
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aaqib Ayub | -- | 3439 | 2024-03-13 15:20:11 | | | |
| 2 | Wendy Huang | Meta information modification | 3439 | 2024-03-14 05:42:03 | | |
Video Upload Options
Disinfection is described as a process that eliminates many or all pathogenic microorganisms on inanimate objects, with the exception of bacterial endospores. Disinfection is usually carried out by chemical or physical means. Among other settings, disinfection is of utmost importance in hospital environments due to pathogens living on hospital surfaces being the direct cause for hospital-acquired infections (HAIs). However, the presence of a wide range of pathogens and biofilms, combined with the indiscriminate use of antibiotics, presents infection control teams in healthcare facilities with ongoing challenges in the selection of biocides and application methods. This necessitates the development of biocides and innovative disinfection methods that overcome the shortcomings of conventional methods. The use of hydrogen peroxide vapour to be a superior alternative to conventional methods. Hydrogen peroxide vapour to be very close to an ideal disinfectant due to its proven efficacy against a wide range of microorganisms, safety to use, lack of toxicity concerns and good material compatibility.
1. Introduction
2. Hydrogen Peroxide Vapour as a Biocide
- (i)
-
Efficacy: A significant number of in vitro and in vivo studies have demonstrated the efficiency of H2O2, both in liquid and vapour phases, against organisms ranging from highly resistant bacterial endospores to enveloped viruses [18][31][33][34][35][36]. According to these studies, antimicrobial activities depend on the concentration of H2O2, the exposure time and the method of application.
- (ii)
-
Safety: Hydrogen peroxide is applied to the skin for wound disinfection and used in acne products in liquid form at a low concentration of less than 3% w/w; this concentration level is considered very safe for use on human skin [29][37]. However, with an increase in concentration, a decreased tissue compatibility has been reported [29][37]. The safety of H2O2 is entirely dependent on how it is used. Owing to the absence (or to the low toxicity effects), H2O2 is seen as an excellent option for replacing more toxic chemicals like formaldehyde, which is known to be carcinogenic, and ethylene oxide, which has high toxicity and carcinogenicity concerns [38][39][40]. A major advantage of modern hydrogen peroxide vapour systems is that they can be easily set up and operated remotely, thus eliminating contact with the operator and reducing risk. The permissible exposure limit weighted over 8 h by the OSHA (Occupational Safety and Health Administration) in the United States is 1 ppm, whereas an immediate danger to life or health is posed at 75 ppm [41].
- (iii)
-
Environmental impact: The environmental impact of hydrogen peroxide is entirely dependent on how it is used. HPV slowly decomposes into water and oxygen and, because of this, it is considered safe for the environment [42]. As a result, no harmful residues are left on surfaces. The relatively unstable peroxide bond leads to its natural decomposition.
- (iv)
-
Ease of use: Factors that impact the ease of use of H2O2 are its concentration and method of application. For example, hydrogen peroxide is highly effective when used in vapour form as it can easily reach crevices and other hard-to-reach areas. This can also be ideal for large-area decontamination as multiple machines can be used at the same time. Modern, no-touch HPV systems reduce the number of labour hours when compared with traditional decontamination methods, leading to a reduction in labour costs.
- (v)
-
Stability: Hydrogen peroxide is stable in water and other formulations, depending on its purity and storage conditions. It is important that hydrogen peroxide is stored under conditions recommended by the manufacturer. Dissociation of hydrogen peroxide can take place if stored incorrectly. This will reduce the concentration of hydrogen peroxide in the solution and will have an impact on its antimicrobial efficacy.
- (vi)
-
Compatibility with surface materials: Hydrogen peroxide can be safe to surfaces, depending on how it is used. Being an oxidising agent, it can oxidise certain metallic and plastic surfaces when used in higher concentrations in liquid form [31]. However, these effects can be prevented when H2O2 is used in vapour form, which is considered to be gentle to surfaces and electrical equipment that are key parts of hospital environments. Boyce et al. [43] studied the impact of microcondensation HPV room decontamination on hospital physiological monitors over an 8-year period and observed that there was no increase in maintenance service calls; in fact, a rather unexplained decrease in maintenance was apparent. Furthermore, a recent study by Sher and Mulder [44] on the use of vapour-phase and aerosolised hydrogen peroxide for disinfection of dental surgery areas found no damage to any surface in these surgery areas. The effect of HPV on three metallic materials was characterised by Gale et al. [45], and no systematic effects were seen on the tensile strength or post-HPV-treated corrosion resistance of the alloys tested. Microstructural changes were seen to be confined to the areas adjacent to the exposed surface and were considered to be relatively small [45].
