
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lalit Kaurani | -- | 3023 | 2024-03-14 08:18:16 | | | |
| 2 | Fanny Huang | Meta information modification | 3023 | 2024-03-15 10:25:27 | | |
Video Upload Options
Depression is a major contributor to the overall global burden of disease. The discovery of biomarkers for diagnosis or prediction of treatment responses and as therapeutic agents is a current priority. Previous studies have demonstrated the importance of short RNA molecules in the etiology of depression. The most extensively researched of these are microRNAs, a major component of cellular gene regulation and function. MicroRNAs function in a temporal and tissue-specific manner to regulate and modify the post-transcriptional expression of target mRNAs. They can also be shuttled as cargo of extracellular vesicles between the brain and the blood, thus informing about relevant mechanisms in the CNS through the periphery.
1. Introduction
1.1. General Introduction to Depression
1.2. Current Therapeutic Approaches
1.3. General Introduction to miRNAs
2. MicroRNAs and Depression
2.1. miRNAs in the Pathophysiology of Depression
2.2. miRNAs as Diagnostic Biomarkers
2.2.1. Blood-Derived miRNAs: Refining the Complex Landscape of Depression Biomarkers
| Refs. | Tissue Source | miRNAs | Status (Patient vs. Control) | Antidepressant Treatment | Regulation by Treatment | miRNAs |
|---|---|---|---|---|---|---|
| [28][39] | Whole blood | miR-1202 | Down | Citalopram | Up | miR-1202 |
| [35][36] | Whole blood | - | - | Escitalopram (12 weeks) |
