Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ronald B. Brown | -- | 1487 | 2024-03-13 14:07:57 | | | |
| 2 | Peter Tang | Meta information modification | 1487 | 2024-03-14 03:53:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Brown, R.B. Polypharmacy in Older Patients with COVID-19. Encyclopedia. Available online: https://encyclopedia.pub/entry/56223 (accessed on 08 February 2026).
Brown RB. Polypharmacy in Older Patients with COVID-19. Encyclopedia. Available at: https://encyclopedia.pub/entry/56223. Accessed February 08, 2026.
Brown, Ronald B.. "Polypharmacy in Older Patients with COVID-19" Encyclopedia, https://encyclopedia.pub/entry/56223 (accessed February 08, 2026).
Brown, R.B. (2024, March 13). Polypharmacy in Older Patients with COVID-19. In Encyclopedia. https://encyclopedia.pub/entry/56223
Brown, Ronald B.. "Polypharmacy in Older Patients with COVID-19." Encyclopedia. Web. 13 March, 2024.
Copy Citation
Polypharmacy is the use of multiple and potentially inappropriate medications (PIMs) that lack sufficient evidence of benefits and/or increase the risks of adverse drug reactions. It is an increasing problem among older adults. The global polypharmacy prevalence is 34.6% in patients with COVID-19, and polypharmacy in COVID-19 increases with age.
polypharmacy
potentially inappropriate medications
COVID-19
comorbid conditions
clinical trials
absolute risk reduction
relative risk reduction
number needed to treat
1. Introduction
Polypharmacy is the use of multiple and potentially inappropriate medications (PIMs) that lack sufficient evidence of benefits and/or increase the risks of adverse drug reactions [1]. The prevalence of polypharmacy is high among older populations [2], which increases health risks in older adults who are already burdened with chronic diseases and infectious illnesses such as COVID-19. Recently, polypharmacy in older adults with cardiovascular disease has almost doubled annual U.S. healthcare expenditures and tripled pharmacy costs [3]. The risk factors for polypharmacy in older adults include inappropriate prescribing by physicians [4], but other contributing factors need to be investigated to address this growing healthcare problem.
A cross-sectional study of seven European cities recently found that polypharmacy is common among relatively healthy older adults and that factors such as being a woman, older age, a greater body mass index, and a greater number of comorbidities were associated with increased odds of polypharmacy [5]. Despite having a high level of medication knowledge, public service workers in France who used “multiple sources of trustworthy information” had increased exposure to polypharmacy, including the overuse of antibiotics and hypnotics that induce sleep [6]. The position statement of the International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP) asserts that, when prescribing without definitive benefits, “less is more” for many older adults [7]. IGRIMUP advocates for “a shift in medical education, research, and diagnostic frameworks, and re-examination of the measures used as quality indicators.”
2. Polypharmacy in Older Adults with COVID-19
Harvard researchers estimated that, during the COVID-19 pandemic, adults over 65 years of age accounted for 80% of hospitalized COVID-19 cases and had a 23 times higher risk of death than people under 65 years [8]. A meta-regression analysis of approximately 190,000 patients with COVID-19 found that the global polypharmacy prevalence is 34.6% and that polypharmacy increases with age in patients with COVID-19 [9]. The authors also noted that people with polypharmacy have an increased risk and severity of COVID-19, along with more kidney problems, more drug interactions, and an increased mortality.
Researchers found that almost one-third of older adult patients with COVID-19 in a Malaysian population had been prescribed a PIM [10], based on Beers assessment tool criteria [11] and criteria from the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP) [12]. Beers 2019 criteria are intended to “improve medication selection; educate clinicians and patients; reduce adverse drug events; and serve as a tool for evaluating quality of care, cost, and patterns of drug use of older adults” [11].
