Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Pierluigi Scalia and Version 2 by Peter Tang.
Insulin receptor overexpression is a common event in human cancer. Its overexpression is associated with a relative increase in the expression of its isoform A (IRA), a shorter variant lacking 11 aa in the extracellular domain, conferring high affinity for the binding of IGF-II along with added intracellular signaling specificity for this ligand. Since IGF-II is secreted by the vast majority of malignant solid cancers, where it establishes autocrine stimuli, the co-expression of IGF-II and IRA in cancer provides specific advantages such as apoptosis escape, growth, and proliferation to those cancers bearing such a co-expression pattern.
- IGF(I/II/1R), Insulin-like Growth factor (1 or 2 or receptor)
- IRA/IR-A
- insulin receptor isoform A
- IGF binding protein
- mannose 6 phosphate receptor
- Transferrin
- vitronectin
- hypoxia-inducible factor
- Von Hippel-Lindau gene product
- OCT
1. The Insulin–IGF Ligand and Receptor System in Cancer
The family of the insulin and IGF ligands and receptors are known for their central metabolic and growth-related functions spanning throughout phylogenetically distant organisms [1][2][1,2]. Up to the late 90s, the working model for the role of insulin, IGFs, and their receptors in cancer was based on a scenario dominated by two cousin receptors (the IGF-IR and the insulin receptor) used by their own ligands (IGF-I for the IGF-I receptor and insulin for the insulin receptor), with the IGF-I receptor being considered the sole active mediator of the IGF-I and IGF-II effects, making the latter a favorite target for halting the actions of IGFs in cancer [3][4][3,4]. This paradigm (the IGF1R mandatory transducer hypothesis) has undergone many changes over time with the realization, first, that the IGFIR is able to form hybrid variants with the insulin receptor [5][6][5,6] and, second, that the insulin receptor (IR) could mediate IGF-specific effects. Indeed, genetic evidence of the permissive role of the insulin receptor in a number of developmental and body-size effects mediated by IGF-II had been shown in genetic studies conducted both in null mice [7] and in transgenic mouse models [8]. However, cellular studies were not able to reproduce such a result in vitro until a specific isoform of the insulin receptor, lacking 12 aa in the extracellular portion corresponding to exon 11 (IRA), was shown to be the high-affinity receptor for IGF-II in both fetal and cancer cells [9]. This finding, besides changing a long-rooted view, also presented a distinct role for the insulin receptor far beyond defining it as a pure metabolic and growth permissive mediator. A number of subsequent studies have also demonstrated insulin and IGF ligand-specific differences in their activation of the IRA. In particular, such differences have been demonstrated at the gene expression level [10] and at the signaling level [11][12][11,12]. In this regard, it is worth noting that IGF-II has been found to be able to bind and transduce signals via both the homo-tetrameric, high-affinity RTKs (IGF1R and IRA) and via its hetero-tetrameric (IGF1R/IRA) hybrid receptor in cancer [13].
2. IGF-II is a Bona Fide Oncogenic Ligand Tightly Regulated Under Development and a Commonly Selected Self-Stimulatory Signal in Cancer
In comparison to IGF-I (the main growth-hormone-induced ligand and physiological effect mediator) and as discussed below, IGF-II undergoes different and extensive regulation at the genetic, epigenetic, and post-transcriptional levels. Interestingly, the escape from such tight regulation, as observed in cancer, offers IGF-II distinctive advantages over IGF-I, mainly linked to its ability to activate specific developmental, cellular, and cancer-promoting signals via Insulin receptor A. Overall, IGF-II (a) has a wider possibility of transcriptional regulation and control at the gene promoter level via its four promoters and 10 exons [14][15][15,16], all of which produce a pre-pro-hormone and four isoform variants; (b) is epigenetically regulated via DNA-methylation-dependent and -methylation-independent mechanisms [16][17][18][19][20][21][22][17,18,19,20,21,22,23] with a paternal-restricted expression pattern which is typically lost in cancer (loss of imprinting), causing increased/biallelic expression and bloodstream secretion levels [23][24][25][26][27][28][29][24,25,26,27,28,29,30]; (c) displays post-translational variants derived via differential processing of its pre-pro-hormone leading, to an O-glycosylated high molecular weight form (also known as Big-IGF2) [30][31][31,32] retaining its binding and signaling activity for IRA [32][33] but with acquired capability to elude physiological binders such as the high-affinity scavenging receptor also known as igf2R (binding mannose 6-phosphate as well) and IGFBP3 [33][34]. This and the potential clinical implications have also been reviewed in References [34][35][35,36]. Finally, (d) additional types of regulation of the igf2 transcript linked to non-coding RNA products have also been demonstrated, adding a layer of additional regulation for the igf2 gene [36][37][38][39][40][41][42][37,38,39,40,41,42,43]. The escape from any of these regulatory mechanisms make IGF-II and, more so, its cancer-secreted variant (Big)IGF-II, an ideal autocrine signal for highly demanding cellular requirements such as those found throughout the tumorigenic process [32][33][43][33,34,44]. No less important, (e) IGF-II binds to the IGF1R, the Insulin receptor isoform A, and their hybrid tetra-dimeric forms under different physiological and pathological contexts to exert ligand-receptor-specific cellular effects [7][9][44][45][7,9,14,45]. The contextual roles mentioned above for IGF-I and IGF-II ligands in cancer are graphically summarized in Figure 1.