Up | miR-130b, miR-505, miR-29b-2, miR-26b, miR-22, miR-26a, miR-664, miR-494, let-7d, let-7g, let-7e, let-7f, miR-629, miR-106b, miR-103, miR-191, miR-128, miR-502-3p, miR-374b, miR-132, miR-30d, miR-500, miR-589, miR-183, miR-574-3p, miR-140-3p, miR-335, miR-361-5p |
| Down | miR-34c-5p, miR-770-5p | |||||
| [37] | Whole blood | miR-132-3p, miR-124-3p | Up | Citalopram (8 weeks) |
Up | miR-124 |
| Down | miR-132-3p | |||||
| [41] | Whole blood | - | - | Duloxetine (8 weeks) |
Up | miR-3688, miR-5695 |
| [42] | Whole blood | - | - | Escitalopram (2 weeks) |
Up | miR-103a-3p, miR-103b, miR-106a-5p, miR-106b-3p, miR-140-3p, miR-145-5p, miR-148b-3p, miR-151a-5p, miR-15a-5p, miR-15b-5p, miR-17-5p, miR-182-5p, miR-185-3p, miR-185-5p, miR-186-5p, miR-20a-5p, miR-20b-5p, miR-210-3p, miR-25-3p, miR-30a-5p, miR-30b-5p, miR-3158-3p, miR-3158-5p, miR-324-5p, miR-331-5p, miR-500a-3p, miR-502-3p, miR-532-5p, miR-550a-3p, miR-584-5p, miR-589-5p, miR-660-5p, miR-93-5p |
| Down | miR-1301-3p, miR-191-3p, miR-200b-3p, miR-222-3p, miR-25-5p, miR-27a-3p, miR-30c-1-3p, miR-3168, miR-328-3p, miR-505-5p, miR-744-5p, miR-92a-1-5p | |||||
| [43] | Plasma | miR-144-5p | Down | Personalized (8 weeks) |
Up | miR-144-5p |
| [44] | Serum | miRNA-34a-5p, miRNA-221-3p | Up | Paroxetine (8 weeks) |
Down | miRNA-34a-5p, miRNA-221-3p |
| miRNA-451a | Down | Up | miRNA-451a | |||
| [33] | Neuron-derived EV | - | - | Escitalopram (8 weeks) |
Up (responders) |
miR-30d-5p and miR-486-5p |
2.2.2. Fluid-Based miRNA Landscapes: Serum, Plasma, and Cerebrospinal Fluid
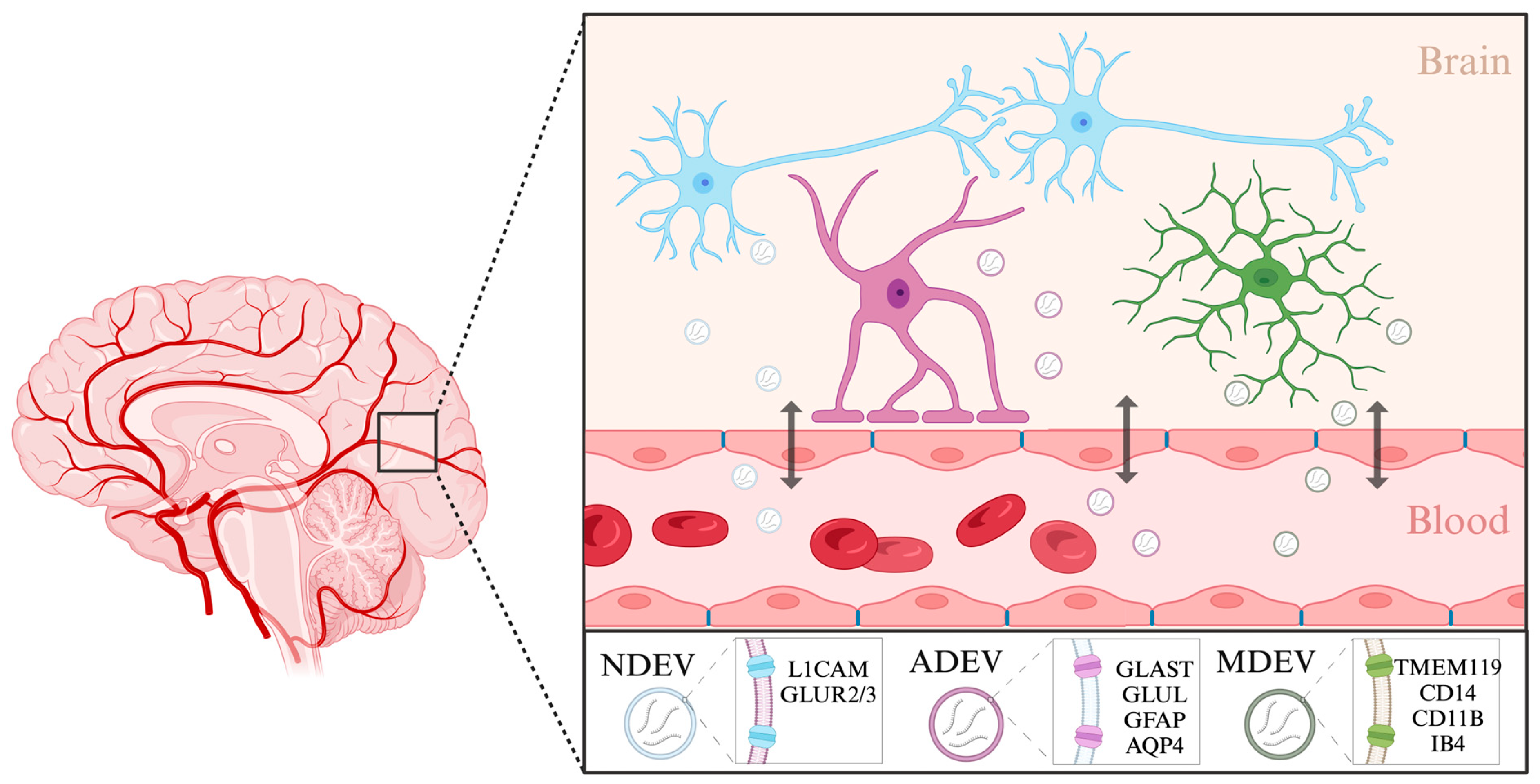
2.2.3. Extracellular Vesicles: Emerging Protagonists in the Biomarker Landscape of Depression
References
- Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx); Institute of Health Metrics and Evaluation: Seattle, WA, USA, 2021.
