Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pierluigi Scalia | -- | 2021 | 2024-03-13 10:16:42 | | | |
| 2 | Peter Tang | Meta information modification | 2021 | 2024-03-13 10:20:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Scalia, P.; Giordano, A.; Williams, S.J. IGF-II–Insulin Receptor Isoform-A Autocrine Signal in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/56195 (accessed on 07 February 2026).
Scalia P, Giordano A, Williams SJ. IGF-II–Insulin Receptor Isoform-A Autocrine Signal in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/56195. Accessed February 07, 2026.
Scalia, Pierluigi, Antonio Giordano, Stephen J. Williams. "IGF-II–Insulin Receptor Isoform-A Autocrine Signal in Cancer" Encyclopedia, https://encyclopedia.pub/entry/56195 (accessed February 07, 2026).
Scalia, P., Giordano, A., & Williams, S.J. (2024, March 13). IGF-II–Insulin Receptor Isoform-A Autocrine Signal in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/56195
Scalia, Pierluigi, et al. "IGF-II–Insulin Receptor Isoform-A Autocrine Signal in Cancer." Encyclopedia. Web. 13 March, 2024.
Copy Citation
Insulin receptor overexpression is a common event in human cancer. Its overexpression is associated with a relative increase in the expression of its isoform A (IRA), a shorter variant lacking 11 aa in the extracellular domain, conferring high affinity for the binding of IGF-II along with added intracellular signaling specificity for this ligand. Since IGF-II is secreted by the vast majority of malignant solid cancers, where it establishes autocrine stimuli, the co-expression of IGF-II and IRA in cancer provides specific advantages such as apoptosis escape, growth, and proliferation to those cancers bearing such a co-expression pattern.
IGF(I/II/1R), Insulin-like Growth factor (1 or 2 or receptor)
IRA/IR-A
insulin receptor isoform A
IGF binding protein
mannose 6 phosphate receptor
Transferrin
vitronectin
hypoxia-inducible factor
Von Hippel-Lindau gene product
OCT
1. The Insulin–IGF Ligand and Receptor System in Cancer
The family of the insulin and IGF ligands and receptors are known for their central metabolic and growth-related functions spanning throughout phylogenetically distant organisms [1][2]. Up to the late 90s, the working model for the role of insulin, IGFs, and their receptors in cancer was based on a scenario dominated by two cousin receptors (the IGF-IR and the insulin receptor) used by their own ligands (IGF-I for the IGF-I receptor and insulin for the insulin receptor), with the IGF-I receptor being considered the sole active mediator of the IGF-I and IGF-II effects, making the latter a favorite target for halting the actions of IGFs in cancer [3][4]. This paradigm (the IGF1R mandatory transducer hypothesis) has undergone many changes over time with the realization, first, that the IGFIR is able to form hybrid variants with the insulin receptor [5][6] and, second, that the insulin receptor (IR) could mediate IGF-specific effects. Indeed, genetic evidence of the permissive role of the insulin receptor in a number of developmental and body-size effects mediated by IGF-II had been shown in genetic studies conducted both in null mice [7] and in transgenic mouse models [8]. However, cellular studies were not able to reproduce such a result in vitro until a specific isoform of the insulin receptor, lacking 12 aa in the extracellular portion corresponding to exon 11 (IRA), was shown to be the high-affinity receptor for IGF-II in both fetal and cancer cells [9]. This finding, besides changing a long-rooted view, also presented a distinct role for the insulin receptor far beyond defining it as a pure metabolic and growth permissive mediator. A number of subsequent studies have also demonstrated insulin and IGF ligand-specific differences in their activation of the IRA. In particular, such differences have been demonstrated at the gene expression level [10] and at the signaling level [11][12]. In this regard, it is worth noting that IGF-II has been found to be able to bind and transduce signals via both the homo-tetrameric, high-affinity RTKs (IGF1R and IRA) and via its hetero-tetrameric (IGF1R/IRA) hybrid receptor in cancer [13].
2. IGF-II is a Bona Fide Oncogenic Ligand Tightly Regulated Under Development and a Commonly Selected Self-Stimulatory Signal in Cancer
In comparison to IGF-I (the main growth-hormone-induced ligand and physiological effect mediator) and as discussed below, IGF-II undergoes different and extensive regulation at the genetic, epigenetic, and post-transcriptional levels. Interestingly, the escape from such tight regulation, as observed in cancer, offers IGF-II distinctive advantages over IGF-I, mainly linked to its ability to activate specific developmental, cellular, and cancer-promoting signals via Insulin receptor A. Overall, IGF-II (a) has a wider possibility of transcriptional regulation and control at the gene promoter level via its four promoters and 10 exons [14][15], all of which produce a pre-pro-hormone and four isoform variants; (b) is epigenetically regulated via DNA-methylation-dependent and -methylation-independent mechanisms [16][17][18][19][20][21][22] with a paternal-restricted expression pattern which is typically lost in cancer (loss of imprinting), causing increased/biallelic expression and bloodstream secretion levels [23][24][25][26][27][28][29]; (c) displays post-translational variants derived via differential processing of its pre-pro-hormone leading, to an O-glycosylated high molecular weight form (also known as Big-IGF2) [30][31] retaining its binding and signaling activity for IRA [32] but with acquired capability to elude physiological binders such as the high-affinity scavenging receptor also known as igf2R (binding mannose 6-phosphate as well) and IGFBP3 [33]. This and the potential clinical implications have also been reviewed in References [34][35]. Finally, (d) additional types of regulation of the igf2 transcript linked to non-coding RNA products have also been demonstrated, adding a layer of additional regulation for the igf2 gene [36][37][38][39][40][41][42]. The escape from any of these regulatory mechanisms make IGF-II and, more so, its cancer-secreted variant (Big)IGF-II, an ideal autocrine signal for highly demanding cellular requirements such as those found throughout the tumorigenic process [32][33][43]. No less important, (e) IGF-II binds to the IGF1R, the Insulin receptor isoform A, and their hybrid tetra-dimeric forms under different physiological and pathological contexts to exert ligand-receptor-specific cellular effects [7][9][44][45]. The contextual roles mentioned above for IGF-I and IGF-II ligands in cancer are graphically summarized in Figure 1.