3. Mechanism of Biocidal Action
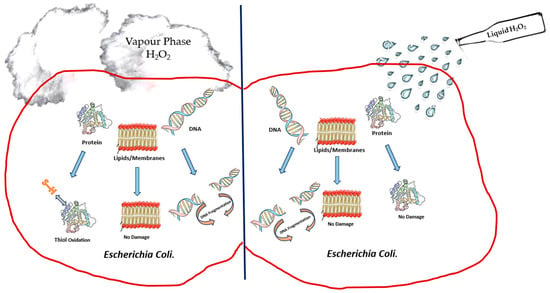
References
- Haque, M.; Sartelli, M.; McKimm, J.; Abu Bakar, M. Health care-associated infections—An overview. Infect. Drug Resist. 2018, 11, 2321–2333.
- Gaynes, R.; Horan, T.; Mayhall, C. Hospital Epidemiology and Infection Control; Williams & Wilkins: Baltimore, MD, USA, 1996.
- Mcdonnell, G. The Use of Hydrogen Peroxide for Disinfection and Sterilization Applications. In PATAI’S Chemistry of Functional Groups; Wileys: Hoboken, NJ, USA, 2009; pp. 1–34.
- Susan, H.; Karen, S.; Lisa, S. English National Point Prevalence Survey on Healthcare-Associated Infections and Antimicrobial Use 2011; Health Protection Agency: London, UK, 2012.
- Stone, P.W. Economic burden of healthcare-associated infections: An American perspective. Expert. Rev. Pharmacoecon Outcomes Res. 2009, 9, 417–422.
- Talon, D. The role of the hospital environment in the epidemiology of multi-resistant bacteria. J. Hosp. Infect. 1999, 43, 13–17.
- Andersson, D.I.; Levin, B.R. The biological cost of antibiotic resistance. Curr. Opin. Microbiol. 1999, 2, 489–493.
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180.
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130.
- Abreu, A.C.; Tavares, R.R.; Borges, A.; Mergulhão, F.; Simões, M. Current and emergent strategies for disinfection of hospital environments. J. Antimicrob. Chemother. 2013, 68, 2718–2732.
- Hota, B. Contamination, disinfection, and cross-colonization: Are hospital surfaces reservoirs for nosocomial infection? Clin. Infect. Dis. 2004, 39, 1182–1189.
- Simões, M. Antimicrobial strategies effective against infectious bacterial biofilms. Curr. Med. Chem. 2011, 18, 2129–2145.
- Hanna, H.; Raad, I.; Gonzalez, V.; Umphrey, J.; Tarrand, J.; Neumann, J.; Champlin, R. Control of nosocomial Clostridium difficile transmission in bone marrow transplant patients. Infect. Control Hosp. Epidemiol. 2000, 21, 226–228.
- Dancer, S.J. Mopping up hospital infection. J. Hosp. Infect. 1999, 43, 85–100.
- Weber, D.J.; Rutala, W.A.; Miller, M.B.; Huslage, K.; Sickbert-Bennett, E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. Am. J. Infect. Control 2010, 38 (Suppl. 1), S25–S33.
- Saint, S.; Kaufman, S.R.; Thompson, M.; Rogers, M.A.; Chenoweth, C.E. A reminder reduces urinary catheterization in hospitalized patients. Jt. Comm. J. Qual. Patient Saf. 2005, 31, 455–462.
- Saint, S.; Lipsky, B.A. Preventing Catheter-Related Bacteriuria: Should We? Can We? How? Arch. Intern. Med. 1999, 159, 800–808.
- Jeanes, A.; Rao, G.; Osman, M.; Merrick, P. Eradication of persistent environmental MRSA. J. Hosp. Infect. 2005, 61, 85–86.