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858.
- Kessler, R.C.; Bromet, E.J. The Epidemiology of Depression across Cultures. Annu. Rev. Public Health 2013, 34, 119–138.
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; de Girolamo, G.; de Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N.; et al. Cross-National Epidemiology of DSM-IV Major Depressive Episode. BMC Med. 2011, 9, 90.
- Nihalani, N.; Simionescu, M.; Dunlop, B.W. Depression: Phenomenology, Epidemiology, and Pathophysiology. In Depression; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-0-429-24993-8.
- Kuehner, C. Why Is Depression More Common among Women than among Men? Lancet Psychiatry 2017, 4, 146–158.
- Van de Velde, S.; Bracke, P.; Levecque, K. Gender Differences in Depression in 23 European Countries. Cross-National Variation in the Gender Gap in Depression. Soc. Sci. Med. 2010, 71, 305–313.
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312.
- Heim, C.; Binder, E.B. Current Research Trends in Early Life Stress and Depression: Review of Human Studies on Sensitive Periods, Gene–Environment Interactions, and Epigenetics. Exp. Neurol. 2012, 233, 102–111.
- Belmaker, R.H.; Agam, G. Major Depressive Disorder. N. Engl. J. Med. 2008, 358, 55–68.
- Hasin, D.S.; Sarvet, A.L.; Meyers, J.L.; Saha, T.D.; Ruan, W.J.; Stohl, M.; Grant, B.F. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry 2018, 75, 336–346.
- Cuijpers, P.; Noma, H.; Karyotaki, E.; Vinkers, C.H.; Cipriani, A.; Furukawa, T.A. A Network Meta-Analysis of the Effects of Psychotherapies, Pharmacotherapies and Their Combination in the Treatment of Adult Depression. World Psychiatry 2020, 19, 92–107.
- Cuijpers, P.; Stringaris, A.; Wolpert, M. Treatment Outcomes for Depression: Challenges and Opportunities. Lancet Psychiatry 2020, 7, 925–927.
- Gurtan, A.M.; Sharp, P.A. The Role of miRNAs in Regulating Gene Expression Networks. J. Mol. Biol. 2013, 425, 3582.
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most Mammalian mRNAs Are Conserved Targets of microRNAs. Genome Res. 2009, 19, 92–105.
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA Function in Animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37.
- Selbach, M.; Schwanhäusser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread Changes in Protein Synthesis Induced by microRNAs. Nature 2008, 455, 58–63.
- Griffiths-Jones, S.; Grocock, R.J.; van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: MicroRNA Sequences, Targets and Gene Nomenclature. Nucleic Acids Res. 2006, 34, D140–D144.
- de Rie, D.; Abugessaisa, I.; Alam, T.; Arner, E.; Arner, P.; Ashoor, H.; Åström, G.; Babina, M.; Bertin, N.; Burroughs, A.M.; et al. An Integrated Expression Atlas of miRNAs and Their Promoters in Human and Mouse. Nat. Biotechnol. 2017, 35, 872–878.
- Smalheiser, N.R.; Lugli, G.; Zhang, H.; Rizavi, H.; Cook, E.H.; Dwivedi, Y. Expression of microRNAs and Other Small RNAs in Prefrontal Cortex in Schizophrenia, Bipolar Disorder and Depressed Subjects. PLoS ONE 2014, 9, e86469.
- Wang, W.; Kwon, E.J.; Tsai, L.-H. MicroRNAs in Learning, Memory, and Neurological Diseases. Learn. Mem. 2012, 19, 359–368.
- Issler, O.; Chen, A. Determining the Role of microRNAs in Psychiatric Disorders. Nat. Rev. Neurosci. 2015, 16, 201–212.
- Dwivedi, Y. MicroRNAs in Depression and Suicide: Recent Insights and Future Perspectives. J. Affect. Disord. 2018, 240, 146–154.
