Disinfection is described as a process that eliminates many or all pathogenic microorganisms on inanimate objects, with the exception of bacterial endospores. Disinfection is usually carried out by chemical or physical means. Among other settings, disinfection is of utmost importance in hospital environments due to pathogens living on hospital surfaces being the direct cause for hospital-acquired infections (HAIs). However, the presence of a wide range of pathogens and biofilms, combined with the indiscriminate use of antibiotics, presents infection control teams in healthcare facilities with ongoing challenges in the selection of biocides and application methods. This necessitates the development of biocides and innovative disinfection methods that overcome the shortcomings of conventional methods. The use of hydrogen peroxide vapour to be a superior alternative to conventional methods. Hydrogen peroxide vapour to be very close to an ideal disinfectant due to its proven efficacy against a wide range of microorganisms, safety to use, lack of toxicity concerns and good material compatibility.
- disinfection
- hospital-acquired infections
- biocides
- hydrogen peroxide vapour
- pathogens
- mechanism
1. Introduction
2. Hydrogen Peroxide Vapour as a Biocide
Hydrogen peroxide is used as a disinfectant/sterilant by being applied directly in the form of an aqueous solution at a concentration ranging from 3 to 9% (w/w) [25][26]; it is formulated with different chemicals in water or gas, in an aerosolised form or in a vapour form [26][27]. The use of H2O2 as a biocide is found in multiple industries, including the food and beverage sector, agriculture, hospitals, the pharmaceutical and cosmetic sector, the water supply industry, and the public and commercial disinfection industry [3][27][4,28]. Its use in the food and beverage sector in a liquid form is targeted for disinfection and sterilisation of food contact surfaces that are used for milk and juice storage and for the preservation of water, milk and juices [27][28]. The use of H2O2 in the pharmaceutical and cosmetic industry takes the form of liquid formulations at concentrations ranging from 3 to 9% (v/v) in various products including wound applicants, oral disinfection in dentistry, contact lens disinfection, and as a preservative in cosmetics [28][29][30][29,30,31]. Furthermore, higher concentrations of hydrogen peroxide solutions are used in the manufacturing of foam rubber, organic compounds, rocket fuel, and bleach for paper and textiles [30][31]. Examples of use in commercial sterilisation and in the water industry include industrial effluent treatment, algae control in water and wastewater deodorisation. The use of H2O2 in vapour form is found widely in the healthcare sector for disinfection and sterilisation [31][32]. In addition to its use against bacteria, hydrogen peroxide in vapour form is shown to be effective against a variety of organisms including certain types of hard-to-kill nematode worms and prions, thus finding use in animal husbandry as well [26][32][27,33]. This widespread use of H2O2 in multiple industries is due to it being considered an “ideal” biocide depending on how it is used [3][4]. An “ideal” biocide as defined by McDonnell [26][27] as one that must be safe to use, easy to store, easy to apply and have a long-lasting effect, as well as being environmentally friendly and being chemically compatible with the surface it is applied on. An assessment of hydrogen peroxide vapour (HPV) against the attributes of an ideal biocide can be outlined as follows:- (i)
-
Efficacy: A significant number of in vitro and in vivo studies have demonstrated the efficiency of H2O2, both in liquid and vapour phases, against organisms ranging from highly resistant bacterial endospores to enveloped viruses [18][31][33][34][35][36][19,32,34,35,36,37]. According to these studies, antimicrobial activities depend on the concentration of H2O2, the exposure time and the method of application.
- (ii)
-
Safety: Hydrogen peroxide is applied to the skin for wound disinfection and used in acne products in liquid form at a low concentration of less than 3% w/w; this concentration level is considered very safe for use on human skin [29][37][30,38]. However, with an increase in concentration, a decreased tissue compatibility has been reported [29][37][30,38]. The safety of H2O2 is entirely dependent on how it is used. Owing to the absence (or to the low toxicity effects), H2O2 is seen as an excellent option for replacing more toxic chemicals like formaldehyde, which is known to be carcinogenic, and ethylene oxide, which has high toxicity and carcinogenicity concerns [38][39][40][39,40,41]. A major advantage of modern hydrogen peroxide vapour systems is that they can be easily set up and operated remotely, thus eliminating contact with the operator and reducing risk. The permissible exposure limit weighted over 8 h by the OSHA (Occupational Safety and Health Administration) in the United States is 1 ppm, whereas an immediate danger to life or health is posed at 75 ppm [41][42].
