Bioprosthetic heart valves (BHV) have been increasingly implanted instead of mechanical valves in patients undergoing surgical aortic valve replacement (SAVR). Structural valve deterioration (SVD) is a common issue at follow-up and can justify the need for a reintervention. In the evolving landscape of interventional cardiology, valve-in-valve transcatheter aortic valve replacement (ViV TAVR) has emerged as a remarkable innovation to address the complex challenges of patients previously treated with SAVR and has rapidly gained prominence as a feasible technique especially in patients at high surgical risk. On the other hand, the expanding indications for TAVR in progressively younger patients with severe aortic stenosis pose the crucial question on the long-term durability of transcatheter heart valves (THVs), as patients might outlive the bioprosthetic valve.
1. Introduction
Over the last decades, bioprosthetic heart valves (BHV) have been increasingly implanted instead of mechanical valves in patients undergoing surgical aortic valve replacement (SAVR).
Deciding between a mechanical and biologic aortic prosthesis is a daily discussion between the patient and surgeon. Data in the literature are controversial, with no unanimous watershed on indication for type of prothesis to implant in candidates for surgery. Indeed, American Heart Association (AHA) Guidelines [
1] recommend mechanical aortic prosthesis in the absence of contraindications to anticoagulation in patients younger than 50 years of age (Class of Recommendation COE 2a, Level of evidence LOE B-R). This threshold raises to 60 years in the European Guidelines (COR IIa LOE B) [
2].
Although the main obstacle to mechanical prostheses is the difficult management of a life-long warfarin therapy, often contraindicated in some patients [
3], BHVs tend to fail early and often requires reintervention [
4].
On the other side, TAVR is established for the treatment of severe symptomatic aortic stenosis (AS) in patients at high surgical risk and recently showed favorable outcomes in patients at lower surgical risk [
6,
7]. In the evolving landscape of interventional cardiology, (ViV) TAVR has emerged as a remarkable innovation to address the complex challenges of patients previously treated with SAVR and has rapidly gained prominence as a feasible technique in the management of failed surgical BHVs. ViV TAVR is considered to be a safe and effective therapeutic option in patients at high surgical risk [
1,
2].
2. ViV TAVR vs. Redo-SAVR: Evidence, Indications and Patients’ Selection
To date, no randomized clinical trial (RCT) has compared ViV TAVR and redo-SAVR in patients with failed surgical BHVs. Several observational studies showed the feasibility of ViV TAVR with both balloon-expandable (BE) and self-expandable (SE) THVs in patients who underwent previous SAVR with BHVs [
10,
11,
12,
13].
Overall, even in absence of RCT, these data indicate ViV TAVR as a safe and effective strategy for patients deemed at high or prohibitive surgical risk.
Redo-SAVR is still considered in peculiar conditions:
- -
-
patients at intermediate or low surgical risk;
- -
-
young individuals with longer life expectancy (because no data are available on long-term durability of ViV TAVR);
- -
-
patients with complex anatomical features for ViV TAVR, such as a high risk for coronary obstruction (without possibility of performing BASILICA) or with small anatomies.
- -
-
in cases of non-structural BHV dysfunction, such as patient-prosthesis mismatch (PPM) or severe PVL (percutaneous approach might be reasonable in cases of PPM when a balloon valve fracture might be performed within a stented surgical valve or in cases of PVL suitable for a percutaneous closure).
3. Lifetime Management of Patients with AS: TAV Surgical Explant vs. Redo-TAVR for Failed THVs
Over the last years, TAVR has been increasingly adopted for the treatment of severe AS, with a progressive decrease in the mean age and surgical risk of patients [
8]. Given the longer life expectancy of these patients, the incidence of THV failure is likely to increase in the next years.
The main treatment options for failed THVs are TAVR surgical explant and redo-TAVR within the previously implanted THV. The choice between the two treatment modalities should be tailored to the individual anatomical and clinical characteristics of the patient, including mechanism and timing of THV failure, aortic root anatomy, risk of coronary obstruction, type of failed THV and surgical risk profile.
In certain circumstances, such as endocarditis or severe PPM, redo-TAVR is not feasible and TAVR surgical explant is the only therapeutic option. TAVR explant is technically more complex and has a higher rate of perioperative complications compared to more common SAVR.