- Zhang, Y.; Zhao, Y.; Tian, C.; Wang, J.; Li, W.; Zhong, C. Differential Exosomal microRNA Profile in the Serum of a Patient with Depression. Eur. J. Psychiatry 2018, 32, 105–112.
- Kohen, R.; Dobra, A.; Tracy, J.H.; Haugen, E. Transcriptome Profiling of Human Hippocampus Dentate Gyrus Granule Cells in Mental Illness. Transl. Psychiatry 2014, 4, e366.
- Li, Y.-J.; Xu, M.; Gao, Z.-H.; Wang, Y.-Q.; Yue, Z.; Zhang, Y.-X.; Li, X.-X.; Zhang, C.; Xie, S.-Y.; Wang, P.-Y. Alterations of Serum Levels of BDNF-Related miRNAs in Patients with Depression. PLoS ONE 2013, 8, e63648.
- Yuta, Y.; Roy, B.; Dwivedi, Y. Altered miRNA Landscape of the Anterior Cingulate Cortex Is Associated with Potential Loss of Key Neuronal Functions in Depressed Brain. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2020, 40, 70.
- Lopez, J.P.; Lim, R.; Cruceanu, C.; Crapper, L.; Fasano, C.; Labonte, B.; Maussion, G.; Yang, J.P.; Yerko, V.; Vigneault, E.; et al. miR-1202 Is a Primate-Specific and Brain-Enriched microRNA Involved in Major Depression and Antidepressant Treatment. Nat. Med. 2014, 20, 764–768.
- Issler, O.; Haramati, S.; Paul, E.D.; Maeno, H.; Navon, I.; Zwang, R.; Gil, S.; Mayberg, H.S.; Dunlop, B.W.; Menke, A.; et al. MicroRNA 135 Is Essential for Chronic Stress Resiliency, Antidepressant Efficacy, and Intact Serotonergic Activity. Neuron 2014, 83, 344–360.
- Belzeaux, R.; Bergon, A.; Jeanjean, V.; Loriod, B.; Formisano-Tréziny, C.; Verrier, L.; Loundou, A.; Baumstarck-Barrau, K.; Boyer, L.; Gall, V.; et al. Responder and Nonresponder Patients Exhibit Different Peripheral Transcriptional Signatures during Major Depressive Episode. Transl. Psychiatry 2012, 2, e185.
- Kaurani, L.; Besse, M.; Methfessel, I.; Methi, A.; Zhou, J.; Pradhan, R.; Burkhardt, S.; Kranaster, L.; Sartorius, A.; Habel, U.; et al. Baseline Levels of miR-223-3p Correlate with the Effectiveness of Electroconvulsive Therapy in Patients with Major Depression. Transl. Psychiatry 2023, 13, 294.
- Burrows, K.; Figueroa-Hall, L.; Stewart, J.; Alarbi, A.; Kuplicki, R.; Hannafon, B.; Tan, C.; Risbrough, V.; McKinney, B.; Ramesh, R.; et al. Exploring the Role of Neuronal-Enriched Extracellular Vesicle miR-93 and Interoception in Major Depressive Disorder. Res. Sq. 2023; preprint.
- Saeedi, S.; Nagy, C.; Ibrahim, P.; Théroux, J.-F.; Wakid, M.; Fiori, L.M.; Yang, J.; Rotzinger, S.; Foster, J.A.; Mechawar, N.; et al. Neuron-Derived Extracellular Vesicles Enriched from Plasma Show Altered Size and miRNA Cargo as a Function of Antidepressant Drug Response. Mol. Psychiatry 2021, 26, 7417–7424.
- He, C.; Bai, Y.; Wang, Z.; Fan, D.; Wang, Q.; Liu, X.; Zhang, H.; Zhang, H.; Zhang, Z.; Yao, H.; et al. Identification of microRNA-9 Linking the Effects of Childhood Maltreatment on Depression Using Amygdala Connectivity. Neuroimage 2021, 224, 117428.
