Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leyre Pérez-Álvarez | -- | 3748 | 2024-03-14 09:14:35 | | | |
| 2 | Lindsay Dong | Meta information modification | 3748 | 2024-03-14 09:31:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rosales-Murillo, S.; Sánchez-Bodón, J.; Hernández Olmos, S.; Ibarra-Vázquez, M.; Guerrero-Ramírez, L.; Pérez-Álvarez, L.; Vilas-Vilela, J. Anthocyanin-Based Polymers for Healthcare Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/56245 (accessed on 07 February 2026).
Rosales-Murillo S, Sánchez-Bodón J, Hernández Olmos S, Ibarra-Vázquez M, Guerrero-Ramírez L, Pérez-Álvarez L, et al. Anthocyanin-Based Polymers for Healthcare Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/56245. Accessed February 07, 2026.
Rosales-Murillo, S.s., Julia Sánchez-Bodón, S.l. Hernández Olmos, M.f. Ibarra-Vázquez, L.g. Guerrero-Ramírez, L. Pérez-Álvarez, J.l. Vilas-Vilela. "Anthocyanin-Based Polymers for Healthcare Applications" Encyclopedia, https://encyclopedia.pub/entry/56245 (accessed February 07, 2026).
Rosales-Murillo, S., Sánchez-Bodón, J., Hernández Olmos, S., Ibarra-Vázquez, M., Guerrero-Ramírez, L., Pérez-Álvarez, L., & Vilas-Vilela, J. (2024, March 14). Anthocyanin-Based Polymers for Healthcare Applications. In Encyclopedia. https://encyclopedia.pub/entry/56245
Rosales-Murillo, S.s., et al. "Anthocyanin-Based Polymers for Healthcare Applications." Encyclopedia. Web. 14 March, 2024.
Copy Citation
Anthocyanins are a specific group of molecules found in nature that have recently received increasing attention due to their interesting biological and colorimetric properties that have been successfully applied in several fields such as food preservation and biomedicine. Meanwhile, the incorporation of anthocyanins into polymeric systems has become an interesting strategy to widen the applicability of these molecules and develop new smart and functional polymers in the above-cited areas.
anthocyanins
polymers
food packaging
healthcare
1. Introduction
Anthocyanins are non-toxic flavonoids pigments that are widely distributed in nature, offering attractive colors to the flower petals and fruits of some plants, such as bright orange, pink, scarlet, red, mauve, violet and blue. The term “anthocyanin” comes from the Greek words anthos (“flower”) and kyanos (“blue”), which was originally used to describe the blue pigment in cornflowers. Anthocyanins are positively charged plant-derived molecules classified as the largest group of water-soluble pigments. They can be found accumulated in the vacuoles of epidermal or subepidermal cells in plant organs such as roots and leaves [1]. They are present in fruits such as blueberries, strawberries, bananas, apples, blackberries, and grapes, to name a few, as well as in vegetables like purple cabbage, red onions, and eggplants [2].
These compounds belong to the flavonoid family. Flavonoids are crucial for plant development and proper functioning due to their role in attracting animals for oviposition and protecting against UV light or infections by phytopathogenic organisms [3]. In addition, flavonoids present remarkable properties related to human health, including antioxidant, anti-inflammatory, antidiabetic, chemopreventive, and antimutagenic properties, among others [4]. Consequently, their ongoing consumption is regarded as a beneficial health promotion practice and a preventive measure against numerous diseases. However, anthocyanins face a challenge in terms of absorption by the human body. The body rapidly metabolizes and excretes anthocyanins, reducing their beneficial activity [5]. Beyond their low bioavailability, anthocyanins also have low stability and are highly sensitive to external factors, making them susceptible to decomposition [6].
Within anthocyanins’ characteristics, they are known to be water- and alcohol-soluble organic compounds that have a three-ring heteroaromatic polyphenolic skeleton. Anthocyanins are glycosylated analogues, mainly at the C3 position, of anthocyanidins, both based on the 2-phenyl-benzopyrilium chromophore structure (flavylium ion in Figure 1), which shows an extended π conjugation, as well as the presence of a positive charge and several free -OH groups. These characteristics allow anthocyanins to absorb light in the visible region, which generates a great variety of dye colors, making them one of the most important natural pigments besides chlorophyll.
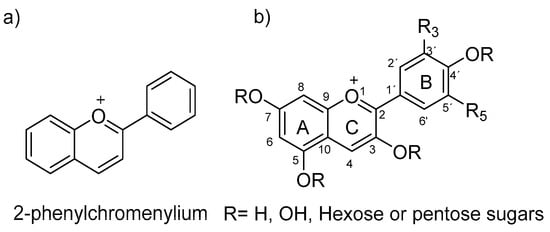
Figure 1. (a) Flavylium ion structure; (b) common numbering of flavylium ion.
Anthocyanidins possess an almost planar chemical structure characterized by variations in the number and positioning of hydroxyl groups and/or methoxy groups. These structural differences play a crucial role in determining the natural availability of a diverse array of anthocyanins and anthocyanidins [7]. Anthocyanidin glycosides are 3-monoglucosides and 3,5-diglucosides, consisting of the most common sugar, glucose; however, rhamnose, xylose, galactose arabinose, and rutinose can also appear [8].
Depending on conditions such as temperature, light, solvent, metal ions, and mainly pH, anthocyanins undergo structural modifications accompanied by photophysical and chemical changes. In fact, different proton concentrations can lead these molecules to hydrated open ring or quinoidal forms [9]. Anthocyanins are very stable at acidic pHs, but as the pH approaches neutral, their stability dramatically decreases, leading to complete degradation at pHs above 7. The kinetics of these changes is one of the main factors that determines the final color of these compounds [10].
