di muro | -- | 2770 | 2024-03-14 14:12:39 | | | |
| 2 | Lindsay Dong | -371 word(s) | 2399 | 2024-03-15 07:34:34 | | |
Video Upload Options
Bioprosthetic heart valves (BHV) have been increasingly implanted instead of mechanical valves in patients undergoing surgical aortic valve replacement (SAVR). Structural valve deterioration (SVD) is a common issue at follow-up and can justify the need for a reintervention. In the evolving landscape of interventional cardiology, valve-in-valve transcatheter aortic valve replacement (ViV TAVR) has emerged as a remarkable innovation to address the complex challenges of patients previously treated with SAVR and has rapidly gained prominence as a feasible technique especially in patients at high surgical risk. On the other hand, the expanding indications for TAVR in progressively younger patients with severe aortic stenosis pose the crucial question on the long-term durability of transcatheter heart valves (THVs), as patients might outlive the bioprosthetic valve.
1. Introduction
2. ViV TAVR vs. Redo-SAVR: Evidence, Indications and Patients’ Selection
- -
-
patients at intermediate or low surgical risk;
- -
-
young individuals with longer life expectancy (because no data are available on long-term durability of ViV TAVR);
- -
-
patients with complex anatomical features for ViV TAVR, such as a high risk for coronary obstruction (without possibility of performing BASILICA) or with small anatomies.
- -
-
in cases of non-structural BHV dysfunction, such as patient-prosthesis mismatch (PPM) or severe PVL (percutaneous approach might be reasonable in cases of PPM when a balloon valve fracture might be performed within a stented surgical valve or in cases of PVL suitable for a percutaneous closure).
3. Lifetime Management of Patients with AS: TAV Surgical Explant vs. Redo-TAVR for Failed THVs
4. Pre-Procedural Planning
4.1. TAVR in SAVR
4.2. TAVR in TAVR
5. Procedural Scenarios and Possible Complications Management
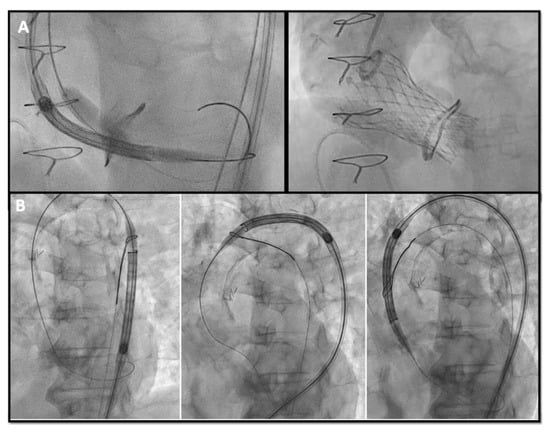
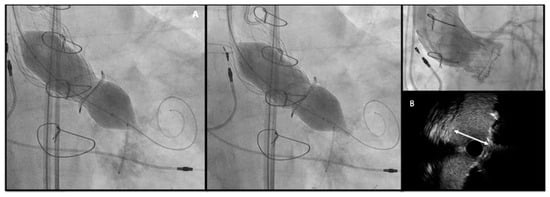
6. Main Concerns to Consider during VIV Procedures
-
Patient prosthesis mismatch (PPM)
-
Risk of coronary artery obstruction (CAO)
- -
-
Lower implantation of the THV: Preferring a SEF due to the possibility of re-capturing or checking coronary flow before definite deployment;
- -
-
Chimney snorkel stenting technique: Wiring the coronary artery and putting a stent on standby to be eventually implanted after THV deployment if the coronary flow is inadequate at angiographic control (Figure 3);
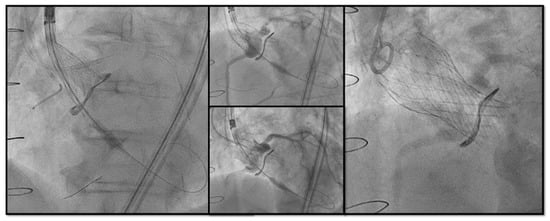 Figure 3. Double Chimney Stenting Technique with 4.0 × 33 Everolimus eluting stent implantation at ostial RCA and 4.0 × 18 at proximal left main before Corevalve Evolut Pro + 23 mm valve-in-valve implantation in a Mitroflow 23.