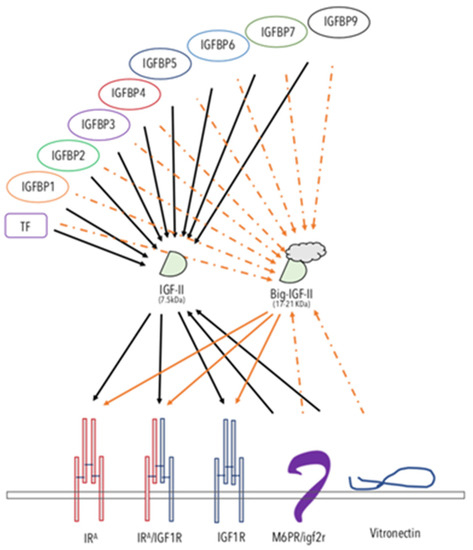
Figure 1. The IGF-II-binding/-neutralizing and -transducing system. The schematic figure summarizes the interactions reported in the literature for IGF-II. The known soluble, extracellular and/or membrane-bound IGF-II-binding proteins are displayed. The solid arrows represent experimentally supported interactions. The dashed arrows represent interactions that have been shown to either be impaired or not yet experimentally confirmed. Arrows from a ligand to its RTKs indicate activating–transducing properties. Arrows towards IGF-II indicate a binding–neutralizing effect. The overview of the comprehensive IGF ligands system role in cancer is shown in Figure 1.
3. The IGF-II Binders: A Fine-Tuned System for the Control of IGF-II Levels in the Extracellular and Tumor Microenvironment
Igf2R/m6pR. The non-transducing/scavenger high-affinity-binding membrane-bound protein known as igf2 receptor (reviewed in Reference [46]), initially thought to be an IGF-II biological mediator, exerts, indeed, most of its IGF-related effects by neutralizing IGF-II and subtracting it from other transducing interactions (namely from the IRA and the IGF1R receptor tyrosine kinases). The key evidence for such a view comes from the demonstration of the absence of a TK domain in its cloned structure [47] and from the oncogenic effect shown by null mutation of igf2rR/m6pR in mice [48]. Indeed, the tumor-suppressing effect of the igf2R/m6pR can be interpreted as further demonstration of the oncogenic potential of IGF-II when present in high levels in vertebrates either at focal tissue levels and/or in the whole organism bloodstream.
The IGFBPs 1-7 and 9. Insulin-like growth factor-II has been shown to bind to most of the soluble extracellular proteins of the IGFBP family, as reviewed elsewhere [49][50][51][49,50,51]. The cumulative effect of IGF-II binding proteins towards the IGF-II levels in the bloodstream might mitigate its increased exposure to local tissues. As a result, some authors have proposed the use of recombinant fragments of IGFBPs as tools to counteract IGF-II oncogenicity. However, the fact that cancer-secreted IGF-II has been found to interact poorly with IGFBPs [33][52][53][34,52,53] might be seen as an escape mechanism for all those cancers using IGF-II as an autocrine growth factor to sustain/maintain their malignant growth features. These potential limits should be taken into consideration.
Transferrin (TF). TF has been shown to be a constitutive component of the 150kDa trimeric IGF binding protein complex found in the bloodstream [51]. Its binding to IGFs (I and II) is less strong than other IGF–IGFBP interactions (where the highest affinity is shown with IGFBP3), and its physiological role is still to be determined.
Vitronectin (VTN). VTN is a constitutive component of the extracellular matrix, involved in cell-to-cell interactions [54][55][54,55]. VTN has been known to bind integrin (ITN) alpha5beta3 and, as such, has been also referred as to integrin receptor [56]. Interestingly, VTN, which bears a somatomedin-like domain, binds IGF-II with high affinity [57][58][57,58]. Although the physiological and pathological roles of VTN interaction with IGF-II are still to be determined, some evidence points at a suppressing role of VTN on IGF-II-induced proliferation and migration via interference with the IGF-II mitogenic signaling (Scalia et al., manuscript in preparation).