Generally, a molecule with more -OH groups generates a more intense blue color, while in those with -OR groups, the coloration turns red. As for the glycosyl substituents, their presence decreases the coplanarity of the B-ring, so they tend to be less stable, and the coloration is usually not as intense.
If, in addition to sugar, there is an acyl radical in the molecule, they are called acylated anthocyanins. An increase in glucosidic substitution and acylation with cinnamic acids allows them to be more stable and retain their characteristic color at alkaline pHs [11].
Anthocyanins have received increasing attention from researchers in recent years mainly due to their importance in two main application sectors: the food industry and healthcare. Regarding food technology, the utility of anthocyanins extends beyond their role as a natural colorant to enhance the organoleptic properties of food. Their inherently reactive nature has led to their consideration as colorimetric indicators for assessing food quality in advanced smart food packaging technologies. Regarding healthcare and pharmaceutical applications, a large amount of highly interesting health-promoting effects of anthocyanins have been revealed in the last decade. Thus, the nutraceutical effect of the anthocyanin or anthocyanin-rich food intake has attracted research interest. However, their low stability and bioavailability hinder an efficient effect of anthocyanins in the human body. Consequently, research efforts have focused on the development of strategies to improve their stability, bioavailability, and color preservation. Among these strategies, encapsulation has become the most effective and explored one. Encapsulation is based on the development of a protective coverage of anthocyanins, which, in addition to improving their stability and bioavailability, could also provide advanced properties or performance (like selective or controlled release).
Accordingly, there exists a real need for immobilizing anthocyanins within appropriate substrates that fulfill the wide range of physical and chemical requirements specified for the mentioned applications. Among these substrate systems, polymers are versatile materials that show unique properties in a wide range of forms, like films, emulsions, hydrogels, mats, and nanoparticles, among others, that are of great interest in the food-packaging and healthcare sectors. In the light of this combination, the incorporation of anthocyanins within polymeric matrices has proliferated in recent years as a promising approach to utilize the synergistically positive properties of both substrates and active agents.
2. Anthocyanin-Loaded Polymers: Preparation and Characterization Methods
2.1. Films
Anthocyanin-loaded polymer films are obtained mainly by solution casting, a simple and cheap technique that uses aqueous solutions or organic solvents to dissolve anthocyanins as the active agent and polymers as the substrate for immobilization [12]. In brief, polymer/s, plasticizer, and an anthocyanin extract are sequentially dissolved in solution at temperatures lower than 80 °C for a short period of time to minimize the degradation of the anthocyanins. Finally, the obtained solution is incubated onto flat Petri dishes at ~25–40 °C for 24–48 h. Other more sophisticated and expensive techniques have also been reported to fabricate anthocyanin films, such as electrospinning and layer-by-layer and 3D printing [13]. Chitosan, cellulose, starch, zein, gelatin, pectin, agarose, xanthan gum, and combinations of these have been the most commonly employed polymer systems for the development of anthocyanin-loaded films. Glycerol and sorbitol are the most popular plasticizers used in anthocyanin-based films [14].
Regarding films for monitoring food spoilage, it has been shown that combining polymers improves the physical properties of the material. For example, Nadi et al. [15] showed that combining basil seed gum with chitosan and adding red cabbage extract as a colorimetric indicator improves properties such as solubility, water vapor permeability, and flexibility in the material.
The incorporation of anthocyanins into films can modify the technological and functional properties of the film. These changes depend on the physical or chemical interactions between the film-forming polymer and the anthocyanin extract, which can lead to structural modifications. The interaction between biopolymers and anthocyanin-rich extracts depends on the nature, chemical characteristics, and concentration of both the polymer and extract, as well as the structural properties of the active components. The addition of anthocyanin-rich extracts can influence the thickness, color, opacity, solubility, water vapor permeability, and mechanical properties of the films [16]. Thickness is typically analyzed because, in most cases, it is modified and, since it hinders the penetration of water, it is a key parameter to take into consideration [13].
2.2. Mats and Fibers
It is known that nanofibrous mats have a high surface-to-volume ratio, small pore size, and high porosity compared to polymer films, which makes electrospun nanofibrous mats very attractive materials for the manufacture of substrates for the immobilization of anthocyanins [17].
The electrospinning process involves the application of an electric field to create fine polymer fibers from a solution containing anthocyanins. Anthocyanins can decrease the conductivity of the polymer solution, and consequently, as Jiang et al. [18] observed by SEM microscopy, heterogeneous fibers are formed.
Anthocyanin nanofiber mats made by electrospinning are used as tools in the field of active/smart packaging where they have been studied to improve their stability, simplify the slow and controlled release of antimicrobial and antioxidant substances [19], and as spoilage sensors [20]. When it comes to healthcare applications, nanofibrous mats of anthocyanins have found their major application as smart wound dressings that can monitor the wound healing process [21].
2.3. Hydrogels
Hydrogels have been widely used as a method of encapsulating water-soluble active ingredients. The thermo-degradation and stability against sudden pH changes of anthocyanins could be inhibited by encapsulating them within hydrogel systems. This has been demonstrated in the process of simulated digestion and may be very useful in the prevention of intestinal diseases [22][23].