Figure 3. Double Chimney Stenting Technique with 4.0 × 33 Everolimus eluting stent implantation at ostial RCA and 4.0 × 18 at proximal left main before Corevalve Evolut Pro + 23 mm valve-in-valve implantation in a Mitroflow 23. - -
-
Orthotopic Snorkel Stenting Technique: Re-cannulation and wiring after THV release to have a more physiologic stenting through the prosthesis valve frame structure [27]
- -
-
BASILICA: Intentional laceration of surgical leaflets with an electrified guidewire to create a communication between the sinus and neo-sinus [28].
7. Conclusions
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227.
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632.
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or Biologic Prostheses for Aortic-Valve and Mitral-Valve Replacement. N. Engl. J. Med. 2017, 377, 1847–1857.
- Pibarot, P.; Herrmann, H.C.; Wu, C.; Hahn, R.T.; Otto, C.M.; Abbas, A.E.; Chambers, J.; Dweck, M.R.; Leipsic, J.A.; Simonato, M.; et al. Standardized Definitions for Bioprosthetic Valve Dysfunction Following Aortic or Mitral Valve Replacement: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 545–561.
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705.
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715.
- Latib, A.; Ielasi, A.; Montorfano, M.; Maisano, F.; Chieffo, A.; Cioni, M.; Mussardo, M.; Bertoldi, L.; Shannon, J.; Sacco, F.; et al. Transcatheter valve-in-valve implantation with the Edwards Sapien in patients with bioprosthetic heart valve failure: The Milan experience. EuroIntervention 2012, 7, 1275–1284.
- Gurvitch, R.; Cheung, A.; Ye, J.; Wood, D.A.; Willson, A.B.; Toggweiler, S.; Binder, R.; Webb, J.G. Transcatheter valve-in-valve implantation for failed surgical bioprosthetic valves. J. Am. Coll. Cardiol. 2011, 58, 2196–2209.
- Bedogni, F.; Laudisa, M.L.; Pizzocri, S.; Tamburino, C.; Ussia, G.P.; Petronio, A.S.; Napodano, M.; Ramondo, A.; Presbitero, P.; Ettori, F.; et al. Transcatheter valve-in-valve implantation using Corevalve Revalving System for failed surgical aortic bioprostheses. JACC Cardiovasc. Interv. 2011, 4, 1228–1234.
- Eggebrecht, H.; Schäfer, U.; Treede, H.; Boekstegers, P.; Babin-Ebell, J.; Ferrari, M.; Möllmann, H.; Baumgartner, H.; Carrel, T.; Kahlert, P.; et al. Valve-in-valve transcatheter aortic valve implantation for degenerated bioprosthetic heart valves. JACC Cardiovasc. Interv. 2011, 4, 1218–1227.
- Tarantini, G.; Nai Fovino, L.; Gersh, B.J. Transcatheter aortic valve implantation in lower-risk patients: What is the perspective? Eur. Heart J. 2018, 39, 658–666.
- Tarantini, G.; Dvir, D.; Tang, G.H.L. Transcatheter aortic valve implantation in degenerated surgical aortic valves. EuroIntervention 2021, 17, 709–719.
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857.
- Keshishi, M.; Fatima, R.; Seidman, M.A.; Butany, J.; Ouzounian, M.; Chung, J. Comparison of modes of failure and clinical outcomes between explanted porcine and bovine pericardial bioprosthetic valves. Cardiovasc. Pathol. 2023, 65, 107516.
- Bapat, V.N.; Attia, R.; Thomas, M. Effect of valve design on the stent internal diameter of a bioprosthetic valve: A concept of true internal diameter and its implications for the valve-in-valve procedure. JACC Cardiovasc. Interv. 2014, 7, 115–127.
- Landes, U.; Sathananthan, J.; Witberg, G.; De Backer, O.; Sondergaard, L.; Abdel-Wahab, M.; Holzhey, D.; Kim, W.K.; Hamm, C.; Buzzatti, N.; et al. Transcatheter Replacement of Transcatheter Versus Surgically Implanted Aortic Valve Bioprostheses. J. Am. Coll. Cardiol. 2021, 77, 1–14.