- (iii)
-
Environmental impact: The environmental impact of hydrogen peroxide is entirely dependent on how it is used. HPV slowly decomposes into water and oxygen and, because of this, it is considered safe for the environment [42][43]. As a result, no harmful residues are left on surfaces. The relatively unstable peroxide bond leads to its natural decomposition.
- (iv)
-
Ease of use: Factors that impact the ease of use of H2O2 are its concentration and method of application. For example, hydrogen peroxide is highly effective when used in vapour form as it can easily reach crevices and other hard-to-reach areas. This can also be ideal for large-area decontamination as multiple machines can be used at the same time. Modern, no-touch HPV systems reduce the number of labour hours when compared with traditional decontamination methods, leading to a reduction in labour costs.
- (v)
-
Stability: Hydrogen peroxide is stable in water and other formulations, depending on its purity and storage conditions. It is important that hydrogen peroxide is stored under conditions recommended by the manufacturer. Dissociation of hydrogen peroxide can take place if stored incorrectly. This will reduce the concentration of hydrogen peroxide in the solution and will have an impact on its antimicrobial efficacy.
- (vi)
-
Compatibility with surface materials: Hydrogen peroxide can be safe to surfaces, depending on how it is used. Being an oxidising agent, it can oxidise certain metallic and plastic surfaces when used in higher concentrations in liquid form [31][32]. However, these effects can be prevented when H2O2 is used in vapour form, which is considered to be gentle to surfaces and electrical equipment that are key parts of hospital environments. Boyce et al. [43][44] studied the impact of microcondensation HPV room decontamination on hospital physiological monitors over an 8-year period and observed that there was no increase in maintenance service calls; in fact, a rather unexplained decrease in maintenance was apparent. Furthermore, a recent study by Sher and Mulder [44][45] on the use of vapour-phase and aerosolised hydrogen peroxide for disinfection of dental surgery areas found no damage to any surface in these surgery areas. The effect of HPV on three metallic materials was characterised by Gale et al. [45][46], and no systematic effects were seen on the tensile strength or post-HPV-treated corrosion resistance of the alloys tested. Microstructural changes were seen to be confined to the areas adjacent to the exposed surface and were considered to be relatively small [45][46].
3. Mechanism of Biocidal Action
Hydrogen peroxide in liquid and gaseous forms has been shown to provide excellent antimicrobial activities against a broad spectrum of organisms. However, there is a lack of knowledge of the mechanism underlying its biocidal action; in spite of its demonstrated effectiveness in destroying infectious microorganisms, there remains a need to critically understand its mechanism by performing studies that simultaneously measure damage to all bacterial cell components and assess the correlation of this damage with a reduction in viable cell count [52][53]. The main mechanism leading to decontamination through the use of hydrogen peroxide has been thought to be via the deactivation of microorganisms through the oxidation of macromolecules that form viral and cellular structure/function, such as lipids, carbohydrates, proteins and nucleic acids [26][31][27,32]. However, in a study by Linley et al. [56][95] on the mechanism of cytotoxicity and genotoxicity of H2O2, it was proposed that the mechanism is due to localised formation of short-lived hydroxyl radicals through the intracellular reaction between Fe2+ ions and H2O2 (known as the Fenton’s reaction). Evidence for the Fenton’s reaction leading to the biocidal action of H2O2 on bacterial cells was sought by Repine et al. [57][96], who grew S. aureus bacteria in a nutrient broth with increased concentrations of iron. This approach effectively increased the iron content in S. aureus cells, and this was associated with a significant enhancement in the killing of bacterial cells when they were exposed to H2O2. The destruction of the cell walls of bacteria is dependent on the overall extent of peroxide-induced damage and on the effect on target cells, which have the ability to repair DNA damage. This implies that bacterial strains that are exposed to H2O2 have a reduced ability to repair DNA damage and are, therefore, more susceptible to be killed from exposure to H2O2 [57][96]. Since viruses have no repair mechanisms, McDonell [31][32] has suggested that excessive damage to viral