Recent research has demonstrated the versatility of these materials that allow the easy incorporation of multiple active agents. For instance, Lotfinia et al. [24] combined alginate in the form of a hydrogel with honey and red cabbage extract, and could observe improved mechanical properties, the antibacterial activity attributed to the honey, as well as the antioxidant properties and good activity against pH changes from the red cabbage.
Polysaccharides have been traditionally employed in anthocyanin hydrogel preparations due to the simplicity of their corresponding gelation process. This is the case for alginate, which forms cold gels through Ca2+-induced cross-linking [25], or starch [26], another widely used material for these gel-based encapsulation systems.
2.4. Polyelectrolyte Complexes
Polyelectrolytes are macromolecular materials that possess repeating units and dissociate into highly charged polymeric molecules in aqueous solution, forming either positively or negatively charged polymeric chains [27]. There are numerous compounds that can serve as biopolyelectrolytes such as proteins, polysaccharides, and their derivatives. Biopolyelectrolyte complexes can be formed by the titration of a biopolyelectrolyte solution in another biopolyelectrolyte solution with the opposite charge under agitation. Among the advantages of this methodology are the simplicity, quickness, and the fact that it does not require high energy, chemical crosslinkers, specialized equipment, or organic solvents. An advantage of these materials is that polymeric chains can generate dense interconnecting networks, which is useful for inhibiting the penetration of polar reactive compounds and the attack on charged anthocyanins [28].
2.5. Nanoparticles
As in the general case of polymeric nanoparticle preparations, the methods commonly used for the preparation of anthocyanin nanocarriers can be classified as the emulsification cross-linking method, ionic cross-linking method, covalent cross-linking method, and self-assembly methods.
The emulsification crosslinking method is a widely used method. A carrier particle is usually large (tens to hundreds of microns) with a highly homogeneous particle distribution. Anthocyanin nanoparticles are divided into ionically crosslinked and covalently crosslinked depending on the crosslinking method. Covalently cross-linked carriers are more stable. Studies have shown that the type of polymer employed has a relevant effect on the crosslinking efficiency.
Ionic nanoparticles could be easily formulated through complexation of two different charged biopolymers in diluted solutions. Biopolymers such as alginate, chitosan, whey, and soy protein are commonly extruded as a carrier solution through a needle or nozzle into a gelation solution containing the specified ions. This is a mild process that has shown a high encapsulation efficiency and effective protection of anthocyanins under gastric conditions [29].
2.6. Emulsions
Emulsion-based systems are intended to overcome the low stability and bioavailability of anthocyanins. Among the most used systems are nanoemulsions and microemulsions that have been studied in vivo and in vitro as anthocyanin delivery systems [30][31][32][33].
Nanoemulsions are colloidal dispersions formed by droplets of a liquid dispersed in another liquid that, despite being immiscible, can be stabilized with a surfactant layer. In nanoemulsions, particles showing sizes from 20 to 500 nm can avoid emulsification, flocculation, or precipitation during storage [34][35][36]. Some of the benefits of nanoemulsions are the control of the release rate and the avoidance of decomposition or degradation of the encapsulated anthocyanins [37].
Microemulsions are mainly composed of water, oil, surfactants, and cosurfactants, and have better flowability, more uniform particle dispersion, and stronger stability than emulsions. Microemulsion particles have sizes between 10 and 100 nm, which can provide stable and uniformly dispersed systems [38], which are useful properties for improving the absorption and bioavailability of anthocyanins and enable easy and multiple routes of administration [39][40].
There are several studies that report the development of nanoemulsions and microemulsions with extracts from different sources of anthocyanins, such as mangosteen peel, Brazilian berry, purple sweet potato, cranberry, red cabbage, blueberry, and jaboticaba peel, among others, showing nanoparticles that are stable for long periods of time, ranging from 30 days to 3 months [32][37][38][41][42].
2.7. Self-Assembled Liposomes, Proteins, Peptides, and Phospholipids
Today, liposomes, phospholipids, and proteins are used as anthocyanin nanocarriers and are formed by direct self-assembly. Proteins, as was commented above, have the drawback of being very sensitive to pH changes or temperature. Liposomes also have low stability and their phospholipids are prone to oxidation during long-term storage. Phospholipids have a lot of benefits including the ability to protect sensitive ingredients, increase the bioavailability of nutrients and the efficacy of food additives, and confine undesirable flavors [43].
Amphiphilic peptides with a hydrophobic tail and a hydrophilic head are also used in molecular self-assembly. These amphiphilic peptides have good biocompatibility, biodegradable self-assembly, and chemical variability, which leads to a variety of nanostructures. Indeed, the addition of peptides to foods containing anthocyanins is recognized as the simplest way to improve the color stability of anthocyanins [37][43][44].
2.8. Microencapsulates
Microencapsulation consists of the incorporation of the active agent within the encapsulants by noncovalent interactions formed during a mixing process that can include additional coating layers [29]. The final drying step generally takes place by a freeze-drying or spray-drying methodology. Microencapsulation is an alternative to keep the properties of the anthocyanins intact and offer food processors a means of protecting sensitive food components [45][46][47][48][49][50]. However, during the spray drying process, some heat-sensitive anthocyanins may lose their activity or degrade and, as a consequence of the rapid dehydration, they may change their crystal structure. Consequently, freeze-drying is often preferred to mitigate these potential issues and preserve the integrity of the anthocyanins [51].
Microencapsulation allows the encapsulation of anthocyanins within a wide variety of encapsulating agents, such as polysaccharides, starches, inulin, maltodextrin or dextrose, corn syrups, arabic gum, mesquite gum, lipids, and proteins [52].