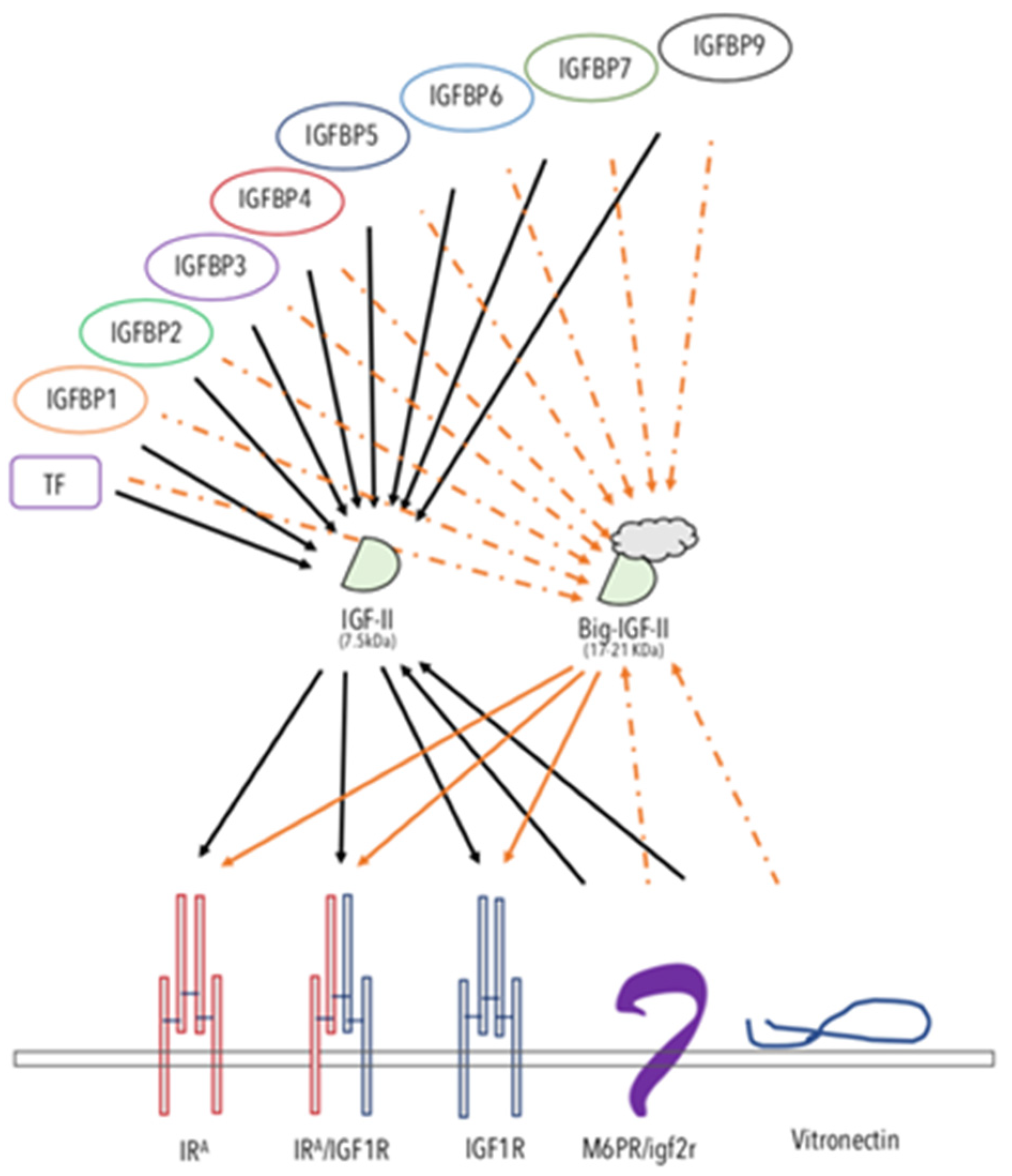
Figure 1. The IGF-II-binding/-neutralizing and -transducing system. The schematic figure summarizes the interactions reported in the literature for IGF-II. The known soluble, extracellular and/or membrane-bound IGF-II-binding proteins are displayed. The solid arrows represent experimentally supported interactions. The dashed arrows represent interactions that have been shown to either be impaired or not yet experimentally confirmed. Arrows from a ligand to its RTKs indicate activating–transducing properties. Arrows towards IGF-II indicate a binding–neutralizing effect. The overview of the comprehensive IGF ligands system role in cancer is shown in Figure 1.