A Canadian population-based cohort study that analyzed data from the Quebec Integrated Chronic Disease Surveillance System found that the use of 5–9 medications by older adults with COVID-19 was associated with a 1.11 relative risk of hospitalizations compared to the use of 0–4 medications, and the relative risks ranged up to 1.62 for 20 or more medications [13]. Deaths among patients with COVID-19 were also associated with a relative risk of 1.13 for 5–9 medications, ranging up to 1.97 for 20 or more. Importantly, the researchers of the cohort study had adjusted for the confounding effects of age and comorbid chronic diseases, suggesting that polypharmacy may impose an iatrogenic burden on patients.
Of relevance, the most common chronic conditions in U.S. adults 65 years and older receiving Medicare benefits in 2010 were high blood pressure (61%), high cholesterol (48%), ischemic heart disease (34%), arthritis (31%), and diabetes (28%) [14]. Similarly, a more recent retrospective cohort study of older Italian adults hospitalized with COVID-19 found that the most common comorbidities included high blood pressure, cardiovascular disease, dementia or cerebrovascular disease, and diabetes [15].
3. RCT Analyses for Medications in Polypharmacy
This section presents a small sample of analyses of data reported in clinical trials for medications to treat the most common chronic conditions in older adults, including older adults with COVID-19. The types of medications analyzed include an antihypertensive for high blood pressure, a statin for high cholesterol, an anticoagulant for ischemic heart disease, and an antihyperglycemic agent for diabetes. A comprehensive review and meta-analysis of RCTs for each type of medication are beyond the scope of the present research. Accordingly, the analyses in this research are not representative of the body of literature and are purposively selected from a convenience sample to demonstrate examples of large discrepancies between reported RRRs and unreported ARRs. More research of a wider selection of medications commonly used by older adults is needed to verify the important implications of the analyses in the present research.
The ARRs in the medication clinical trial analyses presented below are calculated by subtracting the rate of events in the trial’s treatment group from the rate of events in the placebo group. The RRRs are calculated by dividing the trial’s ARR by the rate of events in the placebo group. The number needed to treat (NNT), the number of people who need to be treated to reduce one event, is calculated as the reciprocal of the ARR, and 95% confidence intervals are calculated using formulas provided elsewhere [16].
Importantly, relative risk measures are useful in uncontrolled observational studies to compare proportions of associations between exposures and disease risks, while absolute risk measures are useful to evaluate the strength of causative relationships in experimentally controlled studies, such as RCTs [17]. Furthermore, RCTs with identical RRRs can have different ARRs depending on differences in the baseline risk or placebo group event rate. For example, an RCT with a 50% RRR will have an ARR of only 1% if the baseline risk is 2%. However, the RRR remains at 50% if the ARR and baseline risk double to 2% and 4%, respectively.
4. Determinants and Effects of Polypharmacy
The determinants and effects of polypharmacy apply to COVID-19 as well as chronic conditions in older adults. Figure 1 is a directed acyclic graph (DAG) created by the present author to show the relationship of the findings in the present research. The DAG shows that medication clinical trial reporting bias and unreported ARRs are upstream determinants leading to the downstream effects of biased medication benefits, poor clinical decision making, PIMs, and patient adverse effects from polypharmacy. Obviously, patients, clinicians, public health administrators and policy makers, pharmacists, pharmaceutical companies, researchers, educators, regulators, consumers, and other people involved with prescription medications are all eventually affected by upstream outcome reporting bias in medication RCTs.