- Blythe, D.; Keenlyside, D.; Dawson, S.; Galloway, A. Environmental contamination due to methicillin-resistant Staphylococcus aureus (MRSA). J. Hosp. Infect. 1998, 38, 67–69.
- French, G.L.; Otter, J.A.; Shannon, K.; Adams, N.; Watling, D.; Parks, M. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): A comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J. Hosp. Infect. 2004, 57, 31–37.
- Williams, G.J.; Denyer, S.P.; Hosein, I.K.; Hill, D.W.; Maillard, J.Y. The development of a new three-step protocol to determine the efficacy of disinfectant wipes on surfaces contaminated with Staphylococcus aureus. J. Hosp. Infect. 2007, 67, 329–335.
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398.
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40.
- Vickery, K.; Deva, A.; Jacombs, A.; Allan, J.; Valente, P.; Gosbell, I.B. Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J. Hosp. Infect. 2012, 80, 52–55.
- Henry, M.C.; Wheeler, J.; Mofenson, H.C.; Caraccio, T.R.; Marsh, M.; Comer, G.M.; Singer, U.J. Hydrogen peroxide 3% exposures. J. Toxicol. Clin. Toxicol. 1996, 34, 323–327.
- McDonnell, G.E. Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance; John Wiley & Sons: Hoboken, NJ, USA, 2017.
- Block, S.S. Disinfection, Sterilization, and Preservation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001.
- Caplin, J.L.S. Special Issues in Dentistry. In Russell, Hugo & Ayliffe’s; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 537–549.
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571.
- National Center for Biotechnology Information. Hydrogen Peroxide; PubChem: Bethesda, MD, USA, 2023.
- Fichet, G.; Antloga, K.; Comoy, E.; Deslys, J.P.; McDonnell, G. Prion inactivation using a new gaseous hydrogen peroxide sterilisation process. J. Hosp. Infect. 2007, 67, 278–286.
- Bates, C.; Pearse, R. Use of hydrogen peroxide vapour for environmental control during a Serratia outbreak in a neonatal intensive care unit. J. Hosp. Infect. 2005, 61, 364–366.
- Otter, J.; Barnicoat, M.; Down, J.; Smyth, D.; Yezli, S.; Jeanes, A. Hydrogen peroxide vapour decontamination of a critical care unit room used to treat a patient with Lassa fever. J. Hosp. Infect. 2010, 75, 335–337.
- Heckert, R.A.; Best, M.; Jordan, L.T.; Dulac, G.C.; Eddington, D.L.; Sterritt, W.G. Efficacy of vaporized hydrogen peroxide against exotic animal viruses. Appl. Env. Microbiol. 1997, 63, 3916–3918.
- Al-Adham, I.; Haddadin, R.; Collier, P. Types of microbicidal and microbistatic agents. In Russell, Hugo & Ayliffe’s: Principles and Practice of Disinfection, Preservation and Sterilization; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 5–70.
- Heck, D.H.A.; Casanova, M.; Starr, T.B. Formaldehyde Toxicity—New Understanding. Crit. Rev. Toxicol. 1990, 20, 397–426.
- da Cunha Mendes, G.C.; da Silva Brandão, T.R.; Miranda Silva, C.L. Ethylene oxide potential toxicity. Expert. Rev. Med. Devices 2008, 5, 323–328.
- Klapes, N.A.; Vesley, D. Vapor-phase hydrogen peroxide as a surface decontaminant and sterilant. Appl. Env. Microbiol. 1990, 56, 503–506.
- HYDROGEN PEROXIDE USA: Occupational Safety and Health Administration. 2023. Available online: https://www.osha.gov/chemicaldata/630 (accessed on 20 December 2023).
- Abdollahi, M.; Hosseini, A. Hydrogen Peroxide. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 967–970.
- Boyce, J.M.; Havill, N.L.; Cianci, V.; Flanagan, G. Compatibility of Hydrogen Peroxide Vapor Room Decontamination with Physiological Monitors. Infect. Control Hosp. Epidemiol. 2014, 35, 92–93.
- Sher, M.; Mulder, R. Comparison of Aerosolized Hydrogen Peroxide Fogging with a Conventional Disinfection Product for a Dental Surgery. J. Contemp. Dent. Pract. 2020, 21, 1308.