Redo-TAVR is a reasonable therapeutic strategy in patients with THV failure, especially in those with SVD. It is an area of ongoing research, but, nowadays, the data on long-term clinical outcomes are still scarce. There are two main observational studies assessing the safety and efficacy of redo-TAVR in the real world. In an analysis from the Medicare database on 617 patients undergoing redo-TAVR in the U.S. between 2012 and 2017 (0.46% of all TAVR procedures), the mortality rates at 30 days and 1 year were 6.0% and 22.0%, respectively.
4. Pre-Procedural Planning
4.1. TAVR in SAVR
The main limitations of ViV TAVR are related to a lack of space in the aortic root or to the mechanical complications potentially deriving from the deflection of surgical bioprosthetic valve leaflets. The former lead to high gradients and significant prosthesis-patient mismatch (PPM) and the latter to coronary obstruction or disruption [
22]. Echocardiography and ECG-gated computer tomography (ECG-gated CT) allow us to assess surgical bioprosthetic valve dysfunction and the best planning for redo intervention.
Structural valve deterioration (SVD) is defined as intrinsic permanent changes to the prosthetic valve, including wear and tear, leaflet disruption, flail leaflet, leaflet fibrosis and/or calcification, and strut fracture, manifested as stenosis and/or regurgitation [
23].
Bovine pericardial valves tend to develop stenosis, while porcine valves tend to develop regurgitation [
24]. Each model comes with a manufacturer labelled size which, in most cases, does not represent the true internal diameter (ID) of the prosthesis [
25]. Stented bioprostheses usually have internally mounted leaflets generating a smaller true inner diameter (ID) than the one reported on the label and this can lead to oversize the ViV-TAVR.
Conversely, stentless bioprostheses have less risk of higher gradients, but the lack of fluoroscopic markers increases the risk of malpositioning, leaks and coronary obstruction.
4.2. TAVR in TAVR
Preprocedural planning of a redo-TAVR requires a detailed analysis of the failed THV characteristics and patients’ anatomy. Current data are encouraging, but more data are needed on the best approach to plan and perform redo-TAVR [
28]. Redo-TAVR is indicated for SVD according to the Valve Academic Research Consortium-3 definitions cited above [
23]. As per surgical valves, careful analysis of the index THV characteristics, mechanism of failure and a detailed assessment of the patient anatomy is needed.
The specific index THV dimensions given by manufacturers in the technical sizing chart are helpful to plan redo TAVR, but only ECG-gated CT will give the actual dimensions specific to the patient.
THV can be classified in balloon-expandable valves (BEV), mechanically expandable valves (MEV), and self-expanding valves (SEV). BEV and MEV have low stent frames and their leaflets are in intra-annular position, whereas SEV may have low or high stent frames and their leaflets are intra-annular and supra-annular, respectively.
When the redo THV is deployed, the failed THV leaflets are ‘pinned open’ creating a ‘neoskirt’. The neoskirt height will depend on the length of the stent frame and the height of the implant. These technical aspects have implications for the risk of coronary obstruction and must be considered when choosing the valve to use [
30].
High risk features for TAVR in TAVR, such as endocarditis/valve thrombosis, severe PPM or significant paravalvular leaks not amenable of percutaneous treatment, and presence of other cardiac lesions needing cardiac surgery can be assessed in transthoracic and transoesophageal echocardiography [
30].
5. Procedural Scenarios and Possible Complications Management
Optimal procedural execution, following a step-by-step codified approach, is crucial for the success of ViV procedures.
Most of ViV TAVR can be performed under conscious sedation with local anesthesia at site of puncture. Femoral access is preferred in most of the anatomies with 5 mm accepted as the minimum lumen diameter for the smallest transcatheter aortic valves (THV). A lumen diameter lower than 5 mm is associated with increased risks and the risks raise steeply when the sheath-to-femoral-artery ratio (SFAR) is more than 1.05 [
32].
Regardless of the artery size, one should carefully consider the extent and the distribution of arterial wall calcifications. When the arterial wall is elastic with minor calcifications, a SFAR of 1.2 is acceptable and does not carry major risks. On the other hand, when the arterial wall is highly calcific, especially with circumferential distribution of calcium, the SFAR cut-off becomes less permissive.