2.9. Specific Characterization of Anthocyanin-Loaded Polymer
The characterization of anthocyanin-loaded polymers is crucial for understanding their structure and properties. Several techniques are used to obtain significant information on these materials.
UV-Vis spectroscopy is used to analyze the absorption spectra of these materials, providing information about their specific composition, color, stability, and optimum absorption range [53].
FTIR-ATR is also a useful technique because it provides information regarding the interactions between the anthocyanin and the polymer matrix. The main changes are reported in two regions, between 1500 and 1600 cm−1, corresponding to the C=C bonds, and around 3200 cm−1, corresponding to the vibrations of the C-H bonds. In both cases, if the signals are shifted and/or widened, it confirms the immobilization of the anthocyanins in the polymer due the formation of strong interactions with the polymers [12][54][55].
Using Thermo-Gravimetric Analysis (TGA), several studies have shown that the addition of anthocyanins to polymeric matrices or the encapsulation of anthocyanins in a polymer provides greater stability with respect to pure compounds [56][57][58].
3. Anthocyanin-Based Polymers for Healthcare Applications
Owing to the vibrant and diverse color spectrum of anthocyanins, they have found multiple applications as natural dyes in various healthcare-related fields. These natural compounds present an intriguing potential as safe and biocompatible alternatives to synthetic dyes. Due to their ability to exhibit color shifts under different conditions, their potential use as biosensors has garnered attention in recent years. In addition, anthocyanins show excellent antioxidant and anti-inflammatory effects as well as potential in the prevention of cancer, neurodegenerative diseases, and diabetes, making them very interesting natural bioactive compounds. However, their limited bioavailability remains as one of the main disadvantages for this family of compounds. Therefore, the development of systems to increase the bioavailability and, consequently, the efficacy of anthocyanins is desirable.
3.1. Biosensors
Anthocyanins exhibit changes in their chemical structure in response to varying hydrogen ion concentrations. This characteristic has garnered extensive attention for utilizing anthocyanin pigments as key components in biocompatible pH sensors. Riaz et al. [59] studied the use of anthocyanins on contact lenses to monitor the ocular pH, a crucial parameter in evaluating ocular health post-eye surgery in conditions like keratoconjunctivitis and ocular rosacea. For this purpose, commercially available lacreon and lacreon-free contact lenses were functionalized with the anthocyanins obtained from Brassica oleracea by soaking and drop casting methods. The lacreon contact lenses demonstrated enhanced and uniformly distributed coloration that was noticeable to the naked eye within the physiological pH range of 6.5 and 7.5.
Similarly, Alsahag et al. [60] developed an economical, reversible, eco-friendly wound dressing based on anthocyanins in order to monitor wound healing progress. This innovative bioassay detected pH changes in a simulated wound solution, which were indicated by shifts in color. They employed anthocyanins sourced from Brassica oleracea and L. var capitate that were integrated onto a carboxymethyl cellulose/polyvinyl alcohol composite. The comfort and durability of these composites were confirmed through favorable colorfastness, air permeability, and bend length. In terms of biological properties, these composites exhibited non-cytotoxic effects and enhanced antimicrobial properties. This colorimetric assay offers an affordable, used-friendly sensor for monitoring wound healing progress. In contrast to prior electric-based sensing tools requiring complex equipment, this chromogenic sensor enables onsite wound pH measurements without intricate procedures.
Another example of anthocyanin-based materials used as biosensors was reported by Al-Qahtani et al. [61]. They encapsulated both natural anthocyanin and urease enzyme in a calcium alginate biopolymer, which were then immobilized within the fabric. The resulting bio-chromic sensor offered quick responses with a detection limit of 300–1000 ppm for urea. This innovative reversible sensor employs urease to convert urea to ammonia, allowing for easy urea detection via the encapsulated anthocyanin pH indicator from red cabbage. This method provides an efficient, eco-friendly, and selective colorimetric approach for measuring urea leve
3.2. Nanoencapsulated Delivery Systems
Taking into account the inherent toxicity associated with numerous synthetic drug compounds, the discovery and development of novel and efficient bioactive natural compounds like anthocyanins is highly desirable. However, since these natural phytochemicals are remarkably sensitive to external environmental factors, resulting in a notably brief half-life, as has been described above, the development of innovative delivery systems that can provide stability to anthocyanins without compromising their bioactivity is crucial. Indeed, it is essential to achieve kinetic and thermodynamic stability while simultaneously enhancing solubility and improving bioavailability [62].
Among the diverse array of alternatives explored, encapsulation has garnered substantial attention in recent decades, showcasing immense potential in various sectors, including the pharmaceutical and nutraceutical industries [63]. Furthermore, nanoencapsulation strategies exhibit interactions, such as Van der Waals forces, hydrophobic interactions, and hydrogen bonding, between nanocarriers and natural compounds like anthocyanins, ensuring enhanced material stability and increased bioavailability. Indeed, these advantages endow nanomaterials with the remarkable ability to easily traverse the blood–brain barrier, thereby magnifying the therapeutic potential of the encapsulated molecules [62].