- Żurawek, D.; Turecki, G. The miRNome of Depression. Int. J. Mol. Sci. 2021, 22, 11312.
- Bocchio-Chiavetto, L.; Maffioletti, E.; Bettinsoli, P.; Giovannini, C.; Bignotti, S.; Tardito, D.; Corrada, D.; Milanesi, L.; Gennarelli, M. Blood microRNA Changes in Depressed Patients during Antidepressant Treatment. Eur. Neuropsychopharmacol. 2013, 23, 602–611.
- Fang, Y.; Qiu, Q.; Zhang, S.; Sun, L.; Li, G.; Xiao, S.; Li, X. Changes in miRNA-132 and miR-124 Levels in Non-Treated and Citalopram-Treated Patients with Depression. J. Affect. Disord. 2018, 227, 745–751.
- Li, J.; Meng, H.; Cao, W.; Qiu, T. MiR-335 Is Involved in Major Depression Disorder and Antidepressant Treatment through Targeting GRM4. Neurosci. Lett. 2015, 606, 167–172.
- Fiori, L.M.; Lopez, J.P.; Richard-Devantoy, S.; Berlim, M.; Chachamovich, E.; Jollant, F.; Foster, J.; Rotzinger, S.; Kennedy, S.H.; Turecki, G. Investigation of miR-1202, miR-135a, and miR-16 in Major Depressive Disorder and Antidepressant Response. Int. J. Neuropsychopharmacol. 2017, 20, 619–623.
- Funatsuki, T.; Ogata, H.; Tahara, H.; Shimamoto, A.; Takekita, Y.; Koshikawa, Y.; Nonen, S.; Higasa, K.; Kinoshita, T.; Kato, M. Changes in Multiple microRNA Levels with Antidepressant Treatment Are Associated with Remission and Interact with Key Pathways: A Comprehensive microRNA Analysis. Int. J. Mol. Sci. 2023, 24, 12199.
- Belzeaux, R.; Fiori, L.M.; Lopez, J.P.; Boucekine, M.; Boyer, L.; Blier, P.; Farzan, F.; Frey, B.N.; Giacobbe, P.; Lam, R.W.; et al. Predicting Worsening Suicidal Ideation With Clinical Features and Peripheral Expression of Messenger RNA and MicroRNA During Antidepressant Treatment. J. Clin. Psychiatry 2019, 80, 18m12556.
- Yrondi, A.; Fiori, L.M.; Frey, B.N.; Lam, R.W.; MacQueen, G.M.; Milev, R.; Müller, D.J.; Foster, J.A.; Kennedy, S.H.; Turecki, G. Association Between Side Effects and Blood microRNA Expression Levels and Their Targeted Pathways in Patients With Major Depressive Disorder Treated by a Selective Serotonin Reuptake Inhibitor, Escitalopram: A CAN-BIND-1 Report. Int. J. Neuropsychopharmacol. 2020, 23, 88–95.
- Wang, X.; Sundquist, K.; Hedelius, A.; Palmér, K.; Memon, A.A.; Sundquist, J. Circulating microRNA-144-5p Is Associated with Depressive Disorders. Clin. Epigenet. 2015, 7, 69.
- Kuang, W.-H.; Dong, Z.-Q.; Tian, L.-T.; Li, J. MicroRNA-451a, microRNA-34a-5p, and microRNA-221-3p as Predictors of Response to Antidepressant Treatment. Braz. J. Med. Biol. Res. 2018, 51, e7212.
- Lopez, J.P.; Fiori, L.M.; Cruceanu, C.; Lin, R.; Labonte, B.; Cates, H.M.; Heller, E.A.; Vialou, V.; Ku, S.M.; Gerald, C.; et al. MicroRNAs 146a/b-5 and 425-3p and 24-3p Are Markers of Antidepressant Response and Regulate MAPK/Wnt-System Genes. Nat. Commun. 2017, 8, 15497.