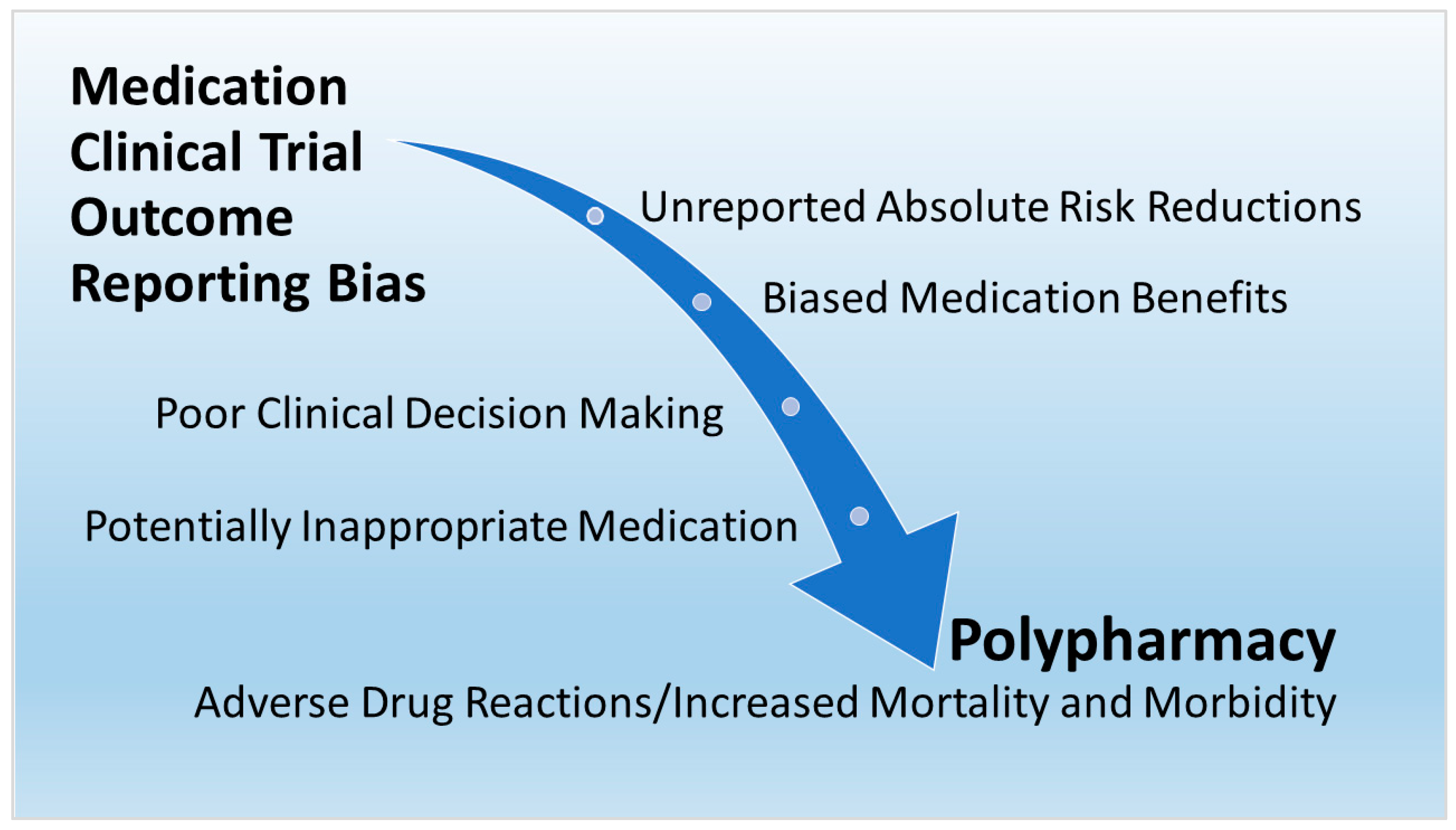
Figure 1. Upstream determinants and downstream effects of polypharmacy.
A recent qualitative study that interviewed pharmacists and general practitioners explored the determinants of polypharmacy, but none of the interviewed participants appeared to challenge the reliability of the reported clinical trial outcomes of the medications that they prescribed and dispensed to patients [18]. An American national action plan to eliminate medication overload confirms that “health care providers do not have clear, accurate, up-to-date information on the harms and benefits of medications when making prescribing decisions”, and “clinicians and patients lack critical information and skills they need to appraise the evidence” [19]. The present research provides critical information from analyses of unreported outcomes in medication clinical trials to help clinicians and patients reappraise evidence and improve medication decision making. Considering the pervasiveness of outcome reporting bias throughout the research literature, much more work lies ahead in establishing preventive measures to reduce outcome reporting bias in published RCTs [20].