Owing to the defined chemical structure, synthetic polymers offer control over their physical and chemical properties compared to natural polymers. Conversely, bio-based polymeric nanoencapsulations offer key advantages for use in biomedical applications as they present remarkable biocompatibility across a wide range of concentrations and are often cost-effective [64]. In nanomedicine, these polymers predominantly fall into two subcategories: polysaccharides and proteins. It is important to highlight that certain polysaccharides are commonly used alongside anthocyanins due to their inherent charged form. This ionization enables effective interaction with oppositely charged phospholipids, effectively preventing phospholipid hydrolysis under acidic pH conditions or in the presence of enzymes. Chitosan and pectin stand out among these polysaccharides as the most commonly used due to their non-toxic, eco-friendly, biodegradable nature and their high biocompatibility. Apart from these inherent advantages, they demonstrate considerable potential as an efficient drug delivery system, particularly for targeting the colon. For instance, Zhao et al. [65] studied the activity of anthocyanins encapsulated in pectin and chitosan and the controlled release from these nanoparticles, demonstrating enhanced protection for normal rat kidney cells against acrylamide-induced damage. Additionally, they decrease reactive oxygen species as well as matrix metalloproteinases and glutathione levels, providing protection to normal human hepatocyte L02 cells against palmitic acid-induced damage. Nevertheless, it has to be noted that chitosan dissolves only in certain dilute acidic conditions.
Another promising polysaccharide explored for the encapsulation of anthocyanins is chondroitin sulfate. Jeong et al. [66] reported the synthesis of black soybean anthocyanins loaded at different concentrations onto chondroitin sulfate polysaccharides in order to improve the structural stability of this natural antioxidant. When compared to anthocyanins alone, these nanoparticle complexes demonstrated superior inhibition in human cervical cancer HeLa cells. Additionally, Liang et al. [67] demonstrated that the combination of chondroitin sulfate and chitosan with loaded black rice anthocyanins induced apoptosis of human HCT-116 colon cancer cells by providing negative charges to the mitochondria. The results show that the addition of anthocyanin exhibited a noteworthy reduction in cell viability, which altered the mitochondrial structure and, consequently, increased apoptosis of cells.
Hyaluronic acid (HA) stands out as another promising natural polysaccharide that has demonstrated enhanced bioavailability and efficacy in encapsulating anthocyanins. In a study conducted by Liu et al. [68], a black rice anthocyanin-loaded HA nanocarrier was developed to reduce xanthine oxidase (XO) activity, a crucial enzyme involved in the generation of reactive oxygen species and the production of uric acid. In a simulated in vitro analysis, the anthocyanin-embedded HA nanocomplex exhibited a rapid release within the first 12 h, which was maintained consistently until reaching 60% release after 60 h, marking a 54 h difference compared to the non-embedded anthocyanin release system. These results indicate that sustained release could reduce the degradation of active compounds, thereby potentially improving the bioavailability of anthocyanins.
Another polysaccharide example was described by Hanafy et al. [69]. They developed a nanoparticle hydrogel platform based on starch corn, a natural polymer that has garnered attention in recent years. The anthocyanin-loaded starch hydrogel demonstrated promising therapeutic potential in eliminating glycogen from cardiac tissues, overcoming cardiomyopathy, and reducing malondialdehyde levels and collagen fibers. These findings highlights the significance of using biodegradable nanocarriers for encapsulating anthocyanins, showcasing their potential application across various biomedical fields.
On the other hand, much like other drug and active compounds, synthetic polymers have been found to be an excellent approach for the encapsulation of anthocyanins. These polymers offer versatility and enable precise control over the mechanical properties of the material. Among the synthetic polymers, polyesters have been extensively employed in anthocyanin delivery systems. For instance, biodegradable poly(lactic-co-glycolic acid) acid (PLGA) has been used to coat anthocyanins such as pelagonidin, resulting in improved protection against mitochondrial dysfunction [70].
References
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and Purification of Anthocyanins: A Review. J. Agric. Food Res. 2022, 8, 100306.
- Horbowicz, M.; Grzesiuk, A.; DĘBski, H.; Kosson, R. Anthocyanins of Fruits and Vegetables—Their Occurrence, Analysis and Role in Human. Veg. Crops Res. Bull. 2008, 68, 5–22.
- Taylor, L.P.; Grotewold, E. Flavonoids as Developmental Regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323.
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779.
- Fang, J. Bioavailability of Anthocyanins. Drug Metab. Rev. 2014, 46, 508–520.
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967.
- Kossyvaki, D.; Contardi, M.; Athanassiou, A.; Fragouli, D. Colorimetric Indicators Based on Anthocyanin Polymer Composites: A Review. Polymers 2022, 14, 4129.
- Calogero, G.; Bartolotta, A.; Di Marco, G.; Di Carlo, A.; Bonaccorso, F. Vegetable-based dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3244–3294.
- Mirmoeini, S.S.; Moradi, M.; Tajik, H.; Almasi, H.; Gama, F.M. Cellulose/Salep-Based Intelligent Aerogel with Red Grape Anthocyanins: Preparation, Characterization and Application in Beef Packaging. Food Chem. 2023, 425, 136493.
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Thermal and PH Degradation Kinetics of Anthocyanins in Natural Food Colorant Prepared from Black Rice Bran. J. Food Sci. Technol. 2016, 53, 461–470.
- Fossen, T.; Cabrita, L.; Andersen, M. Colour and Stability of Pure Anthocyanins In¯uenced by PH Including the Alkaline Region. Food Chem. 1998, 4, 435–440.
- Li, R.; Wang, S.; Feng, H.; Zhuang, D.; Zhu, J. An Intelligent Chitosan/Gelatin Film via Improving the Anthocyanin-Induced Color Recognition Accuracy for Beef Sub-Freshness Differentiation Monitoring. Food Hydrocoll. 2024, 146, 109219.
- Neves, D.; Andrade, P.B.; Videira, R.A.; de Freitas, V.; Cruz, L. Berry Anthocyanin-Based Films in Smart Food Packaging: A Mini-Review. Food Hydrocoll. 2022, 133, 107885.