nucleic acids should, therefore, be considered important in the overall virucidal effect. However, there is no evidence to support this. Indirect evidence of DNA damage in E. coli following exposure to H2O2 was provided by Imlay and Linn [58][97], who also proposed two kinetically distinguishable modes of killing of bacteria. The killing of bacterial cells at lower H2O2 concentrations was referred to as mode one and was reported to take place by means of DNA damage. Mode-one killing was observed to be maximal at concentrations between 1 and 2 mM of H2O2 [58][97]. Exposure to H2O2 was observed to lead to damage in a dose-dependent manner; this damage could undergo repair during a growth lag, but while cell growth occurred, there was no evidence of septation. The failure to successfully complete the repair of cells would lead to mode-two killing, which was evident at higher H2O2 concentrations. The researcheuthors [58][97] thought that mode-one killing was probably internal, while mode-two killing could be external. If this were indeed the case, mode-one killing would be expected to be diffusion-controlled. However, an earlier investigation by Schwartz et al. [59][98] had suggested otherwise. Brandi et al. [60][99] noted a similar pattern of bimodal killing in their study on the effect of HPV on E. coli. These researcheuthors suggested that cell membrane damage leading to a reduction in cell volume is the major component of mode-two killing, whereas no such effect was seen in mode-one killing. This observation actually strengthens the proposal that the biocidal mechanism upon exposure to H2O2 is due to the Fenton’s reaction via mode-two killing and is dependent on the presence of hydroxyl radicals, unlike mode-one killing. Furthermore, it is important to note that the oxidation–reduction potential (ORP) of hydrogen peroxide in a solution plays a crucial role in the mechanism of antimicrobial action. The extent and the rate of the Fenton’s reaction in a solution will be directly affected by the ORP of the solution. A higher ORP indicates a more oxidising environment, implying a greater tendency for H2O2 to donate electrons and form hydroxyl radicals; hence, a more efficient and potent antimicrobial action could be expected. According to Finnegan et al. [52][53], vaporised hydrogen peroxide interacted differently against amino acids when compared to liquid hydrogen peroxide. These researcheuthors [52][53] observed that liquid hydrogen peroxide at different concentrations was able to oxidise amino acids like cysteine, methionine, lysine, histidine and glycine, whereas vaporised hydrogen peroxide was unable to oxidise amino acids [52][53]. However, vapour-phase hydrogen peroxide was able to degrade aldolase and BSA completely, whereas no impact was observed when hydrogen peroxide was used in the liquid phase. The damage to various macromolecular cell targets upon the treatment of E. coli with liquid- and vapour-phase hydrogen peroxide, as studied by Linley [61][100], is depicted in Figure 1. Similar results showing that vapour-phase hydrogen peroxide was able to degrade protein oxidatively in comparison to liquid-phase hydrogen peroxide were also reported by McDonnell [62][101] in his studies on the neutralisation of bacterial protein toxins. These studies serve to highlight the difference in efficacy between vapour- and liquid-phase hydrogen peroxide. The difficulty with most of these studies in understanding the mechanism of killing of bacteria through the use of hydrogen peroxide vapour is that entire cells are exposed to hydrogen peroxide, and this results in a variety of direct and indirect effects as the causes for cell death.There is still a need for further work to be carried out to improve the current understanding of the exact killing mechanism of vapour-phase hydrogen peroxide. Earlier studies from the 1990s, such as that of Klapes and Vesley [40][41], considered the application of vapour-phase hydrogen peroxide as a sterilant to be “clearly still in its infancy” due to the lack of understanding of the mechanism of action and the factors influencing its effects. Almost twenty years later, Hall et al. [63][62], in their study on using hydrogen peroxide vapour to deactivate Mycobacterium tuberculosis, stated that “the exact mechanism of action of HPV remains to be fully elucidated”.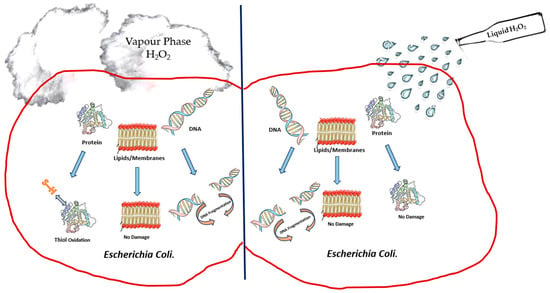 Figure 1.Depiction of damage to cell components ofE. coliupon treatment with liquid-phase hydrogen peroxide and vapour-phase hydrogen peroxide.
Figure 1.Depiction of damage to cell components ofE. coliupon treatment with liquid-phase hydrogen peroxide and vapour-phase hydrogen peroxide.