Overall, the studies on IGF-II physiological binders are in agreement with the genetic studies supporting a distinctive cancer-promoting role for this IGF, differentiating it from its related cousin, IGF-1. The finding that cancer-secreted IGF-II (big-IGF-II) skips the binding control exerted on mature IGF-II by the IGFBPs (as graphically summarized in Figure 1) suggests that more specific targeting strategies should be considered in order to target this factor in its cancer-specific context.
4. Autocrine IGFII and the IRA Isoform Co-Expression in Cancer: At the Root of IGF-I Receptor Block Resistance
A number of historical results obtained in igf1r null murine fibroblasts (r-cells) both in absence or presence (r+) of human IGFIR expression abundantly demonstrated the isolated mitogenic and growth-linked effects of the IGF-I receptor as a key permissive signal for most of the non-IGF RTKs already targeted in therapy [59][60][59,60]. This triggered the development of a number of IGF-IR specific MAbs [61][62][63][64][61,62,63,64] and small molecules [65][66][65,66]) by the pharma industry in the first decade of the new millennium [67]. Although the experimental evidence showing a functional role for the IGF-II/IRA both in embryonal fibroblasts and in cancer has been available since the late 90a, these findings did not seem to affect the rush of drug developers to bring IGF-IR specific blockers to clinical trials. The specific single blocking of IGFIR in phase II clinical studies failed [68][69][68,69]; the extent of the negative impact of anti-IGF1R monotherapy drugs in clinical studies because of the underscoring of the IGF-II/IRA role could have been easily avoided by including IGF-II/IRA testing in the associated companion diagnostics required for the selection of responsive patients [70].
5. The Autocrine IGF-II/IRA System and the Malignant Switch in Solid Tumors: Hints from the Hypoxic Network
Hypoxia is an intrinsic feature of solid cancers’ tridimensional growth, affecting the inner core of the growing tumor tissue at the pre-vascular stage and clearly affecting the extracellular tumoral microenvironment. Under these circumstances, a tight sequential relationship is established between hypoxia and the expression of hypoxia-induced genes, in which HIF isoforms and VHL have been shown to play a major role [71][72][73,74]. Among the factors that have been shown to be induced or upregulated under hypoxic conditions are VEGF, EphB4, and IGF-II [73][74][75][76][77][78][79][80][81][75,76,77,78,79,80,81,82,83]. However, if for VEGF and EphB4 a solid base of supporting evidence has established their role in angiogenesis and cancer blood vessel formation, in the case of IGF-II, its angiogenic role in the literature has been variably and interchangeably associated with the angiogenic role of IGF-I. Indeed, as mentioned before, there is evidence supporting the notion that IGF-I and IGF-II are all but interchangeable molecules under both physiological and pathological conditions, as shown by their differential affinity and signaling properties via the known IGF1R/IR RTKs. All these structural and ligand–receptor interaction differences provide plenty of biological opportunity for their diversified use by the cell under hypoxic conditions (typical of early-stage and overtly malignant cancers). As for the association of IGF-II with the hypoxic tumor microenvironment, what we know from the published literature is that (1) IGF-II, but not IGF-I, is responsible for the hypoglycemic paraneoplastic effects observed in a number of patients affected by aggressive solid cancers (IGF2omas) [34][35][35,36]; (2) that IGF-II (as well as VEGF-A and EphB4) can be upregulated by HIF and hypoxia [81][82][83][83,84,85]; (3) that VEGF, which also exerts autocrine signals [84][86], can be upregulated via the IGF-IR and the IRA [85][86][87][87,88,89] and is under the control of IGF-II and its autocrine loop under hypoxic experimental conditions [44][82][88][14,84,90]. Interestingly, (4) Hypoxia-induced HIF2alpha can regulate IGF-II [89][91] and (5) IGF-II can upregulate HIF1alpha, which is an inducer of VEGF [44][88][14,90]. All these functional links observed in solid cancers can determine a number of coordinated local events towards the acquisition and/or maintenance of angiogenic, invasive, and metastatic potential, and are compatible with an underscored role of IGF-II in the angiogenic switch, supporting its validation as an anti-angiogenic target. It is worth noting the effects that autocrine IGF-II exerts exclusively via the IRA independently of the IGF1R, such as in regards to EphB4 acute protein level regulation in certain cancers such as malignant mesothelioma [44][14], making the IGF-II/IRA signal in these cancers a distinctive, non-redundant ligand–receptor loop with targetable value. This has been observed in vitro and ex vivo using cancer cells exposed to their conditioned media (pH ~6.9–7.2), a feature common to the extracellular conditions found in solid cancer microenvironments in vivo.