- Zheng, Y.-B.; Sheng, X.-M.; Jin, X.; Guan, W. MiR-182-5p: A Novel Biomarker in the Treatment of Depression in CSDS-Induced Mice. Int. J. Neuropsychopharmacol. 2023, 27, pyad064.
- Li, Y.; Li, S.; Yan, J.; Wang, D.; Yin, R.; Zhao, L.; Zhu, Y.; Zhu, X. miR-182 (microRNA-182) Suppression in the Hippocampus Evokes Antidepressant-like Effects in Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 96–103.
- Li, Y.; Wang, N.; Pan, J.; Wang, X.; Zhao, Y.; Guo, Z. Hippocampal miRNA-144 Modulates Depressive-Like Behaviors in Rats by Targeting PTP1B. Neuropsychiatr. Dis. Treat. 2021, 17, 389–399.
- Gheysarzadeh, A.; Sadeghifard, N.; Afraidooni, L.; Pooyan, F.; Mofid, M.R.; Valadbeigi, H.; Bakhtiari, H.; Keikhavani, S. Serum-Based microRNA Biomarkers for Major Depression: MiR-16, miR-135a, and miR-1202. J. Res. Med. Sci. 2018, 23, 69.
- Roy, B.; Ochi, S.; Dwivedi, Y. Potential of Circulating miRNAs as Molecular Markers in Mood Disorders and Associated Suicidal Behavior. Int. J. Mol. Sci. 2023, 24, 4664.
- Shi, Y.; Wang, Q.; Song, R.; Kong, Y.; Zhang, Z. Non-Coding RNAs in Depression: Promising Diagnostic and Therapeutic Biomarkers. eBioMedicine 2021, 71, 103569.
- Wan, Y.; Liu, Y.; Wang, X.; Wu, J.; Liu, K.; Zhou, J.; Liu, L.; Zhang, C. Identification of Differential microRNAs in Cerebrospinal Fluid and Serum of Patients with Major Depressive Disorder. PLoS ONE 2015, 10, e0121975.
- Saeedi, S.; Israel, S.; Nagy, C.; Turecki, G. The Emerging Role of Exosomes in Mental Disorders. Transl. Psychiatry 2019, 9, 122.
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging Role of Extracellular Vesicles in Inflammatory Diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364.
- Hussain, M.T.; Iqbal, A.J.; Norling, L.V. The Role and Impact of Extracellular Vesicles in the Modulation and Delivery of Cytokines during Autoimmunity. Int. J. Mol. Sci. 2020, 21, 7096.
- Kalluri, R.; McAndrews, K.M. The Role of Extracellular Vesicles in Cancer. Cell 2023, 186, 1610–1626.
- Raghav, A.; Singh, M.; Jeong, G.-B.; Giri, R.; Agarwal, S.; Kala, S.; Gautam, K.A. Extracellular Vesicles in Neurodegenerative Diseases: A Systematic Review. Front. Mol. Neurosci. 2022, 15, 1061076.
- Kong, L.; Zhang, D.; Huang, S.; Lai, J.; Lu, L.; Zhang, J.; Hu, S. Extracellular Vesicles in Mental Disorders: A State-of-Art Review. Int. J. Biol. Sci. 2023, 19, 1094–1109.
- Wei, Z.-X.; Xie, G.-J.; Mao, X.; Zou, X.-P.; Liao, Y.-J.; Liu, Q.-S.; Wang, H.; Cheng, Y. Exosomes from Patients with Major Depression Cause Depressive-like Behaviors in Mice with Involvement of miR-139-5p-Regulated Neurogenesis. Neuropsychopharmacology 2020, 45, 1050–1058.
- Levine, A.; Strawn, J.R. Blood Tests of Brain Function: Neuronal Extracellular Vesicles. Biomark. Neuropsychiatry 2022, 7, 100058.
- Mizohata, Y.; Toda, H.; Koga, M.; Saito, T.; Fujita, M.; Kobayashi, T.; Hatakeyama, S.; Morimoto, Y. Neural Extracellular Vesicle-Derived miR-17 in Blood as a Potential Biomarker of Subthreshold Depression. Hum. Cell 2021, 34, 1087–1092.