- Yong, H.; Liu, J. Recent Advances in the Preparation, Physical and Functional Properties, and Applications of Anthocyanins-Based Active and Intelligent Packaging Films. Food Packag. Shelf Life 2020, 26, 100550.
- Nadi, M.; Razavi, S.M.A.; Shahrampour, D. Fabrication of Green Colorimetric Smart Packaging Based on Basil Seed Gum/Chitosan/Red Cabbage Anthocyanin for Real-Time Monitoring of Fish Freshness. Food Sci. Nutr. 2023, 11, 6360–6375.
- de Oliveira Filho, J.G.; Braga, A.R.C.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Pereira, V.A.C.; Egea, M.B. The Potential of Anthocyanins in Smart, Active, and Bioactive Eco-Friendly Polymer-Based Films: A Review. Food Res. Int. 2021, 142, 110202.
- Aman Mohammadi, M.; Mirza Alizadeh, A.; Mohammadi, M.; Mirzakhani, E.; Sabouri, S.; Pourjafar, H.; Hosseini, S.M. Application and Development of Electrospun Nanofibers as an Efficient Platform for the Delivery of Anthocyanin Compounds in the Food Industry. Food Bioproc Tech. 2023.
- Jiang, M.; Zhang, Y. Biopolymer-Based Encapsulation of Anthocyanins as Reinforced Natural Colorants for Food Applications. J. Agric. Food Res. 2023, 11, 100488.
- Khaledian, Y.; Moshtaghi, H.; Shahbazi, Y. Development and Characterization of Smart Double-Layer Nanofiber Mats Based on Potato Starch-Turnip Peel Anthocyanins and Guar Gum-Cinnamaldehyde. Food Chem. 2024, 434, 137462.
- Nath, V.A.; Vijayakumar, R.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Co-Electrospun-Electrosprayed Ethyl Cellulose-Gelatin Nanocomposite PH-Sensitive Membrane for Food Quality Applications. Food Chem. 2022, 394, 133420.
- Pakolpakçıl, A.; Osman, B.; Göktalay, G.; Özer, E.T.; Şahan, Y.; Becerir, B.; Karaca, E. Design and in Vivo Evaluation of Alginate-Based PH-Sensing Electrospun Wound Dressing Containing Anthocyanins. J. Polym. Res. 2021, 28, 50.
- Liu, L.; Zhang, D.; Song, X.; Guo, M.; Wang, Z.; Geng, F.; Zhou, X.; Nie, S. Compound Hydrogels Derived from Gelatin and Gellan Gum Regulates the Release of Anthocyanins in Simulated Digestion. Food Hydrocoll. 2022, 127, 107487.
- Jin, W.; Xiang, L.; Peng, D.; Liu, G.; He, J.; Cheng, S.; Li, B.; Huang, Q. Study on the Coupling Progress of Thermo-Induced Anthocyanins Degradation and Polysaccharides Gelation. Food Hydrocoll. 2020, 105, 105822.
- Lotfinia, F.; Norouzi, M.R.; Ghasemi-Mobarakeh, L.; Naeimirad, M. Anthocyanin/Honey-Incorporated Alginate Hydrogel as a Bio-Based PH-Responsive/Antibacterial/Antioxidant Wound Dressing. J. Funct. Biomater. 2023, 14, 72.
- Mohammadalinejhad, S.; Kurek, M.; Jensen, I.J.; Lerfall, J. The Potential of Anthocyanin-Loaded Alginate Hydrogel Beads for Intelligent Packaging Applications: Stability and Sensitivity to Volatile Amines. Curr. Res. Food Sci. 2023, 7, 100560.
- Cui, C.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Bioactive and Intelligent Starch-Based Films: A Review. Trends Food Sci. Technol. 2021, 116, 854–869.
- Förster, S.; Schmidt, M. Polyelectrolytes in Solution. In Physical Properties of Polymers, Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1995.
- Tan, C.; Huang, M.; Wang, J.; Sun, B. Biopolyelectrolyte Complex (BioPEC)-Based Carriers for Anthocyanin Delivery. Food Hydrocoll. Health 2021, 1, 100037.
- Sharif, N.; Khoshnoudi-Nia, S.; Jafari, S.M. Nano/Microencapsulation of Anthocyanins; a Systematic Review and Meta-Analysis. Food Res. Int. 2020, 132, 109077.
- Li, J.; Guo, C.; Cai, S.; Yi, J.; Zhou, L. Fabrication of Anthocyanin–Rich W1/O/W2 Emulsion Gels Based on Pectin–GDL Complexes: 3D Printing Performance. Food Res. Int. 2023, 168, 112782.
- Baba Shekh, A.O.; Abdul Wahab, R.; Yahya, N.A. Formulation of Roselle Extract Water-in-Oil Nanoemulsion for Controlled Pulmonary Delivery. J. Dispers. Sci. Technol. 2023, 44, 1830–1841.
- Kenari, R.E.; Razavi, R. Encapsulation of Bougainvillea (Bougainvillea spectabilis) Flower Extract in Urtica dioica L. Seed Gum: Characterization, Antioxidant/Antimicrobial Properties, and in Vitro Digestion. Food Sci. Nutr. 2022, 10, 3436–3443.
- Kanha, N.; Surawang, S.; Pitchakarn, P.; Laokuldilok, T. Microencapsulation of Copigmented Anthocyanins Using Double Emulsion Followed by Complex Coacervation: Preparation, Characterization and Stability. LWT 2020, 133, 110154.