The U.S. National Institute on Aging (NIA) notes that scientists are just beginning to explore the new field of deprescribing, and NIA has established a US Deprescribing Research Network to reduce polypharmacy harm in older adults [21]. Future deprescribing research should address outcome reporting bias in RCTs as a key (and often overlooked) upstream factor of polypharmacy. Additionally, “pharmaceutical companies are suspected of putting profits above public interest, using marketing techniques to distort scientific evidence, and actively influencing both physicians and health policy makers” [22].
An editorial in The Journal of the American Medical Association emphasized that patients “need to have trust in treatments recommended by clinicians during the time of COVID-19, including taking medications indefinitely for chronic conditions” [23]. The editorial described how patients must be reassured that their best personal needs are considered above the financial interests of others, but the editorial also pointed out that many factors have contributed to the public’s declining trust in the healthcare system over the past half century, which has been exacerbated by challenges of the COVID-19 pandemic. The editorial further pointed out that regaining the public’s trust requires “a broad, multifaceted approach, including expanding diversity within the scientific community, engaging communities in the design of clinical trials and the interpretation of results, and increasing the number of underrepresented minority groups in clinical trials.” Relevant to polypharmacy discussed in the present research, public health campaigns are urgently needed to disseminate information and educate the public about medication outcome reporting bias as a determinant of the inappropriate use of medications in older adults.
References
- Díez, R.; Cadenas, R.; Susperregui, J.; Sahagún, A.M.; Fernández, N.; García, J.J.; Sierra, M.; López, C. Potentially Inappropriate Medication and Polypharmacy in Nursing Home Residents: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 3808.
- Young, E.H.; Pan, S.; Yap, A.G.; Reveles, K.R.; Bhakta, K. Polypharmacy prevalence in older adults seen in United States physician offices from 2009 to 2016. PLoS ONE 2021, 16, e0255642.
- Kwak, M.J.; Chang, M.; Chiadika, S.; Aguilar, D.; Avritscher, E.; Deshmukh, A.; Goyal, P.; Kim, D.H.; Aparasu, R.; Holmes, H.M. Healthcare Expenditure Associated with Polypharmacy in Older Adults with Cardiovascular Diseases. Am. J. Cardiol. 2022, 169, 156–158.
- Mortazavi, S.S.; Shati, M.; Malakouti, S.K.; Khankeh, H.R.; Mehravaran, S.; Ahmadi, F. Physicians’ role in the development of inappropriate polypharmacy among older adults in Iran: A qualitative study. BMJ Open 2019, 9, e024128.
- de Godoi Rezende Costa Molino, C.; Chocano-Bedoya, P.O.; Sadlon, A.; Theiler, R.; Orav, J.E.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; Kanis, J.A.; Guyonnet, S.; et al. Prevalence of polypharmacy in community-dwelling older adults from seven centres in five European countries: A cross-sectional study of DO-HEALTH. BMJ Open 2022, 12, e051881.
- Lvovschi, V.-E.; Carrouel, F.; du Sartz de Vigneulles, B.; Lamure, M.; Motyka, G.; Fraticelli, L.; Dussart, C. Knowledge, Attitudes and Practices Related to Medication, Antibiotics, and Vaccination among Public Service Population: National Survey Conducted in France. Int. J. Environ. Res. Public Health 2022, 19, 14044.
- Mangin, D.; Bahat, G.; Golomb, B.A.; Mallery, L.H.; Moorhouse, P.; Onder, G.; Petrovic, M.; Garfinkel, D. International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP): Position Statement and 10 Recommendations for Action. Drugs Aging 2018, 35, 575–587.
- Mueller, A.L.; McNamara, M.S.; Sinclair, D.A. Why does COVID-19 disproportionately affect older people? Aging 2020, 12, 9959–9981.