3. The IGF-II Binders: A Fine-Tuned System for the Control of IGF-II Levels in the Extracellular and Tumor Microenvironment
Igf2R/m6pR. The non-transducing/scavenger high-affinity-binding membrane-bound protein known as igf2 receptor (reviewed in Reference [46]), initially thought to be an IGF-II biological mediator, exerts, indeed, most of its IGF-related effects by neutralizing IGF-II and subtracting it from other transducing interactions (namely from the IRA and the IGF1R receptor tyrosine kinases). The key evidence for such a view comes from the demonstration of the absence of a TK domain in its cloned structure [47] and from the oncogenic effect shown by null mutation of igf2rR/m6pR in mice [48]. Indeed, the tumor-suppressing effect of the igf2R/m6pR can be interpreted as further demonstration of the oncogenic potential of IGF-II when present in high levels in vertebrates either at focal tissue levels and/or in the whole organism bloodstream.
The IGFBPs 1-7 and 9. Insulin-like growth factor-II has been shown to bind to most of the soluble extracellular proteins of the IGFBP family, as reviewed elsewhere [49][50][51]. The cumulative effect of IGF-II binding proteins towards the IGF-II levels in the bloodstream might mitigate its increased exposure to local tissues. As a result, some authors have proposed the use of recombinant fragments of IGFBPs as tools to counteract IGF-II oncogenicity. However, the fact that cancer-secreted IGF-II has been found to interact poorly with IGFBPs [33][52][53] might be seen as an escape mechanism for all those cancers using IGF-II as an autocrine growth factor to sustain/maintain their malignant growth features. These potential limits should be taken into consideration.
Transferrin (TF). TF has been shown to be a constitutive component of the 150kDa trimeric IGF binding protein complex found in the bloodstream [51]. Its binding to IGFs (I and II) is less strong than other IGF–IGFBP interactions (where the highest affinity is shown with IGFBP3), and its physiological role is still to be determined.
Vitronectin (VTN). VTN is a constitutive component of the extracellular matrix, involved in cell-to-cell interactions [54][55]. VTN has been known to bind integrin (ITN) alpha5beta3 and, as such, has been also referred as to integrin receptor [56]. Interestingly, VTN, which bears a somatomedin-like domain, binds IGF-II with high affinity [57][58]. Although the physiological and pathological roles of VTN interaction with IGF-II are still to be determined, some evidence points at a suppressing role of VTN on IGF-II-induced proliferation and migration via interference with the IGF-II mitogenic signaling (Scalia et al., manuscript in preparation).
Overall, the studies on IGF-II physiological binders are in agreement with the genetic studies supporting a distinctive cancer-promoting role for this IGF, differentiating it from its related cousin, IGF-1. The finding that cancer-secreted IGF-II (big-IGF-II) skips the binding control exerted on mature IGF-II by the IGFBPs (as graphically summarized in Figure 1) suggests that more specific targeting strategies should be considered in order to target this factor in its cancer-specific context.
4. Autocrine IGFII and the IRA Isoform Co-Expression in Cancer: At the Root of IGF-I Receptor Block Resistance
A number of historical results obtained in igf1r null murine fibroblasts (r-cells) both in absence or presence (r+) of human IGFIR expression abundantly demonstrated the isolated mitogenic and growth-linked effects of the IGF-I receptor as a key permissive signal for most of the non-IGF RTKs already targeted in therapy [59][60]. This triggered the development of a number of IGF-IR specific MAbs [61][62][63][64] and small molecules [65][66]) by the pharma industry in the first decade of the new millennium [67]. Although the experimental evidence showing a functional role for the IGF-II/IRA both in embryonal fibroblasts and in cancer has been available since the late 90a, these findings did not seem to affect the rush of drug developers to bring IGF-IR specific blockers to clinical trials. The specific single blocking of IGFIR in phase II clinical studies failed [68][69]; the extent of the negative impact of anti-IGF1R monotherapy drugs in clinical studies because of the underscoring of the IGF-II/IRA role could have been easily avoided by including IGF-II/IRA testing in the associated companion diagnostics required for the selection of responsive patients [70].