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent Insights into Nanoemulsions: Their Preparation, Properties and Applications. Food Chem. X 2023, 18, 100684.
- Lan, X.; Liu, Y.; Wang, L.; Wang, H.; Hu, Z.; Dong, H.; Yu, Z.; Yuan, Y. A Review of Curcumin in Food Preservation: Delivery System and Photosensitization. Food Chem. 2023, 424, 136464.
- Wulansari, A.; Jufri, M.; Budianti, A. Studies on the Formulation, Physical Stability, and in Vitro Antibacterial Activity of Tea Tree Oil (Melaleuca Alternifolia) Nanoemulsion Gel. Int. J. Appl. Pharm. 2017, 9, 135–139.
- Cheng, Y.; Liu, J.; Li, L.; Ren, J.; Lu, J.; Luo, F. Advances in Embedding Techniques of Anthocyanins: Improving Stability, Bioactivity and Bioavailability. Food Chem. X 2023, 20, 100983.
- Garavand, F.; Jalai-Jivan, M.; Assadpour, E.; Jafari, S.M. Encapsulation of Phenolic Compounds within Nano/Microemulsion Systems: A Review. Food Chem. 2021, 364, 130376.
- Nazareth, M.S.; Shreelakshmi, S.V.; Rao, P.J.; Shetty, N.P. Micro and Nanoemulsions of Carissa Spinarum Fruit Polyphenols, Enhances Anthocyanin Stability and Anti-Quorum Sensing Activity: Comparison of Degradation Kinetics. Food Chem. 2021, 359, 129876.
- Gorantla, S.; Wadhwa, G.; Jain, S.; Sankar, S.; Nuwal, K.; Mahmood, A.; Dubey, S.K.; Taliyan, R.; Kesharwani, P.; Singhvi, G. Recent Advances in Nanocarriers for Nutrient Delivery. Drug Deliv. Transl. Res. 2022, 12, 2359–2384.
- Al-Khayri, J.M.; Asghar, W.; Akhtar, A.; Ayub, H.; Aslam, I.; Khalid, N.; Al-Mssallem, M.Q.; Alessa, F.M.; Ghazzawy, H.S.; Attimarad, M. Anthocyanin Delivery Systems: A Critical Review of Recent Research Findings. Appl. Sci. 2022, 12, 12347.
- Chen, B.H.; Inbaraj, B.S. Nanoemulsion and Nanoliposome Based Strategies for Improving Anthocyanin Stability and Bioavailability. Nutrients 2019, 11, 1052.
- Constantin, O.E.; Stănciuc, N.; Yan, Y.; Ghinea, I.O.; Ungureanu, C.; Cîrciumaru, A.; Wang, D.; Poklar Ulrih, N.; Râpeanu, G. Polymers and Protein-Associated Vesicles for the Microencapsulation of Anthocyanins from Grape Skins Used for Food Applications. J. Sci. Food Agric. 2021, 101, 2676–2686.
- Qiao, L.; Yang, H.; Gao, S.; Li, L.; Fu, X.; Wei, Q. Research Progress on Self-Assembled Nanodrug Delivery Systems. J. Mater. Chem. B 2022, 10, 1908–1922.
- Yavuz-Düzgün, M.; Kareth, S.; Özçelik, B.; Weidner, E. Black Carrot Extract Loaded-Potato Protein Particles by PGSS-Drying: Physico-Chemical Properties and in Vitro Bioaccessability. J. Supercrit. Fluids 2023, 203, 106065.
- Rosales-Chimal, S.; Navarro-Cortez, R.O.; Bello-Perez, L.A.; Vargas-Torres, A.; Palma-Rodríguez, H.M. Optimal Conditions for Anthocyanin Extract Microencapsulation in Taro Starch: Physicochemical Characterization and Bioaccessibility in Gastrointestinal Conditions. Int. J. Biol. Macromol. 2023, 227, 83–92.
- Kurek, M.A.; Majek, M.; Onopiuk, A.; Szpicer, A.; Napiórkowska, A.; Samborska, K. Encapsulation of Anthocyanins from Chokeberry (Aronia Melanocarpa) with Plazmolyzed Yeast Cells of Different Species. Food Bioprod. Process. 2023, 137, 84–92.
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Moscoso-Moscoso, E.; Palomino-Rincón, H.; Taipe-Pardo, F.; Landa, J.P.A.; Arévalo-Quijano, J.C.; Muñoz-Saenz, J.C.; Quispe-Quezada, U.R.; Huamán-Carrión, M.L.; et al. Nanoencapsulation of Phenolic Extracts from Native Potato Clones (Solanum tuberosum spp. Andigena) by Spray Drying. Molecules 2023, 28, 4961.
- Mendes, J.F.; Norcino, L.B.; Manrich, A.; de Oliveira, T.J.P.; Mendes, R.F.; Mattoso, L.H.C. Pectin-Based Color Indicator Films Incorporated with Spray-Dried Hibiscus Extract Microparticles. Food Res. Int. 2022, 162, 111914.
- Nadali, N.; Pahlevanlo, A.; Sarabi-Jamab, M.; Balandari, A. Effect of Maltodextrin with Different Dextrose Equivalents on the Physicochemical Properties of Spray-Dried Barberry Juice (Berberis vulgaris L.). J. Food Sci. Technol. 2022, 59, 2855–2866.
- Yuan, Y.; Fan, Q.; Xu, X.; Wang, O.; Zhao, L.; Zhao, L. Nanocarriers Based on Polysaccharides for Improving the Stability and Bioavailability of Anthocyanins: A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100346.