- Gale, W.F.; Sofyan, N.I.; Gale, H.S.; Sk, M.H.; Chou, S.F.; Fergus, J.W.; Shannon, C.G. Effect of vapour phase hydrogen peroxide, as a decontaminant for civil aviation applications, on microstructure, tensile properties and corrosion resistance of 2024 and 7075 age hardenable aluminium alloys and 304 austenitic stainless steel. Mater. Sci. Technol. 2009, 25, 76–84.
- Boyce, J.M. New approaches to decontamination of rooms after patients are discharged. Infect. Control Hosp. Epidemiol. 2009, 30, 515–517.
- Otter, J.A.; Yezli, S. A call for clarity when discussing hydrogen peroxide vapour and aerosol systems. J. Hosp. Infect. 2011, 77, 83–84.
- Otter, J.A.; Havill, N.L.; Boyce, J.M. Hydrogen Peroxide Vapor Is Not the Same as Aerosolized Hydrogen Peroxide. Infect. Control Hosp. Epidemiol. 2010, 31, 1201–1202.
- Otter, J.A.; French, G.L. Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapor. J. Clin. Microbiol. 2009, 47, 205–207.
- Kahnert, A.; Seiler, P.; Stein, M.; Aze, B.; McDonnell, G.; Kaufmann, S.H. Decontamination with vaporized hydrogen peroxide is effective against Mycobacterium tuberculosis. Lett. Appl. Microbiol. 2005, 40, 448–452.
- Zhu, P.C. New Biocides Development: The Combined Approach of Chemistry and Microbiology; ACS Publications: Washington, DC, USA, 2007.
- Finnegan, M.; Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Mode of action of hydrogen peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010, 65, 2108–2115.
- Pottage, T.; Richardson, C.; Parks, S.; Walker, J.; Bennett, A. Evaluation of hydrogen peroxide gaseous disinfection systems to decontaminate viruses. J. Hosp. Infect. 2010, 74, 55–61.
- Wang, C.G.; Li, Z.; Liu, S.; Ng, C.T.; Marzuki, M.; Jeslyn Wong, P.S.; Tan, B.; Lee, A.; Lim, C.H.; Bifani, P.; et al. N95 respirator decontamination: A study in reusability. Mater. Today Adv. 2021, 11, 100148.
- ISO 22441:2022; Sterilization of Health Care Products—Low Temperature Vaporized Hydrogen Peroxide—Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2022.
- Spaulding, E.H. Chemical disinfection and antisepsis in the hospital. J. Hosp. Res. 1972, 9, 5–31.
- Barbut, F.; Yezli, S.; Otter, J. Activity in vitro of hydrogen peroxide vapour against Clostridium difficile spores. J. Hosp. Infect. 2012, 80, 85–87.
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596.
- Repine, J.; Fox, R.B.; Berger, E. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J. Biol. Chem. 1981, 256, 7094–7096.
- Imlay, J.A.; Linn, S. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J. Bacteriol. 1986, 166, 519–527.
- Schwartz, C.E.; Krall, J.; Norton, L.; McKay, K.; Kay, D.; Lynch, R.E. Catalase and superoxide dismutase in Escherichia coli. J. Biol. Chem. 1983, 258, 6277–6281.
- Brandi, G.; Sestili, P.; Pedrini, M.A.; Salvaggio, L.; Cattabeni, F.; Cantoni, O. The effect of temperature or anoxia on Escherichia coli killing induced by hydrogen peroxide. Mutat. Res. Lett. 1987, 190, 237–240.
- Linley, E. Understanding the Iinteractions of Hydrogen Peroxide with Macromolecules and Microbial Components. Ph.D. Dissertation, Cardiff University, Cardiff, UK, 2012.
- McDonnell, G. Peroxygens and Other Forms of Oxygen: Their Use for Effective Cleaning, Disinfection, and Sterilization. In New Biocides Development; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2007; Volume 967, pp. 292–308.
- Dryden, M.; Parnaby, R.; Dailly, S.; Lewis, T.; Davis-Blues, K.; Otter, J.; Kearns, A. Hydrogen peroxide vapour decontamination in the control of a polyclonal meticillin-resistant Staphylococcus aureus outbreak on a surgical ward. J. Hosp. Infect. 2008, 68, 190–192.