- Akodad, M.; Sellers, S.; Gulsin, G.S.; Tzimas, G.; Landes, U.; Chatfield, A.G.; Chuang, A.; Meier, D.; Leipsic, J.; Blanke, P.; et al. Leaflet and Neoskirt Height in Transcatheter Heart Valves: Implications for Repeat Procedures and Coronary Access. JACC Cardiovasc. Interv. 2021, 14, 2298–2300.
- Hayashida, K.; Lefèvre, T.; Chevalier, B.; Hovasse, T.; Romano, M.; Garot, P.; Mylotte, D.; Uribe, J.; Farge, A.; Donzeau-Gouge, P.; et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc. Interv. 2011, 4, 851–858.
- Bapat, V. Technical pitfalls and tips for the valve-in-valve procedure. Ann. Cardiothorac. Surg. 2017, 6, 541–552.
- Medda, M.; Casilli, F.; Tespili, M.; Bande, M. The “Chaperone Technique”: Valve-in-Valve TAVR Procedure with a Self-Expandable Valve Inside a Degenerated Sutureless Prosthesis. JACC Cardiovasc. Interv. 2020, 13, e15–e18.
- Rodés-Cabau, J.; Abbas, A.E.; Serra, V.; Vilalta, V.; Nombela-Franco, L.; Regueiro, A.; Al-Azizi, K.M.; Iskander, A.; Conradi, L.; Forcillo, J.; et al. Balloon- vs Self-Expanding Valve Systems for Failed Small Surgical Aortic Valve Bioprostheses. J. Am. Coll. Cardiol. 2022, 80, 681–693.
- Ribeiro, H.B.; Rodés-Cabau, J.; Blanke, P.; Leipsic, J.; Kwan Park, J.; Bapat, V.; Makkar, R.; Simonato, M.; Barbanti, M.; Schofer, J.; et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Insights from the VIVID registry. Eur. Heart J. 2018, 39, 687–695.
- Simonato, M.; Azadani, A.N.; Webb, J.; Leipsic, J.; Kornowski, R.; Vahanian, A.; Wood, D.; Piazza, N.; Kodali, S.; Ye, J.; et al. In vitro evaluation of implantation depth in valve-in-valve using different transcatheter heart valves. EuroIntervention 2016, 12, 909–917.
- Allen, K.B.; Chhatriwalla, A.K.; Saxon, J.T.; Huded, C.P.; Sathananthan, J.; Nguyen, T.C.; Whisenant, B.; Webb, J.G. Bioprosthetic valve fracture: A practical guide. Ann. Cardiothorac. Surg. 2021, 10, 564–570.
- Sreedharan, S.; Sellers, S.L.; Ihdayhid, A.R.; Landes, U.; Blanke, P.; Allen, K.B.; Chhatriwalla, A.K.; Pibarot, P.; Wood, D.A.; Webb, J.G.; et al. Bioprosthetic Valve Fracture to Facilitate Valve-in-Valve Transcatheter Aortic Valve Replacement. Structural Heart 2021, 5, 24–38.
- Attisano, T.; Bellino, M.; Vigorito, F.; Maione, A.; Ravera, A.; Pierri, A.; Baldi, C.; Galasso, G.; Vecchione, C.; Bonan, R. Bioprosthetic Valve Fracture After TAVR Complicated by Balloon Rupture: Bail-Out TAVR in TAVR in SAVR. JACC Case Rep. 2022, 4, 1277–1282.
- Burzotta, F.; Kovacevic, M.; Aurigemma, C.; Shoeib, O.; Bruno, P.; Cangemi, S.; Romagnoli, E.; Trani, C. An “Orthotopic” Snorkel-Stenting Technique to Maintain Coronary Patency During Transcatheter Aortic Valve Replacement. Cardiovasc. Revasc Med. 2021, 28s, 94–97.
- Damlin, A.; Meduri, C.; Manouras, A.; Verouhis, D.; Linder, R.; Rück, A.; Settergren, M. BASILICA Procedure Prior to Valve-in-Valve TAVR in a Supra-Annular TAV Prosthesis. JACC Case Rep. 2023, 11, 101777.