- Molina, A.K.; Corrêa, R.C.G.; Prieto, M.A.; Pereira, C.; Barros, L. Bioactive Natural Pigments’ Extraction, Isolation, and Stability in Food Applications. Molecules 2023, 28, 1200.
- Williams, C.A.; Grayer, R.J. Anthocyanins and Other Flavonoids. Nat. Prod. Rep. 2004, 21, 539–573.
- Zhang, K.; Huang, T.S.; Yan, H.; Hu, X.; Ren, T. Novel PH-Sensitive Films Based on Starch/Polyvinyl Alcohol and Food Anthocyanins as a Visual Indicator of Shrimp Deterioration. Int. J. Biol. Macromol. 2020, 145, 768–776.
- Pereira, V.A.; de Arruda, I.N.Q.; Stefani, R. Active Chitosan/PVA Films with Anthocyanins from Brassica Oleraceae (Red Cabbage) as Time-Temperature Indicators for Application in Intelligent Food Packaging. Food Hydrocoll. 2015, 43, 180–188.
- Li, L.; Zhao, Z.; Wei, S.; Xu, K.; Xia, J.; Wu, Q.; Lü, X.; Wang, L. Development and Application of Multifunctional Films Based on Modified Chitosan/Gelatin Polyelectrolyte Complex for Preservation and Monitoring. Food Hydrocoll. 2024, 147, 109336.
- Zhao, R.; Chen, J.; Yu, S.; Niu, R.; Yang, Z.; Wang, H.; Cheng, H.; Ye, X.; Liu, D.; Wang, W. Active Chitosan/Gum Arabic-Based Emulsion Films Reinforced with Thyme Oil Encapsulating Blood Orange Anthocyanins: Improving Multi-Functionality. Food Hydrocoll. 2023, 134, 108094.
- Mao, S.; Ren, Y.; Chen, S.; Liu, D.; Ye, X.; Tian, J. Development and Characterization of PH Responsive Sodium Alginate Hydrogel Containing Metal-Phenolic Network for Anthocyanin Delivery. Carbohydr. Polym. 2023, 320, 121234.
- Riaz, R.S.; Elsherif, M.; Moreddu, R.; Rashid, I.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Anthocyanin-Functionalized Contact Lens Sensors for Ocular PH Monitoring. ACS Omega 2019, 4, 21792–21798.
- Alsahag, M.; Alisaac, A.; Al-Hazmi, G.A.A.; Pashameah, R.A.; Attar, R.M.S.; Saad, F.A.; El-Metwaly, N.M. Preparation of Carboxymethyl Cellulose/Polyvinyl Alcohol Wound Dressing Composite Immobilized with Anthocyanin Extract for Colorimetric Monitoring of Wound Healing and Prevention of Wound Infection. Int. J. Biol. Macromol. 2023, 224, 233–242.
- Al-Qahtani, S.D.; Alzahrani, H.K.; Azher, O.A.; Owidah, Z.O.; Abualnaja, M.; Habeebullah, T.M.; El-Metwaly, N.M. Immobilization of Anthocyanin-Based Red-Cabbage Extract onto Cellulose Fibers toward Environmentally Friendly Biochromic Diagnostic Biosensor for Recognition of Urea. J. Environ. Chem. Eng. 2021, 9, 105493.
- Gonçalves, A.C.; Falcão, A.; Alves, G.; Lopes, J.A.; Silva, L.R. Employ of Anthocyanins in Nanocarriers for Nano Delivery: In Vitro and In Vivo Experimental Approaches for Chronic Diseases. Pharmaceutics 2022, 14, 2272.
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioproc Tech. 2013, 6, 628–647.
- Guterres, S.S.; Paese, K.; Pohlmann, A.R. Polymeric Nanoparticles. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–94. ISBN 9783030165734.
- Zhao, X.; Zhang, X.; Tie, S.; Hou, S.; Wang, H.; Song, Y.; Rai, R.; Tan, M. Facile Synthesis of Nano-Nanocarriers from Chitosan and Pectin with Improved Stability and Biocompatibility for Anthocyanins Delivery: An in Vitro and in Vivo Study. Food Hydrocoll. 2020, 109, 106114.
- Jeong, D.; Na, K. Chondroitin Sulfate Based Nanocomplex for Enhancing the Stability and Activity of Anthocyanin. Carbohydr. Polym. 2012, 90, 507–515.
- Liang, T.; Zhang, Z.; Jing, P. Black Rice Anthocyanins Embedded in Self-Assembled Chitosan/Chondroitin Sulfate Nanoparticles Enhance Apoptosis in HCT-116 Cells. Food Chem. 2019, 301, 125280–125289.
- Liu, Y.; Peng, B. A Novel Hyaluronic Acid-Black Rice Anthocyanins Nanocomposite: Preparation, Characterization, and Its Xanthine Oxidase (XO) -Inhibiting Properties. Front. Nutr. 2022, 9, 879354.
- Hanafy, N.A.N. Starch Based Hydrogel NPs Loaded by Anthocyanins Might Treat Glycogen Storage at Cardiomyopathy in Animal Fibrotic Model. Int. J. Biol. Macromol. 2021, 183, 171–181.
- Unosson, E. Antibacterial Strategies for Titanium Biomaterials; Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1250; Acta Universitatis Upsaliensis: Uppsala, Sweden, 2015; p. 72. ISBN 978-91-554-9241-0.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
14 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