- Ghasemi, H.; Darvishi, N.; Salari, N.; Hosseinian-Far, A.; Akbari, H.; Mohammadi, M. Global prevalence of polypharmacy among the COVID-19 patients: A comprehensive systematic review and meta-analysis of observational studies. Trop. Med. Health 2022, 50, 60.
- Chang, C.T.; Mohd Shariff, S.M.; Abu Bakar, N.S.; Ramzuzzaman, N.S.; Lim, C.K.; Lim, E.Y.J.; Ong, P.S.; Lee, J.M.; Tan, A.Y.; Kamis, S.F.; et al. Polypharmacy and potentially inappropriate medications among hospitalized older adults with COVID-19 in Malaysian tertiary hospitals. J. Pharm. Policy Pract. 2023, 16, 2.
- Fick, D.; Semla, T.; Steinman, M.; Beizer, J.; Brandt, N.; Dombrowski, R.; DuBeau, C.; Pezzullo, L.; Epplin, J.; Flanagan, N.; et al. American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 674–694.
- Gallagher, P.F.; O’Connor, M.N.; O’Mahony, D. Prevention of potentially inappropriate prescribing for elderly patients: A randomized controlled trial using STOPP/START criteria. Clin. Pharm. 2011, 89, 845–854.
- Sirois, C.; Boiteau, V.; Chiu, Y.; Gilca, R.; Simard, M. Exploring the associations between polypharmacy and COVID-19-related hospitalisations and deaths: A population-based cohort study among older adults in Quebec, Canada. BMJ Open 2022, 12, e060295.
- cms.gov. Chronic Conditions among Medicare Beneficiaries, Chartbook, 2012 Edition. Available online: https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/chronic-conditions/downloads/2012Chartbook.pdf (accessed on 17 April 2023).
- Mahmoud, M.; Carmisciano, L.; Tagliafico, L.; Muzyka, M.; Rosa, G.; Signori, A.; Bassetti, M.; Nencioni, A.; Monacelli, F. Patterns of Comorbidity and In-Hospital Mortality in Older Patients with COVID-19 Infection. Front. Med. 2021, 8, 726837.
- Brown, R.B. Outcome Reporting Bias in COVID-19 mRNA Vaccine Clinical Trials. Medicina 2021, 57, 199.
- Brown, R.B. Relative risk reduction: Misinformative measure in clinical trials and COVID-19 vaccine efficacy. Dialogues Health 2022, 1, 100074.
- Taghy, N.; Ramel, V.; Rivadeneyra, A.; Carrouel, F.; Cambon, L.; Dussart, C. Exploring the Determinants of Polypharmacy Prescribing and Dispensing Behaviors in Primary Care for the Elderly—Qualitative Study. Int. J. Environ. Res. Public Health 2023, 20, 1389.
- lowninstitute.org. Eliminating Medication Overload: A National Action Plan. Working Group on Medication Overload. The Lown Institute. Available online: https://lowninstitute.org/reports/eliminating-medication-overload-a-national-action-plan/ (accessed on 19 April 2023).
- Ioannidis, J.P.; Caplan, A.L.; Dal-Ré, R. Outcome reporting bias in clinical trials: Why monitoring matters. BMJ 2017, 356, j408.
- deprescribingresearch.org. U.S. Deprescribing Research Network—National Institue of Aging. Available online: https://deprescribingresearch.org/ (accessed on 19 April 2023).
- Pahus, L.; Suehs, C.M.; Halimi, L.; Bourdin, A.; Chanez, P.; Jaffuel, D.; Marciano, J.; Gamez, A.S.; Vachier, I.; Molinari, N. Patient distrust in pharmaceutical companies: An explanation for women under-representation in respiratory clinical trials? BMC Med. Ethics 2020, 21, 72.
- Baker, D.W. Trust in Health Care in the Time of COVID-19. JAMA 2020, 324, 2373–2375.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
352
Revisions:
2 times
(View History)
Update Date:
14 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