When percutaneous femoral access is not feasible at all, trans-subclavian (TS), trans aortic (TAO), trans-apical (TA), trans-caval (TC) and trans-carotid routes might be considered [
34].
A secondary vascular access, typically radial or contralateral femoral artery, is also required for basal aortography or for cannulation when coronary artery protection is needed (potentially with a stand-by stent on a coronary protection wire).
In case of SHV, the latter is crossed with Terumo straight wire supported by an AL1 or a JR4 catheter, according to different anatomies, then changed with a Safari2™ Pre-Shaped TAVI Guidewires (Boston Scientific, Miami, FL, USA) which allows enough support to advance the new THV.
Sometimes advancing the THV in the SHV can be challenging, especially when the SHV is stenotic. Different techniques and maneuvers to facilitate advancing the THV have been described such as: (A) the “buddy wire technique”, placing two safari wires in the LV to give more support to the advancing device; (B) the “buddy balloon technique” in case of calcific or tortuous segment, if a crystal balloon is used as a buddy to provide taper to the distal end of the delivery system and facilitate crossing; (C) the “snare technique” also described as the chaperone technique [
35], when a snare is inserted through the contralateral femoral artery capturing the safari wire to achieve better alignment of the delivery system within the SHV (
Figure 5).
Figure 5. (A) buddy-balloon technique with VACS II 16 mm balloon inflation parallel to the Corevalve delivery to perform ViV in a Sorin Mitroflow 23 mm; (B) transfemoral TAVR with snare technique in a severe calcific native aortic valve stenosis VIV: valve-in-valve; TAVR: transcatheter aortic valve replacement.
6. Main Concerns to Consider during VIV Procedures
PPM can be considered the “Achilles’ heel” of VIV procedures and it is more frequent with small SHV, such as Mitroflow 21 mm (Sorin Group), Mosaic 19 mm and 21 mm (Medtronic, Minneapolis, MN, USA), TrifectaTM 21 mm (St. Jude Medical, St. Paul, MN, USA).
Self-expanding THV with supra-annular design: When feasible, are the preferred choice in small SHV. Rodés-Cabau et al. [
37], in their randomized trial comparing balloon vs. self-expanding valve systems in failed small SHV, founded an association between supra-annular SEV and improved hemodynamics with lower PPM.
-
Higher THV deployment (even if with a mild higher risk of coronary obstruction or valve embolization): As suggested in the VIVID registry [
27] and confirmed by Simonato et al. [
38] with their in vitro analysis. Considering the two most used THVs, the optimal implantation depth is 0 to 5 mm for the Corevalve and 0–2 mm up to 3.5 mm (0%–10% device frame) for Sapien THV.
-
Bioprosthetic valve fracture (BVF) and bioprosthetic valve remodeling (BVR) [39]: By either fracturing or stretching the surgical valve ring, providing increased THV expansion, better sealing, and post implantation hemodynamics. BVF is performed using a non-compliant balloon such as True Dilatation or Atlas Gold (Bard, Murry Hill, NJ, USA); (II) a high-pressure stopcock and tubing; (III) an indeflator and; (IV) a 60-mL syringe with dilute contrast. After initiating rapid ventricular pacing, the non-compliant balloon is rapidly inflated with 60 mL dilute contrast until fracture occurs. The best confirmation that BVF has occurred is by (A) angiographic modification of balloon waist and THV geometry, (B) a pressure drop in the in deflator or (C) an audible click concomitantly with the fracture.
-
The timing of BVF, either before or after TAVR, remains controversial. BVF after THV implantation leads to better hemodynamics but carries a risk of damaging new prosthesis; BVF before THV implantation ensures better sealing, but may cause embolization of SHV, acute valvular regurgitation and hemodynamic instability [
40,
41]. The general practice is to do BVF after THV if using BEV so the NC balloon simultaneously fully expand the THV and fracture the surgical prosthesis while SEVs may not have enough force to fully expand a degenerated SHV and will benefit from balloon fracture before and if needed, even after implantation (
Figure 6).