5. The Autocrine IGF-II/IRA System and the Malignant Switch in Solid Tumors: Hints from the Hypoxic Network
Hypoxia is an intrinsic feature of solid cancers’ tridimensional growth, affecting the inner core of the growing tumor tissue at the pre-vascular stage and clearly affecting the extracellular tumoral microenvironment. Under these circumstances, a tight sequential relationship is established between hypoxia and the expression of hypoxia-induced genes, in which HIF isoforms and VHL have been shown to play a major role [71][72]. Among the factors that have been shown to be induced or upregulated under hypoxic conditions are VEGF, EphB4, and IGF-II [73][74][75][76][77][78][79][80][81]. However, if for VEGF and EphB4 a solid base of supporting evidence has established their role in angiogenesis and cancer blood vessel formation, in the case of IGF-II, its angiogenic role in the literature has been variably and interchangeably associated with the angiogenic role of IGF-I. Indeed, as mentioned before, there is evidence supporting the notion that IGF-I and IGF-II are all but interchangeable molecules under both physiological and pathological conditions, as shown by their differential affinity and signaling properties via the known IGF1R/IR RTKs. All these structural and ligand–receptor interaction differences provide plenty of biological opportunity for their diversified use by the cell under hypoxic conditions (typical of early-stage and overtly malignant cancers). As for the association of IGF-II with the hypoxic tumor microenvironment, what we know from the published literature is that (1) IGF-II, but not IGF-I, is responsible for the hypoglycemic paraneoplastic effects observed in a number of patients affected by aggressive solid cancers (IGF2omas) [34][35]; (2) that IGF-II (as well as VEGF-A and EphB4) can be upregulated by HIF and hypoxia [81][82][83]; (3) that VEGF, which also exerts autocrine signals [84], can be upregulated via the IGF-IR and the IRA [85][86][87] and is under the control of IGF-II and its autocrine loop under hypoxic experimental conditions [44][82][88]. Interestingly, (4) Hypoxia-induced HIF2alpha can regulate IGF-II [89] and (5) IGF-II can upregulate HIF1alpha, which is an inducer of VEGF [44][88]. All these functional links observed in solid cancers can determine a number of coordinated local events towards the acquisition and/or maintenance of angiogenic, invasive, and metastatic potential, and are compatible with an underscored role of IGF-II in the angiogenic switch, supporting its validation as an anti-angiogenic target. It is worth noting the effects that autocrine IGF-II exerts exclusively via the IRA independently of the IGF1R, such as in regards to EphB4 acute protein level regulation in certain cancers such as malignant mesothelioma [44], making the IGF-II/IRA signal in these cancers a distinctive, non-redundant ligand–receptor loop with targetable value. This has been observed in vitro and ex vivo using cancer cells exposed to their conditioned media (pH ~6.9–7.2), a feature common to the extracellular conditions found in solid cancer microenvironments in vivo.
References
- LeRoith, D.; Kavsan, V.M.; Koval, A.P.; Roberts, C.T., Jr. Phylogeny of the insulin-like growth factors (IGFs) and receptors: A molecular approach. Mol. Reprod. Dev. 1993, 35, 332–336.
- Chan, S.J.; Steiner, D.F. Insulin Through the Ages: Phylogeny of a Growth Promoting and Metabolic Regulatory Hormone. Integr. Comp. Biol. 2000, 40, 213–222.
- Avnet, S.; Sciacca, L.; Salerno, M.; Gancitano, G.; Cassarino, M.F.; Longhi, A.; Zakikhani, M.; Carboni, J.M.; Gottardis, M.; Giunti, A.; et al. Insulin receptor isoform A and insulin-like growth factor II as additional treatment targets in human osteosarcoma. Cancer Res. 2009, 69, 2443–2452.
- Ulanet, D.B.; Ludwig, D.L.; Kahn, C.R.; Hanahan, D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 10791–10798.
- Benyoucef, S.; Surinya, K.H.; Hadaschik, D.; Siddle, K. Characterization of insulin/IGF hybrid receptors: Contributions of the insulin receptor L2 and Fn1 domains and the alternatively spliced exon 11 sequence to ligand binding and receptor activation. Biochem. J. 2007, 403, 603–613.
- Blanquart, C.; Achi, J.; Issad, T. Characterization of IRA/IRB hybrid insulin receptors using bioluminescence resonance energy transfer. Biochem. Pharmacol. 2008, 76, 873–883.
- Louvi, A.; Accili, D.; Efstratiadis, A. Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Dev. Biol. 1997, 189, 33–48.
- Nakae, J.; Kido, Y.; Accili, D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr. Rev. 2001, 22, 818–835.
- Frasca, F.; Pandini, G.; Scalia, P.; Sciacca, L.; Mineo, R.; Costantino, A.; Goldfine, I.D.; Belfiore, A.; Vigneri, R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol. Cell. Biol. 1999, 19, 3278–3288.
- Pandini, G.; Conte, E.; Medico, E.; Sciacca, L.; Vigneri, R.; Belfiore, A. IGF-II binding to insulin receptor isoform A induces a partially different gene expression profile from insulin binding. Ann. N. Y. Acad. Sci. 2004, 1028, 450–456.
- Sacco, A.; Morcavallo, A.; Pandini, G.; Vigneri, R.; Belfiore, A. Differential signaling activation by insulin and insulin-like growth factors I and II upon binding to insulin receptor isoform A. Endocrinology 2009, 150, 3594–3602.
- Vella, V.; Nicolosi, M.L.; Giuliano, M.; Morrione, A.; Malaguarnera, R.; Belfiore, A. Insulin Receptor Isoform A Modulates Metabolic Reprogramming of Breast Cancer Cells in Response to IGF2 and Insulin Stimulation. Cells 2019, 8, 1017.
- Belfiore, A.; Malaguarnera, R.; Vella, V.; Lawrence, M.C.; Sciacca, L.; Frasca, F.; Morrione, A.; Vigneri, R. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr. Rev. 2017, 38, 379–431.
- Mineo, R.; Fichera, E.; Liang, S.J.; Fujita-Yamaguchi, Y. Promoter usage for insulin-like growth factor-II in cancerous and benign human breast, prostate, and bladder tissues, and confirmation of a 10th exon. Biochem. Biophys. Res. Commun. 2000, 268, 886–892.
