Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ronald B. Brown and Version 2 by Peter Tang.
Polypharmacy is the use of multiple and potentially inappropriate medications (PIMs) that lack sufficient evidence of benefits and/or increase the risks of adverse drug reactions. It is an increasing problem among older adults. The global polypharmacy prevalence is 34.6% in patients with COVID-19, and polypharmacy in COVID-19 increases with age.
- polypharmacy
- potentially inappropriate medications
- COVID-19
- comorbid conditions
- clinical trials
- absolute risk reduction
- relative risk reduction
- number needed to treat
1. Introduction
Polypharmacy is the use of multiple and potentially inappropriate medications (PIMs) that lack sufficient evidence of benefits and/or increase the risks of adverse drug reactions [1]. The prevalence of polypharmacy is high among older populations [2], which increases health risks in older adults who are already burdened with chronic diseases and infectious illnesses such as COVID-19. Recently, polypharmacy in older adults with cardiovascular disease has almost doubled annual U.S. healthcare expenditures and tripled pharmacy costs [3]. The risk factors for polypharmacy in older adults include inappropriate prescribing by physicians [4], but other contributing factors need to be investigated to address this growing healthcare problem.
A cross-sectional study of seven European cities recently found that polypharmacy is common among relatively healthy older adults and that factors such as being a woman, older age, a greater body mass index, and a greater number of comorbidities were associated with increased odds of polypharmacy [5]. Despite having a high level of medication knowledge, public service workers in France who used “multiple sources of trustworthy information” had increased exposure to polypharmacy, including the overuse of antibiotics and hypnotics that induce sleep [6]. The position statement of the International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP) asserts that, when prescribing without definitive benefits, “less is more” for many older adults [7]. IGRIMUP advocates for “a shift in medical education, research, and diagnostic frameworks, and re-examination of the measures used as quality indicators.”
2. Polypharmacy in Older Adults with COVID-19
Harvard researchers estimated that, during the COVID-19 pandemic, adults over 65 years of age accounted for 80% of hospitalized COVID-19 cases and had a 23 times higher risk of death than people under 65 years [8][14]. A meta-regression analysis of approximately 190,000 patients with COVID-19 found that the global polypharmacy prevalence is 34.6% and that polypharmacy increases with age in patients with COVID-19 [9][15]. The authors also noted that people with polypharmacy have an increased risk and severity of COVID-19, along with more kidney problems, more drug interactions, and an increased mortality. Researchers found that almost one-third of older adult patients with COVID-19 in a Malaysian population had been prescribed a PIM [10][16], based on Beers assessment tool criteria [11][17] and criteria from the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP) [12][18]. Beers 2019 criteria are intended to “improve medication selection; educate clinicians and patients; reduce adverse drug events; and serve as a tool for evaluating quality of care, cost, and patterns of drug use of older adults” [11][17]. A Canadian population-based cohort study that analyzed data from the Quebec Integrated Chronic Disease Surveillance System found that the use of 5–9 medications by older adults with COVID-19 was associated with a 1.11 relative risk of hospitalizations compared to the use of 0–4 medications, and the relative risks ranged up to 1.62 for 20 or more medications [13][19]. Deaths among patients with COVID-19 were also associated with a relative risk of 1.13 for 5–9 medications, ranging up to 1.97 for 20 or more. Importantly, the researchers of the cohort study had adjusted for the confounding effects of age and comorbid chronic diseases, suggesting that polypharmacy may impose an iatrogenic burden on patients. Of relevance, the most common chronic conditions in U.S. adults 65 years and older receiving Medicare benefits in 2010 were high blood pressure (61%), high cholesterol (48%), ischemic heart disease (34%), arthritis (31%), and diabetes (28%) [14][20]. Similarly, a more recent retrospective cohort study of older Italian adults hospitalized with COVID-19 found that the most common comorbidities included high blood pressure, cardiovascular disease, dementia or cerebrovascular disease, and diabetes [15][21].3. RCT Analyses for Medications in Polypharmacy
This section presents a small sample of analyses of data reported in clinical trials for medications to treat the most common chronic conditions in older adults, including older adults with COVID-19. The types of medications analyzed include an antihypertensive for high blood pressure, a statin for high cholesterol, an anticoagulant for ischemic heart disease, and an antihyperglycemic agent for diabetes. A comprehensive review and meta-analysis of RCTs for each type of medication are beyond the scope of the present respapearchr. Accordingly, the analyses in this researchpaper are not representative of the body of literature and are purposively selected from a convenience sample to demonstrate examples of large discrepancies between reported RRRs and unreported ARRs. More research of a wider selection of medications commonly used by older adults is needed to verify the important implications of the analyses in the present researchpaper. The ARRs in the medication clinical trial analyses presented below are calculated by subtracting the rate of events in the trial’s treatment group from the rate of events in the placebo group. The RRRs are calculated by dividing the trial’s ARR by the rate of events in the placebo group. The number needed to treat (NNT), the number of people who need to be treated to reduce one event, is calculated as the reciprocal of the ARR, and 95% confidence intervals are calculated using formulas provided elsewhere [16][22]. Importantly, relative risk measures are useful in uncontrolled observational studies to compare proportions of associations between exposures and disease risks, while absolute risk measures are useful to evaluate the strength of causative relationships in experimentally controlled studies, such as RCTs [17][23]. Furthermore, RCTs with identical RRRs can have different ARRs depending on differences in the baseline risk or placebo group event rate. For example, an RCT with a 50% RRR will have an ARR of only 1% if the baseline risk is 2%. However, the RRR remains at 50% if the ARR and baseline risk double to 2% and 4%, respectively.4. Determinants and Effects of Polypharmacy
The determinants and effects of polypharmacy apply to COVID-19 as well as chronic conditions in older adults. Figure 1 is a directed acyclic graph (DAG) created by the present author to show the relationship of the findings in the present rpapesearchr. The DAG shows that medication clinical trial reporting bias and unreported ARRs are upstream determinants leading to the downstream effects of biased medication benefits, poor clinical decision making, PIMs, and patient adverse effects from polypharmacy. Obviously, patients, clinicians, public health administrators and policy makers, pharmacists, pharmaceutical companies, researchers, educators, regulators, consumers, and other people involved with prescription medications are all eventually affected by upstream outcome reporting bias in medication RCTs.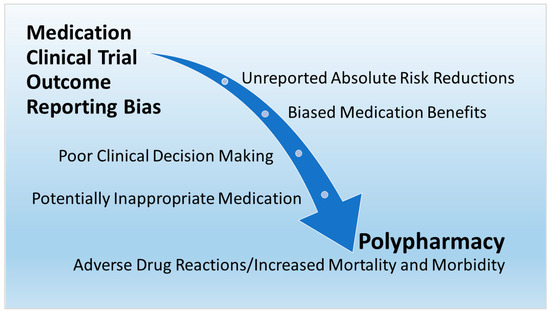
Figure 1.
Upstream determinants and downstream effects of polypharmacy.