- Sun, B.; Dalvi, P.; Abadjian, L.; Tang, N.; Pulliam, L. Blood Neuron-Derived Exosomes as Biomarkers of Cognitive Impairment in HIV. AIDS 2017, 31, F9–F17.
- Lachenal, G.; Pernet-Gallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of Exosomes from Differentiated Neurons and Its Regulation by Synaptic Glutamatergic Activity. Mol. Cell Neurosci. 2011, 46, 409–418.
- Datta Chaudhuri, A.; Dasgheyb, R.M.; DeVine, L.R.; Bi, H.; Cole, R.N.; Haughey, N.J. Stimulus-Dependent Modifications in Astrocyte-Derived Extracellular Vesicle Cargo Regulate Neuronal Excitability. Glia 2020, 68, 128–144.
- Goetzl, E.J.; Mustapic, M.; Kapogiannis, D.; Eitan, E.; Lobach, I.V.; Goetzl, L.; Schwartz, J.B.; Miller, B.L. Cargo Proteins of Plasma Astrocyte-Derived Exosomes in Alzheimer’s Disease. FASEB J. 2016, 30, 3853–3859.
- Wallensten, J.; Nager, A.; Åsberg, M.; Borg, K.; Beser, A.; Wilczek, A.; Mobarrez, F. Leakage of Astrocyte-Derived Extracellular Vesicles in Stress-Induced Exhaustion Disorder: A Cross-Sectional Study. Sci. Rep. 2021, 11, 2009.
- Han, J.; Cho, H.-J.; Park, D.; Han, S. DICAM in the Extracellular Vesicles from Astrocytes Attenuates Microglia Activation and Neuroinflammation. Cells 2022, 11, 2977.
- Long, X.; Yao, X.; Jiang, Q.; Yang, Y.; He, X.; Tian, W.; Zhao, K.; Zhang, H. Astrocyte-Derived Exosomes Enriched with miR-873a-5p Inhibit Neuroinflammation via Microglia Phenotype Modulation after Traumatic Brain Injury. J. Neuroinflamm. 2020, 17, 89.
- Luarte, A.; Nardocci, G.; Chakraborty, A.; Batiz, L.F.; Pino-Lagos, K.; Wyneken, Ú. Astrocyte-Derived Extracellular Vesicles in Stress-Associated Mood Disorders. Does the Immune System Get Astrocytic? Pharmacol. Res. 2023, 194, 106833.
- Gabrielli, M.; Raffaele, S.; Fumagalli, M.; Verderio, C. The Multiple Faces of Extracellular Vesicles Released by Microglia: Where Are We 10 Years After? Front. Cell. Neurosci. 2022, 16, 984690.
- Roseborough, A.D.; Myers, S.J.; Khazaee, R.; Zhu, Y.; Zhao, L.; Iorio, E.; Elahi, F.M.; Pasternak, S.H.; Whitehead, S.N. Plasma Derived Extracellular Vesicle Biomarkers of Microglia Activation in an Experimental Stroke Model. J. Neuroinflamm. 2023, 20, 20.
- Cohn, W.; Melnik, M.; Huang, C.; Teter, B.; Chandra, S.; Zhu, C.; McIntire, L.B.; John, V.; Gylys, K.H.; Bilousova, T. Multi-Omics Analysis of Microglial Extracellular Vesicles From Human Alzheimer’s Disease Brain Tissue Reveals Disease-Associated Signatures. Front. Pharmacol. 2021, 12, 766082.
- Scaroni, F.; Visconte, C.; Serpente, M.; Golia, M.T.; Gabrielli, M.; Huiskamp, M.; Hulst, H.E.; Carandini, T.; De Riz, M.; Pietroboni, A.; et al. miR-150-5p and Let-7b-5p in Blood Myeloid Extracellular Vesicles Track Cognitive Symptoms in Patients with Multiple Sclerosis. Cells 2022, 11, 1551.