- Brouwer-Visser, J.; Huang, G.S. IGF2 signaling and regulation in cancer. Cytokine Growth Factor Rev. 2015, 26, 371–377.
- Frost, J.M.; Monk, D.; Stojilkovic-Mikic, T.; Woodfine, K.; Chitty, L.S.; Murrell, A.; Stanier, P.; Moore, G.E. Evaluation of allelic expression of imprinted genes in adult human blood. PLoS ONE 2010, 5, e13556.
- Reik, W.; Constancia, M.; Dean, W.; Davies, K.; Bowden, L.; Murrell, A.; Feil, R.; Walter, J.; Kelsey, G. Igf2 imprinting in development and disease. Int. J. Dev. Biol. 2000, 44, 145–150.
- Zheng, Q.F.; Xu, B.; Wang, H.M.; Ding, L.H.; Liu, J.Y.; Zhu, L.Y.; Qiu, H.; Zhang, L.; Ni, G.Y.; Ye, J.; et al. Epigenetic alterations contribute to promoter activity of imprinting gene IGF2. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 117–124.
- Hu, J.F.; Oruganti, H.; Vu, T.H.; Hoffman, A.R. The role of histone acetylation in the allelic expression of the imprinted human insulin-like growth factor II gene. Biochem. Biophys. Res. Commun. 1998, 251, 403–408.
- Li, T.; Chen, H.; Li, W.; Cui, J.; Wang, G.; Hu, X.; Hoffman, A.R.; Hu, J. Promoter histone H3K27 methylation in the control of IGF2 imprinting in human tumor cell lines. Hum. Mol. Genet. 2014, 23, 117–128.
- Ishizaki, T.; Yoshie, M.; Yaginuma, Y.; Tanaka, T.; Ogawa, K. Loss of Igf2 imprinting in monoclonal mouse hepatic tumor cells is not associated with abnormal methylation patterns for the H19, Igf2, and Kvlqt1 differentially methylated regions. J. Biol. Chem. 2003, 278, 6222–6228.
- Wolffe, A.P. Transcriptional control: Imprinting insulation. Curr. Biol. 2000, 10, R463–R465.
- Cui, H. Loss of imprinting of IGF2 as an epigenetic marker for the risk of human cancer. Dis. Markers 2007, 23, 105–112.
- Christofori, G.; Naik, P.; Hanahan, D. Deregulation of both imprinted and expressed alleles of the insulin-like growth factor 2 gene during beta-cell tumorigenesis. Nat. Genet. 1995, 10, 196–201.
- Uchida, K.; Kondo, M.; Takeda, S.; Osada, H.; Takahashi, T.; Nakao, A. Altered transcriptional regulation of the insulin-like growth factor 2 gene in human hepatocellular carcinoma. Mol. Carcinog. 1997, 18, 193–198.
- Nakagawa, H.; Chadwick, R.B.; Peltomaki, P.; Plass, C.; Nakamura, Y.; de La Chapelle, A. Loss of imprinting of the insulin-like growth factor II gene occurs by biallelic methylation in a core region of H19-associated CTCF-binding sites in colorectal cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 591–596.
- Cui, H.; Cruz-Correa, M.; Giardiello, F.M.; Hutcheon, D.F.; Kafonek, D.R.; Brandenburg, S.; Wu, Y.; He, X.; Powe, N.R.; Feinberg, A.P. Loss of IGF2 imprinting: A potential marker of colorectal cancer risk. Science 2003, 299, 1753–1755.
- Kaneda, A.; Feinberg, A.P. Loss of imprinting of IGF2: A common epigenetic modifier of intestinal tumor risk. Cancer Res. 2005, 65, 11236–11240.
- Kaneda, A.; Wang, C.J.; Cheong, R.; Timp, W.; Onyango, P.; Wen, B.; Iacobuzio-Donahue, C.A.; Ohlsson, R.; Andraos, R.; Pearson, M.A.; et al. Enhanced sensitivity to IGF-II signaling links loss of imprinting of IGF2 to increased cell proliferation and tumor risk. Proc. Natl. Acad. Sci. USA 2007, 104, 20926–20931.
- Gowan, L.K.; Hampton, B.; Hill, D.J.; Schlueter, R.J.; Perdue, J.F. Purification and characterization of a unique high molecular weight form of insulin-like growth factor II. Endocrinology 1987, 121, 449–458.
- Daughaday, W.H.; Trivedi, B.; Baxter, R.C. Abnormal serum IGF-II transport in non-islet cell tumor hypoglycemia results from abnormalities of both IGF binding protein-3 and acid labile subunit and leads to elevation of serum free IGF-II. Endocrine 1995, 3, 425–428.
- Marks, A.G.; Carroll, J.M.; Purnell, J.Q.; Roberts, C.T., Jr. Plasma distribution and signaling activities of IGF-II precursors. Endocrinology 2011, 152, 922–930.
- Greenall, S.A.; Bentley, J.D.; Pearce, L.A.; Scoble, J.A.; Sparrow, L.G.; Bartone, N.A.; Xiao, X.; Baxter, R.C.; Cosgrove, L.J.; Adams, T.E. Biochemical characterization of individual human glycosylated pro-insulin-like growth factor (IGF)-II and big-IGF-II isoforms associated with cancer. J. Biol. Chem. 2013, 288, 59–68.