Figure 6. (A) Bioprosthetic valve fracture with a True Balloon 20 balloon after valve-in-valve with Corevalve Evolut Pro + n.23 implantation in a Mitroflow 19 (LCA protection without final stent implantation) (B) IVUS shows patency of the VS with a minimum distance between aortic wall and prosthetic leaflet of 2.2 mm at STJ. LCA: left coronary artery; IVUS: intra-vascular ultrasound; STJ: sino-tubular junction distance.
During a VIV procedure, the risk of CAO is four times greater than in TAVR due to the displacement of native valve leaflets towards the coronary ostia. The factors to consider are SHV type, coronary height, sinus of Valsalva (SOV) dimensions and VTC defined by the distance between the virtual ring of the fully expanded THV, simulated into the previous SHV and the coronary ostium.
When the estimated risk of coronary occlusion is high, the following procedural strategy may be considered:
- -
-
Lower implantation of the THV: Preferring a SEF due to the possibility of re-capturing or checking coronary flow before definite deployment;
- -
-
Chimney snorkel stenting technique: Wiring the coronary artery and putting a stent on standby to be eventually implanted after THV deployment if the coronary flow is inadequate at angiographic control (Figure 7);
Figure 7. Double Chimney Stenting Technique with 4.0 × 33 Everolimus eluting stent implantation at ostial RCA and 4.0 × 18 at proximal left main before Corevalve Evolut Pro + 23 mm valve-in-valve implantation in a Mitroflow 23.
- -
-
Orthotopic Snorkel Stenting Technique: Re-cannulation and wiring after THV release to have a more physiologic stenting through the prosthesis valve frame structure [
42]
- -
-
BASILICA: Intentional laceration of surgical leaflets with an electrified guidewire to create a communication between the sinus and neo-sinus [
43].
7. Conclusions
Fifteen years after the first case, ViV-TAVR has now become a safe and effective procedure for many patients with both failed stenotic or regurgitant bioprosthetic valves. Pre-procedural multimodality imaging assessment is crucial for the best THV selection and implantation strategy. However, some pitfalls remain to be overcome, such as device malpositioning, ostial coronary obstruction, and vascular complications. In this sense, the development of new advanced and safer technologies and growing expertise of interventionalists could be the key to optimize percutaneous treatment and make it preferential to a re-do surgery.
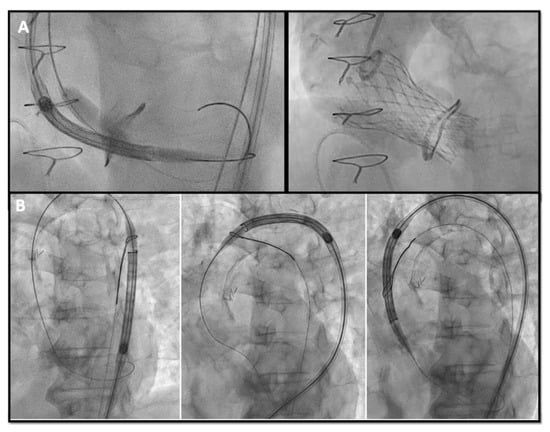
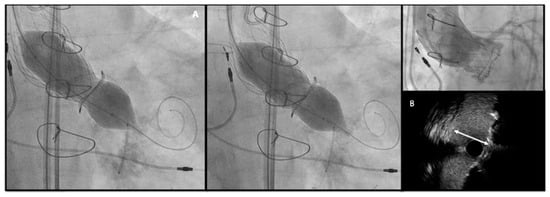
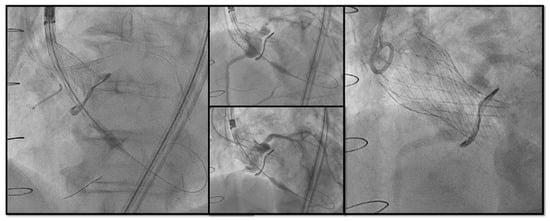 Figure 7. Double Chimney Stenting Technique with 4.0 × 33 Everolimus eluting stent implantation at ostial RCA and 4.0 × 18 at proximal left main before Corevalve Evolut Pro + 23 mm valve-in-valve implantation in a Mitroflow 23.
Figure 7. Double Chimney Stenting Technique with 4.0 × 33 Everolimus eluting stent implantation at ostial RCA and 4.0 × 18 at proximal left main before Corevalve Evolut Pro + 23 mm valve-in-valve implantation in a Mitroflow 23.