- Dynkevich, Y.; Rother, K.I.; Whitford, I.; Qureshi, S.; Galiveeti, S.; Szulc, A.L.; Danoff, A.; Breen, T.L.; Kaviani, N.; Shanik, M.H.; et al. Tumors, IGF-2, and hypoglycemia: Insights from the clinic, the laboratory, and the historical archive. Endocr. Rev. 2013, 34, 798–826.
- Livingstone, C. IGF2 and cancer. Endocr. Relat. Cancer 2013, 20, R321–R339.
- Polesskaya, A.; Cuvellier, S.; Naguibneva, I.; Duquet, A.; Moss, E.G.; Harel-Bellan, A. Lin-28 binds IGF-2 mRNA and participates in skeletal myogenesis by increasing translation efficiency. Genes Dev. 2007, 21, 1125–1138.
- Dai, N.; Rapley, J.; Angel, M.; Yanik, M.F.; Blower, M.D.; Avruch, J. mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ribosomal entry. Genes Dev. 2011, 25, 1159–1172.
- Dai, N.; Christiansen, J.; Nielsen, F.C.; Avruch, J. mTOR complex 2 phosphorylates IMP1 cotranslationally to promote IGF2 production and the proliferation of mouse embryonic fibroblasts. Genes Dev. 2013, 27, 301–312.
- Gao, W.; Gu, Y.; Li, Z.; Cai, H.; Peng, Q.; Tu, M.; Kondo, Y.; Shinjo, K.; Zhu, Y.; Zhang, J.; et al. miR-615-5p is epigenetically inactivated and functions as a tumor suppressor in pancreatic ductal adenocarcinoma. Oncogene 2015, 34, 1629–1640.
- Dai, N.; Ji, F.; Wright, J.; Minichiello, L.; Sadreyev, R.; Avruch, J. IGF2 mRNA binding protein-2 is a tumor promoter that drives cancer proliferation through its client mRNAs IGF2 and HMGA1. Elife 2017, 6, e27155.
- Balzeau, J.; Menezes, M.R.; Cao, S.; Hagan, J.P. The LIN28/let-7 Pathway in Cancer. Front Genet 2017, 8, 31.
- Gailhouste, L.; Liew, L.C.; Yasukawa, K.; Hatada, I.; Tanaka, Y.; Kato, T.; Nakagama, H.; Ochiya, T. MEG3-derived miR-493-5p overcomes the oncogenic feature of IGF2-miR-483 loss of imprinting in hepatic cancer cells. Cell Death Dis. 2019, 10, 553.
- Gallagher, E.J.; LeRoith, D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends Endocrinol. Metab. 2010, 21, 610–618.
- Scalia, P.; Pandini, G.; Carnevale, V.; Giordano, A.; Williams, S.J. Identification of a novel EphB4 phosphodegron regulated by the autocrine IGFII/IR(A) axis in malignant mesothelioma. Oncogene 2019, 38, 5987–6001.
- Sciacca, L.; Costantino, A.; Pandini, G.; Mineo, R.; Frasca, F.; Scalia, P.; Sbraccia, P.; Goldfine, I.D.; Vigneri, R.; Belfiore, A. Insulin receptor activation by IGF-II in breast cancers: Evidence for a new autocrine/paracrine mechanism. Oncogene 1999, 18, 2471–2479.
- Martin-Kleiner, I.; Gall Troselj, K. Mannose-6-phosphate/insulin-like growth factor 2 receptor (M6P/IGF2R) in carcinogenesis. Cancer Lett. 2010, 289, 11–22.
- Oshima, A.; Nolan, C.M.; Kyle, J.W.; Grubb, J.H.; Sly, W.S. The human cation-independent mannose 6-phosphate receptor. Cloning and sequence of the full-length cDNA and expression of functional receptor in COS cells. J. Biol. Chem. 1988, 263, 2553–2562.
- Wise, T.L.; Pravtcheva, D.D. Delayed onset of Igf2-induced mammary tumors in Igf2r transgenic mice. Cancer Res. 2006, 66, 1327–1336.
- Sitar, T.; Popowicz, G.M.; Siwanowicz, I.; Huber, R.; Holak, T.A. Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 13028–13033.
- Daughaday, W.H.; Trivedi, B.; Baxter, R.C. Serum “big insulin-like growth factor II” from patients with tumor hypoglycemia lacks normal E-domain O-linked glycosylation, a possible determinant of normal propeptide processing. Proc. Natl. Acad. Sci. USA 1993, 90, 5823–5827.
- Oesterreicher, S.; Blum, W.F.; Schmidt, B.; Braulke, T.; Kubler, B. Interaction of insulin-like growth factor II (IGF-II) with multiple plasma proteins: High affinity binding of plasminogen to IGF-II and IGF-binding protein-3. J. Biol. Chem. 2005, 280, 9994–10000.
- Daughaday, W.H.; Kapadia, M. Significance of abnormal serum binding of insulin-like growth factor II in the development of hypoglycemia in patients with non-islet-cell tumors. Proc. Natl. Acad. Sci. USA 1989, 86, 6778–6782.
- Baxter, R.C.; Daughaday, W.H. Impaired formation of the ternary insulin-like growth factor-binding protein complex in patients with hypoglycemia due to nonislet cell tumors. J. Clin. Endocrinol. Metab. 1991, 73, 696–702.
- Hayman, E.G.; Pierschbacher, M.D.; Ohgren, Y.; Ruoslahti, E. Serum spreading factor (vitronectin) is present at the cell surface and in tissues. Proc. Natl. Acad. Sci. USA 1983, 80, 4003–4007.
- Hayman, E.G.; Pierschbacher, M.D.; Suzuki, S.; Ruoslahti, E. Vitronectin--a major cell attachment-promoting protein in fetal bovine serum. Exp. Cell Res. Suppl. 1985, 160, 245–258.
- Wang, L.; Zhang, X.; Pang, N.; Xiao, L.; Li, Y.; Chen, N.; Ren, M.; Deng, X.; Wu, J. Glycation of vitronectin inhibits VEGF-induced angiogenesis by uncoupling VEGF receptor-2-alphavbeta3 integrin cross-talk. Cell Death Dis. 2015, 6, e1796.
- Upton, Z.; Cuttle, L.; Noble, A.; Kempf, M.; Topping, G.; Malda, J.; Xie, Y.; Mill, J.; Harkin, D.G.; Kravchuk, O.; et al. Vitronectin: Growth factor complexes hold potential as a wound therapy approach. J. Investig. Dermatol. 2008, 128, 1535–1544.
- Arciniegas, E.; Neves, Y.C.; Carrillo, L.M. Potential role for insulin-like growth factor II and vitronectin in the endothelial-mesenchymal transition process. Differentiation 2006, 74, 277–292.
- Coppola, D.; Ferber, A.; Miura, M.; Sell, C.; D’Ambrosio, C.; Rubin, R.; Baserga, R. A functional insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol. Cell. Biol. 1994, 14, 4588–4595.
- Sell, C.; Dumenil, G.; Deveaud, C.; Miura, M.; Coppola, D.; DeAngelis, T.; Rubin, R.; Efstratiadis, A.; Baserga, R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol. Cell. Biol. 1994, 14, 3604–3612.
- Haluska, P.; Shaw, H.M.; Batzel, G.N.; Yin, D.; Molina, J.R.; Molife, L.R.; Yap, T.A.; Roberts, M.L.; Sharma, A.; Gualberto, A.; et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin. Cancer Res. 2007, 13, 5834–5840.
- Karp, D.D.; Paz-Ares, L.G.; Novello, S.; Haluska, P.; Garland, L.; Cardenal, F.; Blakely, L.J.; Eisenberg, P.D.; Langer, C.J.; Blumenschein, G., Jr.; et al. Phase II study of the anti-insulin-like growth factor type 1 receptor antibody CP-751,871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, 2516–2522.
- Golan, T.; Javle, M. Targeting the insulin growth factor pathway in gastrointestinal cancers. Oncology (Williston Park) 2011, 25, 518–526, 529.
- Brana, I.; Berger, R.; Golan, T.; Haluska, P.; Edenfield, J.; Fiorica, J.; Stephenson, J.; Martin, L.P.; Westin, S.; Hanjani, P.; et al. A parallel-arm phase I trial of the humanised anti-IGF-1R antibody dalotuzumab in combination with the AKT inhibitor MK-2206, the mTOR inhibitor ridaforolimus, or the NOTCH inhibitor MK-0752, in patients with advanced solid tumours. Br. J. Cancer 2014, 111, 1932–1944.
- Haluska, P.; Carboni, J.M.; Loegering, D.A.; Lee, F.Y.; Wittman, M.; Saulnier, M.G.; Frennesson, D.B.; Kalli, K.R.; Conover, C.A.; Attar, R.M.; et al. In vitro and in vivo antitumor effects of the dual insulin-like growth factor-I/insulin receptor inhibitor, BMS-554417. Cancer Res. 2006, 66, 362–371.
- Bitelman, C.; Sarfstein, R.; Sarig, M.; Attias-Geva, Z.; Fishman, A.; Werner, H.; Bruchim, I. IGF1R-directed targeted therapy enhances the cytotoxic effect of chemotherapy in endometrial cancer. Cancer Lett. 2013, 335, 153–159.
- Gariboldi, M.B.; Ravizza, R.; Monti, E. The IGFR1 inhibitor NVP-AEW541 disrupts a pro-survival and pro-angiogenic IGF-STAT3-HIF1 pathway in human glioblastoma cells. Biochem. Pharmacol. 2010, 80, 455–462.
- Baserga, R. The decline and fall of the IGF-I receptor. J. Cell. Physiol. 2013, 228, 675–679.
- Beckwith, H.; Yee, D. Minireview: Were the IGF Signaling Inhibitors All Bad? Mol. Endocrinol. 2015, 29, 1549–1557.
- Buck, E.; Gokhale, P.C.; Koujak, S.; Brown, E.; Eyzaguirre, A.; Tao, N.; Rosenfeld-Franklin, M.; Lerner, L.; Chiu, M.I.; Wild, R.; et al. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): Rationale for cotargeting IGF-1R and IR in cancer. Mol. Cancer Ther. 2010, 9, 2652–2664.
- Maina, E.N.; Morris, M.R.; Zatyka, M.; Raval, R.R.; Banks, R.E.; Richards, F.M.; Johnson, C.M.; Maher, E.R. Identification of novel VHL target genes and relationship to hypoxic response pathways. Oncogene 2005, 24, 4549–4558.
- Pezzuto, A.; Carico, E. Role of HIF-1 in Cancer Progression: Novel Insights. A Review. Curr. Mol. Med. 2018, 18, 343–351.
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985.
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364.
- Sinha, U.K.; Kundra, A.; Scalia, P.; Smith, D.L.; Parsa, B.; Masood, R.; Gill, P.S. Expression of EphB4 in head and neck squamous cell carcinoma. Ear Nose Throat J. 2003, 82, 866, 869–870, 887.
- Xia, G.; Kumar, S.R.; Masood, R.; Zhu, S.; Reddy, R.; Krasnoperov, V.; Quinn, D.I.; Henshall, S.M.; Sutherland, R.L.; Pinski, J.K.; et al. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005, 65, 4623–4632.
- Huang, X.; Yamada, Y.; Kidoya, H.; Naito, H.; Nagahama, Y.; Kong, L.; Katoh, S.Y.; Li, W.L.; Ueno, M.; Takakura, N. EphB4 overexpression in B16 melanoma cells affects arterial-venous patterning in tumor angiogenesis. Cancer Res. 2007, 67, 9800–9808.
- Kumar, S.R.; Scehnet, J.S.; Ley, E.J.; Singh, J.; Krasnoperov, V.; Liu, R.; Manchanda, P.K.; Ladner, R.D.; Hawes, D.; Weaver, F.A.; et al. Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res. 2009, 69, 3736–3745.
- Brantley-Sieders, D.M.; Jiang, A.; Sarma, K.; Badu-Nkansah, A.; Walter, D.L.; Shyr, Y.; Chen, J. Eph/ephrin profiling in human breast cancer reveals significant associations between expression level and clinical outcome. PLoS ONE 2011, 6, e24426.
- Becerikli, M.; Merwart, B.; Lam, M.C.; Suppelna, P.; Rittig, A.; Mirmohammedsadegh, A.; Stricker, I.; Theiss, C.; Singer, B.B.; Jacobsen, F.; et al. EPHB4 tyrosine-kinase receptor expression and biological significance in soft tissue sarcoma. Int. J. Cancer 2015, 136, 1781–1791.
- Furlan, D.; Sahnane, N.; Carnevali, I.; Cerutti, R.; Bertoni, F.; Kwee, I.; Uccella, S.; Bertolini, V.; Chiaravalli, A.M.; Capella, C. Up-regulation of the hypoxia-inducible factor-1 transcriptional pathway in colorectal carcinomas. Hum. Pathol. 2008, 39, 1483–1494.
- Kim, K.W.; Bae, S.K.; Lee, O.H.; Bae, M.H.; Lee, M.J.; Park, B.C. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res. 1998, 58, 348–351.
- Vihanto, M.M.; Plock, J.; Erni, D.; Frey, B.M.; Frey, F.J.; Huynh-Do, U. Hypoxia up-regulates expression of Eph receptors and ephrins in mouse skin. FASEB J. 2005, 19, 1689–1691.
- Masood, R.; Kundra, A.; Zhu, S.; Xia, G.; Scalia, P.; Smith, D.L.; Gill, P.S. Malignant mesothelioma growth inhibition by agents that target the VEGF and VEGF-C autocrine loops. Int. J. Cancer 2003, 104, 603–610.
- Stoeltzing, O.; Liu, W.; Reinmuth, N.; Fan, F.; Parikh, A.A.; Bucana, C.D.; Evans, D.B.; Semenza, G.L.; Ellis, L.M. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am. J. Pathol. 2003, 163, 1001–1011.
- Reinmuth, N.; Fan, F.; Liu, W.; Parikh, A.A.; Stoeltzing, O.; Jung, Y.D.; Bucana, C.D.; Radinsky, R.; Gallick, G.E.; Ellis, L.M. Impact of insulin-like growth factor receptor-I function on angiogenesis, growth, and metastasis of colon cancer. Lab. Investig. 2002, 82, 1377–1389.
- Reinmuth, N.; Liu, W.; Fan, F.; Jung, Y.D.; Ahmad, S.A.; Stoeltzing, O.; Bucana, C.D.; Radinsky, R.; Ellis, L.M. Blockade of insulin-like growth factor I receptor function inhibits growth and angiogenesis of colon cancer. Clin. Cancer Res. 2002, 8, 3259–3269.
- Kwon, Y.W.; Kwon, K.S.; Moon, H.E.; Park, J.A.; Choi, K.S.; Kim, Y.S.; Jang, H.S.; Oh, C.K.; Lee, Y.M.; Kwon, Y.G.; et al. Insulin-like growth factor-II regulates the expression of vascular endothelial growth factor by the human keratinocyte cell line HaCaT. J. Investig. Dermatol. 2004, 123, 152–158.
- Mohlin, S.; Hamidian, A.; Pahlman, S. HIF2A and IGF2 expression correlates in human neuroblastoma cells and normal immature sympathetic neuroblasts. Neoplasia 2013, 15, 328–334.
More
Information
Subjects:
Endocrinology & Metabolism
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
432
Revisions:
2 times
(View History)
Update Date:
13 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




